Abstract
Background
Mammographic breast density is acknowledged as an independent risk factor for breast cancer. Its association with different pathological types and tumors markers is still under evaluation. This study aims to assess the associations of volumetric density grades (VDG) with breast cancer risk in premenopausal and postmenopausal age groups separately. We also aim to assess the association of VDG with hormone receptor status and breast cancer subtypes defined by histology and tumor markers (ER, PR, Her 2-neu and Ki 67).
Results
This retrospective study was done with inclusion of two comparable groups of 185 breast cancer cases and 244 healthy controls. These groups were further divided into pre‑ and postmenopausal subgroups. Mammograms of the cases and controls were evaluated by fully automated volumetric breast density software-VOLPARA and classified into four VDG. The hormone receptor status and breast cancer subtypes defined by histological features and tumor markers in the various VDG were also evaluated. The risk of developing carcinoma was significantly higher in women with high-density breasts (VDG-c + VDG-d) as compared with low-density breasts (VDG-a + VDG-b) in both premenopausal and postmenopausal subgroups. No significant difference was seen in the histopathological characteristics of breast cancer among various VDG.
Conclusions
Our study suggests positive association between high VDG and risk of cancer in both premenopausal and postmenopausal group of Indian women. The hormone receptor status and breast cancer subtypes defined by histology and tumor markers did not reveal any relation to the grades of breast density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Mammographic breast density (MBD) is defined as the relative amount of radio-dense fibro-glandular tissue compared with radiolucent adipose tissue. MBD is acknowledged as an established sovereign breast cancer risk factor [1, 2]. Mammographic density is affected by both heritable and acquired factors with 50–60% of the variance being determined by heritable factors [3, 4]. Other determinants including age, body mass index (BMI), reproductive status, hormone replacement therapy and tamoxifen treatment also influence MBD [3, 5]. The augmented risk of breast cancer lasts for more than or equal to 10 years after density assessment in both pre- and postmenopausal women [6]. However, increased MBD is known to be coupled with decreased mammographic sensitivity [7].
Breast cancer is a diversified disease; different histological subtypes of breast cancer have specific clinical and imaging features that make their diagnosis and management challenging and influence prognosis of the patients [8,9,10,11]. Some of the well-established risk factors for developing breast cancer, such as age, parity, age at first child birth, breast feeding, menopausal status, body mass index, history of hormone therapy and alcohol consumption, have shown associations only with certain histological subtypes, suggesting multifactorial etiology [12,13,14,15]. It is still under evaluation whether breast density differentially affects the risk of certain pathological subtypes of breast cancer. Positive association has been found between high MBD and tumor characteristics that are related to grave prognosis, including larger size, higher grade, estrogen receptor (ER) status and lymph node invasion [16,17,18,19], which could be explained by delay in diagnosis due to reduced sensitivity of mammography and/or aggressive tumor biology [2].
A number of studies have been performed to establish the association of MBD with breast cancer subtype defined by hormone receptor status with the inconsistent results. Most of the studies have primarily investigated the association between MBD and ER status without addressing to Her-2-neu (H2P) or progesterone receptor (PR) status [20, 21]. Few studies found MBD to be associated with both ER positive and ER negative tumors while other found a lack of correlation between MBD and ER status [16, 22, 23]. Similarly, a small case only study found an association between MBD and PR positivity [24], whereas study by Seo et al. [25] found no correlation between high density and human epidermal growth factor receptor 2 (Her2-neu) status.
Breast density assessment can be done using qualitative or quantitative methods. The qualitative method using Breast Imaging Reporting and Data System (BI-RADS) is based on subjective interpretation and has suboptimal reproducibility. To overcome these issues, quantitative methods have been developed for measuring mammographic density in a quantitative manner. One such quantitative density tool is Volpara (Volpara Solutions, Wellington, New Zealand), which is a commercial fully automated product recently developed to measure volumetric breast density. It is based on computerized algorithm that calculates X-ray attenuation at each pixel and converts the attenuation to estimate the tissue composition by creating a density map. By adding these values in the density map, this tool can measure the fibro glandular tissue volume in each breast and breast density is determined as the percentage of fibro-glandular tissue volume. Mapping of that percentage is used to automatically generate volumetric density grade (VDG) a to d, which corresponds to the BI-RADS density categories, A to D [26]. Only few studies have been conducted on the Indian population to look for the association of breast density and cancer risk, and only one study used an automated mammographic volumetric breast density (VBD) assessment in the Indian population [26].
