Abstract
Background
Haemosuccus pancreaticus (HP), also known as pseudohaemobilia, is defined as upper gastrointestinal tract hemorrhage originating from the pancreatic duct into the duodenum via the ampulla of Vater or major pancreatic papilla. Pseudoaneurysm formation from the splenic artery is a common complication of pancreatitis; however, upper gastrointestinal bleed resulting from rupture of splenic artery pseudoaneurysm into the pancreatic duct is unusual and challenging to diagnose.
Case presentation
A 26-year-old patient presented with multiple episodes of hematemesis, melena, and intermittent abdominal pain. A contrast-enhanced computed tomography (CECT) scan was performed that demonstrated chronic calcific pancreatitis with a pseudoaneurysm in the splenic artery in close relation to the main pancreatic duct. The patient was immediately shifted for endovascular management, and the pseudoaneurysm was successfully embolized. Post embolization, the patient developed splenic abscess, which was managed by percutaneous catheter drainage.
Conclusion
Due to its rarity and being challenging to diagnose, the mortality rate of HP is high. A high level of expertise is required to diagnose HP, and it should be considered in all upper gastrointestinal bleed patients associated with acute or chronic pancreatitis. Rapid initial CECT and angiography should be performed to confirm the diagnosis, followed by embolization of the bleeding pseudoaneurysm to eliminate the need for surgery. This case report highlights the challenges in the diagnosis and management of HP.
Similar content being viewed by others
Background
Hemosuccus pancreaticus is a rare cause of life-threatening upper gastrointestinal bleeding. The diagnosis is often delayed or missed due to its low incidence and very few documented cases in the previous literature. The diagnosis remains challenging, so a clinician should always consider hemosuccus pancreaticus in the differential diagnosis of all cases of obscure upper gastrointestinal bleeding, particularly associated with acute or chronic pancreatitis.
Case presentation
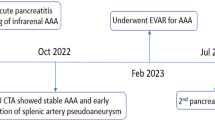
A 26-year-old patient came to our emergency department with abdominal pain, multiple episodes of hematemesis and melena. The bleeding was intermittent and amounted to a maximum of 300 ml per day. Her pulse was 108/min, and her blood pressure was 100/68 mm Hg. Hemoglobin was 2.2 g/dL (reference range: 11–15 g/dL). The patient was treated with blood transfusions and shifted for contrast-enhanced computed tomography (CECT) after stabilizing the vitals. CECT showed features of chronic calcific pancreatitis with evidence of pseudoaneurysm arising from the mid-segment of the splenic artery (Fig. 1). There was no active extravasation of contrast or pseudoaneurysm rupture or direct communication of the pseudoaneurysm with the pancreatic duct. On close inspection, we noticed a hyperdense filling defect (clotted blood) within the dilated main pancreatic duct, which suggests a pseudoaneurysm bleeding into the main pancreatic duct (Fig. 2). The radiological findings were conveyed to the treating gastroenterologists, and the patient was immediately shifted for transarterial embolization after interventional radiology consultation. The celiac and splenic arteries were catheterized using a 5 Fr RC1 (Rosch celiac, Cook, Bloomington, IN, USA) catheter through a 6 Fr introducer sheath inserted from the right common femoral artery. Digital subtraction angiography (DSA) of the splenic artery showed a pseudoaneurysm arising from the mid-segment of the splenic artery without contrast extravasation into the pancreatic duct, which suggests intermittent bleeding (Fig. 3a). The catheter was navigated beyond the pseudoaneurysm, and four detachable coils (Nester embolization coils, Cook) were deployed into the pseudoaneurysm with part of the coil inside the proximal and distal arterial segments (Fig. 3b). The interventional radiology team elected the “sandwich” technique to embolize the distal and proximal flow to the pseudoaneurysm to avoid anterograde and retrograde perfusion. A post- embolization run showed a completely knocked-off splenic artery without filling up the aneurysm (Fig. 3b). The patient was closely followed up for recurrent bleeding. There were no episodes of hematemesis or melena; however, the patient developed splenic abscess, a common complication of embolization (Fig. 4a, b). The patient developed high-grade fever and left hypochondrial pain on the second day of the intervention. Ultrasound was done on the third day, in which splenic abscess was diagnosed. A 10 French malecot catheter was placed within the splenic abscess under CT guidance using the trochar technique (Fig. 5a). The patient was kept on broad-spectrum antibiotics and was discharged after drainage of the splenic abscess (Fig. 5b).