In this study, we aim to-
-
1.
Assess the associations of VDG with breast cancer risk in premenopausal and postmenopausal age groups separately.
-
2.
Assess the association of VDG with hormone receptor status and breast cancer subtypes defined by histology and tumor markers (ER, PR, Her 2-neu and Ki 67).
Methods
Patient selection
This retrospective study in case–control layout was conducted at the Breast Imaging unit of Radiodiagnosis department of our Institute from January 2019 to December 2020. The study was approved by our Institution Ethics committee. All women who had come for screening or diagnostic mammography were included in the study. Women who had undergone mastectomy for breast cancer and had come for surveillance mammography of contralateral breast were also included in the study. Pregnant, lactating women and those with large or bilateral breast lesions were excluded from the study to avoid false density estimation. Women with a history of breast conservation surgery, radiotherapy, chemotherapy or hormonal therapy were also excluded for the same reason. Institute ethics committee waived off the requirement for written informed consent as our study was retrospective in nature.
These patients underwent standard 2D imaging and DBT as a single procedure at the same breast compression on a Digital Mammography Unit (GE Healthcare Senographe Essential 54020/CESM1/SenoClaireA.6) using automatic exposure controls (AECs) called automatic optimization of parameters (AOP). It uses information from the leading part of the detector to vary the scan velocity dynamically, thus adjust anode/filter combinations, peak kilovoltage and target signal-to-noise ratio based on the thickness of compressed breast. Digital mammography was performed in two views: the cranio-caudal (CC) and medio-lateral oblique (MLO) view and tomosynthesis in one view (MLO). Additional views were taken when required. Ultrasound was done whenever required on Supersonic AIXPLORER Multiwave Version 12.2.0808USG scanner.
Volumetric breast density (VBD) assessment
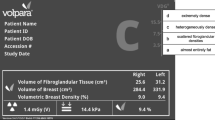
Quantitative volumetric assessment was done by using software Volpara Density (Imaging software version 3.3.2, Volpara Algorithm version Number1.5.4.0, Wellington, New Zealand). Volpara works by selecting a reference point of known breast composition, such as fatty breast tissue just anterior to the chest wall. Then, it works backward and calculates the attenuation at each pixel appertaining to the reference pixel. It calculates the types of tissue that must have been present between the pixel and the x-ray source. Then, the volume of fibro glandular tissue in cubic centimeters, the volume of breast tissue in cubic centimeters and their ratio are obtained to acquire quantitative VBD. The volumetric density grade (VDG) is the VBD threshold at various levels and was determined automatically in each case and was noted. VBD of less than 3.5% is VDG-a, 3.5–7.4% is VDG-b, 7.5–15.5% is VDG-c, and ≥ 15.5% to VDG-d. (Fig. 1)
As per the presentation, mammographic and ultrasonographic findings in each case, BI-RADS assessment category was assigned. Two hundred and fifty-six masses with suspicious features were identified, and underwent trucut biopsy and their pathological findings were available to us for analysis. Out of these 256 lesions, 193 came out to be malignant. We could not get accurate demographic data (like age at menarche, age at first child birth and menopausal status) in 8 of the histologically confirmed cases of malignancy, hence excluded them from study. Remaining 185 patients with biopsy proven malignancy and complete demographic details constituted the “case group.” Two hundred and forty-seven women with no suspicious lesion on mammogram or ultrasound constituted the comparison group. Out of these, three females had undergone hysterectomy for various reasons, hence excluded from the study. Remaining 244 women with negative mammogram and ultrasound constituted the “control group.”
Since menopause is an event which has major influence on the changes in breast tissue, both the groups (cases and controls) were reevaluated after dichotomizing the study subjects (185 cases + 244 controls) into “premenopausal” and “postmenopausal” subgroups. “Menopause” was defined as the time when the woman had missed menses for 12 consecutive months in absence of any physiological, surgical or medical condition that may cause bleeding to stop. A total of 188 women were enrolled in premenopausal (79 cases + 109 controls) and 241 (106 cases + 135 controls) in postmenopausal subgroups.
The ER, PR and Her2-neu status was derived from core biopsies or postoperative specimens and were available for 127 patients. Based on these hormone receptor status, breast carcinomas were classified into three groups- luminal type (ER+, PR+, Her2±), Her2 positive (ER−, PR−, Her2+) and triple-negative breast cancers (ER−, PR−, Her2−) as these cancer groups have therapeutic and prognostic implications. Ki67 index scoring was performed in clinically low-stage luminal like carcinomas (like in T1N0 disease) as only in those cases it could have changed the treatment plan. Cases with > 14% positive nuclei were classified as high Ki-67 expression, and those with < 14% were classified as low Ki-67 expression. It was available only for 14 patients. Hence, further classification of luminal type breast carcinomas into Luminal A and Luminal B was not done.
Statistical analysis
It was carried out on SPSS Version 21.0 statistical Analysis Software. Cross-tab and frequency were used to calculate different parameters among cases and controls and t-test to estimate mean and SD. Mann–Whitney U test was used to compare different parameters between cases and controls. Nonparametric-one sample test was applied for comparison between premenopausal and postmenopausal subgroups. Pearson’s correlation coefficient was measured to find out difference in the distribution of VDG among cases and controls. Bivariate Pearson correlation was measured to look for association between different VDG types and occurrence of carcinoma in premenopausal and postmenopausal women. Chi-square test was applied to evaluate the relation between VDG versus hormone receptor status and breast cancer subtypes defined by histology and tumor markers.
Results
A total of 185 biopsy proven breast cancer patients constituted the “case” group and compared with a group of 244 “controls.” Cross-tab and frequency were used to calculate different parameters among cases and controls and t-test to estimate mean and SD. The mean age of control group was 48.7 ± 10.1 years and that of case group was 50.3 ± 11.02 years. The mean age at menarche in control group was 13.4 ± 0.92 years and that of case group was 13.4 ± 0.76 years. The mean age at first child birth in control group was 20.9 ± 6.7 years and that of case group were 20.7 ± 5.97 years. Premenopausal status was seen in 42.7% of controls and 44.6% of cases. Nulliparity was observed in 6.5% of controls and 5.9% of cases. Exclusive breast feeding was present in 80.3% of controls and 80.0% of cases. All the aforementioned factors were compared in cases and controls by using Mann–Whitney U test. We found no significant difference in distribution of subjects in the two groups and thus the two groups were comparable. (Table 1)
Among the controls, most common breast density type was VDG-b (45.49%) followed by VDG-c (25%), VDG-d (17.21%) and VDG-a (12.29%). Among the cases, the frequency of VDG-c (57.83%) was the highest, followed by VDG-d (26.48%). For computation of correlation, VDG-a and VDG-b were amalgamated together to form low-density breasts (LDB), and VDG-c and VDG-d as high-density breasts (HDB). Pearson’s correlation coefficient was measured and significant difference was found in the distribution of VDG among cases and controls, i.e., more controls had VDG-a (P = 0.009) and VDG-b (P = 0.002) while more cases had VDG-c density (P = 0.015). Distribution of VDG-d among the two groups was insignificant (P 0.065). Similarly more controls had LDB whereas more cases had HDB with Odd’s ratio (OR-2; 95% CI) (P = 0.005 each). (Table 2)
Among premenopausal women, VDG-c was most commonly seen (in 38.29%) followed by grade d (in 31.91%). VDG-b was seen in 27.65% and VDG-a in 2.1% of premenopausal population. In other words, high-density breasts were present in 70.20% and low density in 29.80% of this group. In postmenopausal subgroup VDG-c was again the most common entity (39.83%) followed by grade b (36.09%). VDG-d and VDG-a was noted in 12.86% and 11.20% of these women. Or, we can say, high density was present in 52.69% and low density in 47.29% of the postmenopausal subgroup. Nonparametric-one sample test was applied and significant difference was found in the distribution of VDG-a (P = 0.014) and VDG-b (P = 0.042) between premenopausal and postmenopausal subgroups, i.e., more postmenopausal women had VDG-a and VDG-b type densities. Distribution of VDG-c and VDG-d among the two subgroups was insignificant (P = 0.211 and 0.646, respectively) (Table 3).