Discussion
Haemosuccus pancreaticus is defined as upper gastrointestinal tract hemorrhage from the ampulla of Vater via the pancreatic duct. The term haemosuccus pancreaticus was coined by Sandblom in 1970 [1]. The most common etiology is a pseudoaneurysm of the peripancreatic arteries due to acute or chronic pancreatitis [2, 3]. It could arise from splenic (60–65%), common hepatic, gastroduodenal, or pancreaticoduodenal artery [4]. HP could be due to rupture of a pseudoaneurysm or an aneurysm of the peripancreatic artery into the duct or may be due to bleeding of the intact or aneurysm-containing artery to the pseudocyst communicating with the duct. Pseudoaneurysm formation is most commonly secondary to chronic pancreatitis and occurs in 10% of this population [4]. Chronic local inflammation is thought to lead to an increased local release of elastase, with either autodigestion of peripancreatic vessels or erosion of a concomitant pseudocyst into the artery [5]. Other rare causes of HP are peripancreatic tumor hemorrhage, congenital abnormality, trauma, and iatrogenic (endoscopic ultrasound-guided fine-needle aspiration cytology) [6]. HP mainly presents with upper gastrointestinal bleeding and colic pain. The bleeding is usually intermittent and repetitive. However, some acute cases manifested as severe hematemesis or shock and needed immediate blood transfusions [7]. The characteristic colic pain results from the increased intraductal pressure caused by obstruction of the Wirsung duct due to clot formation.
In HP, endoscopy can detect blood in the duodenum or active bleeding via the papilla in only 30% of patients [8, 9]. It is difficult to diagnose on endoscopy due to intermittent hemorrhage in most cases and its anatomical location [10, 11]. A review of literature, in patients with intermittent bleeding in nature, the source of bleeding may not be identifiable. Even though endoscopy may be normal in HP, it helps rule out other causes of upper gastrointestinal bleeding (peptic ulcers, erosive gastritis, and varices) [9, 12].
Ultrasound with Doppler could be used to find pseudocysts or aneurysms of the peripancreatic artery. The classical yin-yang flow appearance with a “to-and-fro” waveform can be appreciated within the pseudoaneurysm on Color Doppler.
CECT is an excellent modality for demonstrating pancreatic pathology and demonstrating features of chronic pancreatitis and its complications. It may show the culprit pseudoaneurysm or pseudocyst, possibly demonstrating active bleeding, along with hyperdense material (i.e., fresh blood, clots) in the pancreatic ducts. The characteristic finding of clotted blood in the pancreatic duct, known as the sentinel clot, is seldom seen on multiphase CECT [13].
Ultimately, angiography remains the gold standard for diagnosis and therapy. Angiography identifies the causative artery and allows for delineation of the arterial anatomy and therapeutic intervention [9, 14]. HP is diagnosed on clinical, endoscopic, and radiological findings, and a definitive diagnosis can be established with angiography.
Interventional radiological procedures and surgery are the primary treatment modalities. If the patient is hemodynamically stable, interventional procedures (coil or glue embolization) are effective as an initial treatment in 67–100% of cases [15]. Chandra Mohan et al. have mentioned a high overall success rate of 75–100% [13]. However, embolization of the splenic artery using coils, gelfoam, or glue may lead to splenic infarction, abscess, or septic complications. In cases where extensive collateral blood supply is present, a “sandwich” coil embolization method is preferred to prevent continued retrograde flow to the pseudoaneurysm. In contrast, a pseudoaneurysm in an expendable end artery may be treated by coil embolization of the afferent vessels alone [16]. Benz et al. had used an uncovered metal stent to treat HP, in which the stent was placed across the aneurysmic segment of the splenic artery [17]. This report suggests that implanting a metal stent may be an effective treatment for HP with low complication rates.
For hemodynamically unstable patients, emergency operations (surgical debridement and ligation) are inevitable. Most surgical series have documented a success rate of 70% to 85%, with mortality rates of 20–25% and rebleeding rates of 0–5% [18, 19].
Conclusions
To conclude, a high level of expertise is required to diagnose HP, and it should be considered in all upper gastrointestinal bleed patients associated with acute or chronic pancreatitis. Multiple diagnostic modalities could be helpful; however, rapid initial angiography should be performed to confirm the diagnosis. Early diagnosis and prompt treatment are pertinent in reducing the mortality rate.
Availability of data and materials
Not applicable.