In premenopausal and postmenopausal subgroups, malignancy was present in 42.02% and 43.98% of the study population. In premenopausal subgroup, women with VDG-c had maximum number of carcinoma cases (40 out of 72, i.e., 55.5%) followed by VDG-d (29 out of 60, i.e., 48.33%) and VDG-b (10 out of 52, i.e., 19.2%). Only 4 premenopausal women had VDG-a, but none of them had breast carcinoma (zero out of 4). In postmenopausal subgroup, women with VDG-c had highest number of carcinoma cases (67 out of 96, i.e., 69.79%) followed by VDG-d (20 out of 31, i.e., 64.51%), VDG-b (18 out of 87, i.e., 20.68%) and VDG-a (1 out of 27, i.e., 0.03%). Bivariate Pearson correlation was measured and no significant difference was found between different VDG types and occurrence of carcinoma in premenopausal (P = 0.228) or postmenopausal women (P = 0.297).
In premenopausal subgroup, women with HDB (VDG-c plus VDG-d) had more number of carcinoma cases (69 out of 132, i.e., 52.27%) compared with LDB (VDG-a plus VDG-b) (10 out of 56, i.e., 17.85%). Similarly, in postmenopausal subgroup, women with HDB had more number of carcinoma cases (87 out of 127, i.e., 68.50%) compared with LDB (19 out of 114, i.e., 16.66%). Chi-square test was applied and statistically significant difference was found between HDB and risk of developing carcinoma in both premenopausal and postmenopausal subgroups with OR = 2.98 and 2.63, respectively, with 95% CI, (P < 0.001 and P = 0.003, respectively).
In the present study, the infiltrating ductal carcinoma was the most common histopathological type, (n = 175, 94.59%) followed by malignant phyllodes (1.0%, n = 2). Invasive lobular carcinoma, mucinous carcinoma, invasive papillary carcinoma, DCIS, carcino-sarcoma, primary lymphoma of breast, neuroendocrine tumor and Paget's disease were equally distributed in 0.5% cases (n = 1 for each). Out of 175 cases of infiltrating ductal carcinoma (n = 175), maximum cases were seen in VDG-c (n = 104) followed by VDG-d (n = 45), VDG-b (n = 25). Only one woman who had VDG-a type breast had malignancy and it was infiltrating ductal carcinoma. Malignant phyllodes was seen in only 2 cases and both the cases belonged to breasts with VDG-d. Chi-square test was applied and no statistically significant difference in distribution of VDG was found among different histological types (P = 0.312) (Table 4).
The ER, PR and Her2-neu receptor status was available for 127 patients out of 185 cases of cancer breast. ER, PR and Her2 positivity was noted in 43.30% (n = 55), 42.51% (n = 54) and 29.82% (n = 38) of the cases, respectively. Out of the total ER+ cases, maximum were from VDG-c (n = 29, 52.72%) followed by VDG-d (n = 18, 32.72%), VDG-b (n = 7, 12.72%) and VDG-a (n = 1, 1.8%). Similarly, PR+ status was most commonly seen in VDG-c (n = 25, 46.29%) followed by VDG-d (n = 21, 38.9%), VDG-b (n = 7, 12.9%) and VDG-a (n = 1, 1.8%). Her 2 neu expression was most commonly seen in VDG-c (n = 26, 68.4%) followed by VDG-d (n = 8, 21%), and VDG-b (n = 4, 10.5%). It was absent in the only case seen in VDG-a. Chi-square test was applied and no statistically significant difference in distribution of VDG was found as compared to hormone receptor status (P = 0.263 for ER, P = 0.197 for PR and P = 0.75 for Her2-neu). High Ki-67 expression (> 14%) was noted in 11 patients and low Ki-67 expression (< 14%) was seen in 3 patients.
Based on the hormone receptor status, breast carcinomas were classified into three groups—Luminal type (n = 59, 46.45%), Her2 positive (n = 31, 24.4%) and Triple-Negative Breast cancers (n = 37, 29.13%). Ki-67 index was available for 14 patients. Luminal type breast cancer was most commonly distributed in VDG-c (n = 30 or 50.8%) followed by VDG-d (n = 20, i.e., 33.9%), VDG-b (n = 8 or 13.55%) and VDG-a (n = 1 or 1.6%). Similarly, Her2 positive breast cancer was most commonly seen in VDG-c (n = 23, i.e., 74.2%) followed by VDG-d (n = 5 or 16.12%) and VDG-b (n = 3, i.e., 9.6%). TNBC was most commonly seen in VDG-c (n = 22, i.e., 59.4%) followed by VDG-d (n = 10 or 27.02%) and VDG-b (n = 5 or16.12%). Only one carcinoma case was present in VDG-a and that was luminal like. Chi-square test was applied and no statistically significant difference in distribution of VDG was found as compared to hormone receptor groups (P = 0.903 for TNBC, P = 0.237 for Her 2 positive tumors and P = 0.867 for Luminal type cancers).
Discussion
MBD is a radiographic depiction of dense fibro-glandular tissue in the breast in comparison to fatty tissue. Wolfe was the first to describe and classify breast density on mammogram and to put forward the association between different parenchymal patterns and breast cancer risk [18]. Since then, several studies have acknowledged MBD as an individualistic risk factor for breast cancer with an inverse relationship to mammographic sensitivity [19,20,21].
In the present study, we found that statistically significant proportion of controls had LDB, VDG-a and VDG-b. Similarly, more cases had HDB and VDG-c. Distribution of VDG-d among the two groups was not statistically significant. These findings were in accordance with the previous study which revealed that dense mammographic patterns (> 50%) were seen in 16.3% of control subjects and 26.7% of cases [28].
Proportion of HDB in premenopausal group was much higher as compared to the postmenopausal subgroup. These findings correlated well with study by Attam et al. [27] Distribution of LDB in pre and postmenopausal women was statistically different, i.e., more postmenopausal women had LDB. Difference in the distribution of HDB among the two subgroups was insignificant (Table 5).
In the present study, statistically significant difference was found between high-density breasts and risk of developing carcinoma in both premenopausal and postmenopausal subgroups with OR = 2.98 and 2.63, respectively, with 95% CI, (P < 0.001 and P = 0.003, respectively). These results are similar to those of previous study which had shown that the increased risk of breast cancer associated with VDG was found in both premenopausal (P interaction = 0.01) and postmenopausal (P interaction = 0.0003) women and it gets strengthened with higher BMI [28]. Other study suggests that the risk of breast cancer increases with the rise of volumetric density grade (VDG) in postmenopausal women (P < 0.001) only. Risk of breast cancer in these women was significantly high in VDG 4 compared with VDG 1/2 regardless of body mass index [29].
These findings differ from previously published articles which describe increased risk only in premenopausal women. Attam et al. [27] found 3.8 times risk of developing breast cancer in Premenopausal women with breast density of 50% or more as compared to women with breast density of < 10%. (OR = 3.86; 95% CI 1.4–10.1). Koshi et al. [30] also found that in premenopausal women, the odds of having breast cancer was significantly higher for Grade 3 and 4 breasts (odds ratio—3.03 and 3.09, respectively) as compared with Grade 1 and 2 breasts. In postmenopausal women with mammographically dense breast, no such increase in risk was established.