Abbreviations
- HP:
-
Haemosuccus pancreaticus
- CECT:
-
Contrast-enhanced computed tomography
- DSA:
-
Digital subtraction angiography
References
Sandblom P (1970) Gastrointestinal hemorrhage through the pancreatic duct. Ann Surg 171(1):61–66
Cahow CE, Gusberg RJ, Gottlieb LJ (1983) Gastrointestinal hemorrhage from pseudoaneurysms in pancreatic pseudocysts. Am J Surg 145(4):534–541
Maus TP (1993) Pseudoaneurysm hemorrhage as a complication of pancreatitis. Mayo Clinic Proc 68(9):895–896
Han B, Song ZF, Sun B (2012) Hemosuccus pancreaticus: a rare cause of gastrointestinal bleeding. Hepatobiliary Pancreat Dis Int HBPD INT 11(5):479–488
Stanley JC, Frey CF, Miller TA, Lindenauer SM, Child CG (1976) Major arterial hemorrhage: a complication of pancreatic pseudocysts and chronic pancreatitis. Arch Surg 111(4):435–440
Keswani RN (2010) Hemosuccus pancreaticus after endoscopic ultrasound-guided fine needle aspiration of a pancreatic cyst. Endoscopy 42(Suppl 2):E79
Rammohan A, Palaniappan R, Ramaswami S, Perumal SK, Lakshmanan A, Srinivasan UP, Ramasamy R, Sathyanesan J (2013) Hemosuccus pancreaticus: 15-year experience from a tertiary care GI bleed centre. ISRN Radiol. https://doi.org/10.5402/2013/191794
Péroux JL, Arput JP, Saint-Paul MC, Dumas R, Hastier P, Caroli FX, Benchimol D, Delmont JP (1994) Wirsungorrhagia complicating chronic pancreatitis associated with a neuroendocrine tumor of the pancreas. Gastroenterol Clin Biol 18(12):1142–1145
Vimalraj V, Kannan DG, Sukumar R, Rajendran S, Jeswanth S, Jyotibasu D, Ravichandran P, Balachandar TG, Surendran R (2009) Haemosuccus pancreaticus: diagnostic and therapeutic challenges. HPB (Oxford) 11(4):345–350
Callinan AM, Samra JS, Smith RC (2004) Hemosuccus pancreaticus. ANZ J Surg 74(5):395–397
Etienne S, Pessaux P, Tuech JJ, Lada P, Lermite E, Brehant O, Arnaud JP (2005) Hemosuccus pancreaticus: a rare cause of gastrointestinal bleeding. Gastroenterol Clin Biol 29(3):237–242
Sugiki T, Hatori T, Imaizumi T, Harada N, Fukuda A, Kamikozuru H, Yazawa T, Noguchi T, Takasaki K (2003) Two cases of hemosuccus pancreaticus in which hemostasis was achieved by transcatheter arterial embolization. J Hepatobiliary Pancreat Surg 10(6):450–454
Mohan SC, Srinivasan S, Paul S, Chung R, Natarajan SK (2020) Hemosuccus pancreatitis due to a ruptured splenic artery pseudoaneurysm - diagnosis and endovascular management. J Radiol Case Rep 14(5):7–15
Koizumi J, Inoue S, Yonekawa H, Kunieda T (2002) Hemosuccus pancreaticus: diagnosis with CT and MRI and treatment with transcatheter embolization. Abdom Imaging 27(1):77–81
Gambiez LP, Ernst OJ, Merlier OA, Porte HL, Chambon JP, Quandalle PA (1997) Arterial embolization for bleeding pseudocysts complicating chronic pancreatitis. Arch Surg 132(9):1016–1021
Arata MA, Cope C (2000) Principles used in the management of visceral aneurysms. Tech Vasc Interv Radiol 3:124–129
Benz CA, Jakob P, Jakobs R, Riemann JF (2000) Hemosuccus pancreaticus–a rare cause of gastrointestinal bleeding: diagnosis and interventional radiological therapy. Endoscopy 32(5):428–431
Bender JS, Bouwman DL, Levison MA, Weaver DW (1995) Pseudocysts and pseudoaneurysms: surgical strategy. Pancreas 10(2):143–147
Heath DI, Reid AW, Murray WR (1992) Bleeding pseudocysts and pseudoaneurysms in chronic pancreatitis. Br J Surg 79(3):281
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BS, ND and SM made the diagnosis and performed the radiological interventional procedure. MK, HK and CK managed the patient clinically. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication of their clinical details and/or clinical images was obtained from the parent of the patient. A copy of the consent form is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sahoo, B., Panigrahi, M.K., Nayak, H.K. et al. Haemosuccus pancreaticus: a diagnostic challenge and its management through interventional radiology. Egypt J Radiol Nucl Med 53, 68 (2022). https://doi.org/10.1186/s43055-022-00744-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00744-w