In the present study, most common histopathological type was infiltrating ductal carcinoma. The ER, PR and Her2 status was available for 127 masses out of 185 cases of cancer breast. Based on the hormone receptor status, breast carcinomas were classified into three hormone receptor groups. Luminal type was most commonly seen. No statistically significant difference in distribution of VDG was found as compared to different histological types, hormone receptor status or hormone receptor groups. These findings support the results of previous studies [30,31,32]. However, there are controversies regarding these results as some studies have suggested that percent mammographic density is positively associated with both luminal A and triple-negative breast cancer [33], while others found more strong association with Her2neu tumors when compared with Luminal A tumors [34].
The strengths of our study are quantitative breast density measurements and presence of comparison groups (cases and controls, premenopausal and postmenopausal) but there are few limitations, viz., small sample size and lack of long term follow-up of controls and non-evaluation of BMI (Table 6).
Conclusion
Our study suggests positive association between high VBD and risk of cancer in both premenopausal and postmenopausal group of Indian women. The hormone receptor status and breast cancer subtypes defined by histology and tumor markers did not reveal any relation to the grades of breast density. This can be explained by the fact that breast cancer is a multifactorial disease which results from a strong interplay between genetic and environmental factors through different pathways.
Availability of data and materials
The datasets used during the current study can be made available from the corresponding author on reasonable request.
Abbreviations
- MBD:
-
Mammographic breast density
- BMI:
-
Body mass index
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- H2P:
-
Her-2neu
- BI-RADS:
-
Breast Imaging Reporting and Data System
- VDG:
-
Volumetric density grade
- VBD:
-
Volumetric breast density
- LDB:
-
Low-density breasts
- HDB:
-
High-density breasts
References
Mc Cormack VA, dos Santos SI (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev 15:1159–1169
Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E et al (2007) Mammographic density and the risk and detection of breast cancer. N Engl J Med 356:227–236
Ursin G, Lillie EO, Lee E, Cockburn M, Schork NJ, Cozen W et al (2009) The relative importance of genetics and environment on mammographic density. Cancer Epidemiol Biomark Prev 18:102–112
Boyd NF, Dite GS, Stone J, Gunasekara A, English DR, Mc Credie MR et al (2002) Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med 347:886–894
Martin LJ, Boyd NF (2008) Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res 10:201. https://doi.org/10.1186/bcr1831
Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA et al (1995) Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst 87(21):1622–1629
Wang AT, Vachon CM, Brandt KR, Ghosh K (2014) Breast density and breast cancer risk: a practical review. Mayo Clin Proc 89:548–557
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K et al (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295:2492–2502
Phipps AI, Li CI, Kerlikowske K, Barlow WE, Buist DS (2010) Risk factors for ductal, lobular, and mixed ductal-lobular breast cancer in a screening population. Cancer Epidemiol Biomark Prev 19:1643–1654
Putti TC, El-Rehim DM, Rakha EA, Paish CE, Lee AH, Pinder SE et al (2005) Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol 18:26–35
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B et al (2007) Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomark Prev 16:439–443
Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE (2004) Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst 96:218–228
Rusiecki JA, Holford TR, Zahm SH, Zheng T (2005) Breast cancer risk factors according to joint estrogen receptor and progesterone receptor status. Cancer Detect Prev 29:419–426
Kwan M, Kushi L, Weltzien E, Maring B, Kutner SE, Fulton RS et al (2009) Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res 11:R31. https://doi.org/10.1186/bcr2261
Aiello EJ, Buist DS, White E, Porter PL (2005) Association between mammographic breast density and breast cancer tumor characteristics. Cancer Epidemiol Biomark Prev 14:662–668
Sala E, Solomon L, Warren R, McCann J, Duffy S, Luben R et al (2000) Size, node status and grade of breast as: association with mammographic parenchymal patterns. Eur Radiol 10:157–161
Ding J, Warren R, Girling A, Thompson D, Easton D (2010) Mammographic density, estrogen receptor status and other breast cancer tumor characteristics. Breast J 16:279–289
Roubidoux MA, Bailey JE, Wray LA, Helvie MA (2004) Invasive cancers detected after breast cancer screening yielded a negativeresult: relationship of mammographic density to tumor prognostic factors. Radiology 230:42–48
Antoni S, Sasco AJ, Dos Santos SI, McCormack V (2013) Is mammographicdensity differentially associated with breast cancer according to receptor status? A meta-analysis. Breast Cancer Res Treat 137:337–347
Arora N, King TA, Jacks LM, Stempel MM, Patil S, Morris E et al (2010) Impact of breast density on the presenting features of malignancy. Ann Surg Oncol 17:211–218
Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K (2004) Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomark Prev 13:2090–2095
Morishita M, Ohtsuru A, Hayashi T, Isomoto I, Itoyanagi N, Maeda S et al (2005) Clinical significance of categorisation of mammographic density for breast cancer prognosis. Int J Oncol 26:1307–1312
Fasching PA, Ekici AB, Adamietz BR, Wachter DL, Hein A, Bayer CM et al (2011) Breast cancer risk—genes, environment and clinics. Geburtshilfe Frauenheilkd 12:1056–1066
Seo BK, Pisano ED, Kuzimak CM, Koomen M, Pavic D, Lee Y et al (2006) Correlation of HER-2/neu overexpression with mammography and age distribution in primary breast carcinomas. Acad Radiol 13:1211–1218
Highnam R, Brady SM, Yaffe MJ, Karssemeijer N, Harvey J (2010) Robust breast composition measurement—Volpara TM. In: Martí J, Oliver A, Freixenet J, Martí R (eds) Digital mammography IWDM 2010. Lecture notes in computer science, vol 6136. Springer, Berlin. https://doi.org/10.1007/978-3-642-13666-5_46
Attam A, Kaur N, Saha S, Bhargava SK (2008) Mammographic density as a risk factor for breast cancer in a low risk population. Indian J Cancer 45:50–53
Engmann NJ, Scott CG, Jensen MR, Winham S, Miglioretti DL, Ma L et al (2019) Combined effect of volumetric breast density and body mass index on breast cancer risk. Breast Cancer Res Treat 177:165–173
Kwon Y, Kang HS, Lee ES, Lee KS, Ro J (2014) High volumetric breast density predicts risk for breast cancer in postmenopausal, but not premenopausal. Korean Women Ann Surg Oncol 21:4124–4132
Koshi S, Singla V, Singh T, Prabhakar N, Bal A, Singh G (2020) Mammographic breast density and breast cancer risk: evaluation using volumetric breast density software. J Cancer Res Ther 16:1258–1264
Moshina N, Ursin G, Hoff SR, Akslen LA, Roman M, Sebuødegård S et al (2015) Mammographic density and histopathologic characteristics of screen-detected tumors in the Norwegian Breast Cancer Screening Program. Acta Radiol Open 17:2058460115604340. https://doi.org/10.1177/2058460115604340
Jung N, Kim W (2018) Association of volumetric breast density with clinical and histopathological factors in 205 breast cancer patients. J Korean Soc Radiol 79(1):18
Ma H, Luo J, Press MF, Wang Y, Bernstein L, Ursin G (2009) Is there a difference in the association between percent mammographic density and subtypes of breast cancer? Luminal A and triple-negative breast cancer. Cancer Epidemiol Biomark Prev 18:479–485
Edwards BL, Atkins KA, Stukenborg GJ, Novicoff WM, Larson KN, Cohn WF et al (2017) The association of mammographic density and molecular breast cancer subtype. Cancer Epidemiol Biomark Prev 26:1487–1492
Acknowledgements
We thank Mrs. Suchita Rai, our Mammography Technologist, for her dedication and tireless efforts to get excellent quality Mammograms for patients’ and our benefit.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
NS conceptualized the project and wrote the manuscript. PJ collected the data. AG helped in data collection and manuscript writing. JM helped in data collection. DS helped in manuscript writing and proof read the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Waived off by the Institute Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, N., Joshi, P., Gupta, A. et al. Evaluation of volumetric breast density as a risk factor for breast carcinoma in pre- and postmenopausal women, its association with hormone receptor status and breast carcinoma subtypes defined by histology and tumor markers. Egypt J Radiol Nucl Med 53, 84 (2022). https://doi.org/10.1186/s43055-022-00759-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00759-3