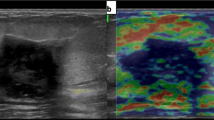
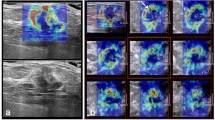
Abstract
Background
Breast cancer is the most common life-threatening cancer in women worldwide. A high number of women are going through biopsy procedures for characterization of breast masses every day and yet 75% of the pathological results prove these masses to be benign. Ultrasound (US) elastography is a non-invasive technique that measures tissue stiffness. It is convenient for differentiating benign from malignant breast tumors. Our study aims to evaluate the role of qualitative ultrasound elastography scoring (ES), quantitative mass strain ratio (SR), and shear wave elasticity ratio (SWER) in differentiation between benign and malignant breast lesions.
Results
Among 51 female patients with 77 histopathologically proved breast lesions, 57 breast masses were malignant and 20 were benign. All patients were examined by B-mode ultrasound then strain and shear wave elastographic examinations using ultrasound machine (Logiq E9, GE Medical Systems) with 8.5–12 MHz high-frequency probes. Our study showed that ES best cut-off point > 3 with sensitivity, specificity, PPV, NPP, accuracy was 94.7%, 85%, 94.7%, 85%, 90.9%, respectively, and AUC = 0.926 at P < 0.001, mass SR the best cut-off point > 4.6 with sensitivity, specificity, PPV, NPP, accuracy was 96.5%, 80%, 93.2%, 88.9%, 92.2%, respectively, and AUC = 0.860 at P < 0.001, SWER the best cut-off value > 4.9 with sensitivity, specificity, PPV, NPP and accuracy was 91.2%, 80%, 92.9%, 76.2%, 93.5%, respectively, and AUC = 0.890 at P < 0.001. The mean mass strain ratio for malignant lesions is 10.1 ± 3.7 SD and for solid benign lesions 4.7 ± 4.3 SD (p value 0.001). The mean shear wave elasticity ratio for malignant lesions is 10.6 ± 5.4 SD and for benign (solid and cystic) lesions 3.6 ± 4.2 SD. Using ROC curve and Youden index, the difference in diagnostic performance between ES, SR and SWER was not significant in differentiation between benign and malignant breast lesions and also was non-significant difference when comparing them with conventional US alone.
Conclusion
ES, SR, and SWER have a high diagnostic performance in differentiating malignant from benign breast lesions with no statistically significant difference between them.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Breast cancer is the most common life-threatening cancer in women worldwide [1]. It accounts for 22.9% of all new female cancers and is considered the most common cancer among women in developed and developing countries and the leading cause of cancer-related mortality accounting for 29.1% of the cancer-related deaths in Egypt in 2008 [2].
A high number of women are going through biopsy procedures for characterization of breast masses every day and yet 75% of these patients the pathological results prove these masses to be benign [3, 4]. Ultrasound (US) elastography is a non-invasive technique that measures tissue stiffness and elasticity [5]. It is convenient for differentiating benign from malignant breast tumors [6]. For breasts, the most frequently used elastographic techniques are strain and shear wave elastography [6].
Strain elastography produces an image based on the relative displacement of the tissue from an external (manual compression of the transducer) or patient source. It is difficult to measure the amount of the force or stress during compression, and the absolute elasticity cannot be calculated [5, 7].
The Tsukuba elasticity score was developed by Itoh and Ueno for the characterization of breast lesions based on color pattern with elastography [8]. US elastography can examine only focal changes, which could be identified with the standard US. A semi-quantitative assessment of elasticity is much more objective. A “strain ratio,” is calculated as a ratio of the strain index of the lesion to the strain index of healthy tissue at the same level [9]. With shear waves that are induced by the acoustic radiation force and propagate transversely in the tissue, SWE can provide a semitransparent color-coded image displaying the shear wave velocity (m/s) or elasticity (kPa) for each pixel in real-time, as the speed of the shear waves can be measured and is linked to the Young modulus (kPa) [7, 10,11,12].
Several clinical studies concluded that sonoelastography was useful for differentiation of benign and malignant breast lesions, with sensitivity of 78.0% to 100% and specificity of 21.0–98.5% [13].
Patients and methods
Our prospective study was conducted in the period between May 2020 and August 2021. Fifty-one female patients with their ages ranged from 25 to 75 years old "mean age 50.3" were recruited from the department of surgical oncology and were examined by B-mode breast ultrasonography and ultrasound elastography, before they underwent histopathological examination by surgical excision (9 cases) or tru cut needle biopsy (rest of cases) of the lesion. The examination was done by ultrasound machine (Logiq E9, GE Medical Systems) with 8.5–12 MHz high-frequency probes. Ethics committee approval was obtained in addition to informed written consent from all patients.
The presenting symptoms were forty-six cases presented with palpable breast mass, two cases presented with bleeding per nipple, two cases presented with breast pain, and one case had an accidentally discovered mass during the breast screening program.
Grayscale B-mode ultrasound
Breast lesions were evaluated first by conventional B-mode ultrasound. Radial scanning of the entire breast and axillary tail of both sides was performed. Longitudinal and transverse images of breast lesions were obtained. Images were classified into five categories according to the BI-RADS system of the American College of Radiology Criteria for ultrasound.
Ultrasound elastography
Mass strain elastographic images were generated using dual-mode the grayscale image on the left and the elastographic image on the right, freehand manual compression by applying the transducer very lightly, perpendicular to the chest wall. The dedicated software provided feedback regarding the use of adequate compression and elastographic quality. Since tissue becomes stiffer when compressed, we avoided compression during shear wave elastography to prevent artifact generation. Color-scale image of strain elastography ranging from red to blue, blue represented stiff tissue and red represented soft tissue, in color-scale image of shear wave elastography red represented stiff tissue and blue represented soft tissue. To calculate the mass strain ratio and shear wave elasticity ratios, the region of interest (ROI) was placed in the hardest portion of lesions on the color-scale images (E2) and another ROI was placed at the reference fat tissue at the same horizontal level and closet to the lesion (E1). ROI was automatically drawn by placing a 2–5 mm circle. Each solid breast mass was given an elasticity score according to the 5 point elasticity Tsukuba score developed by Itoh et al.
Analysis of images
By B-mode ultrasonography, breast lesions were evaluated as regards
Size, shape, echogenicity, borders, margins, posterior acoustic properties, type of the lesions, nature of the lesion, and the lesions were categorized according to BI-RADS score.
By sonoelastography, breast lesions were evaluated as regards
-
Five-point elasticity Tsukuba score for elasticity score by strain elastography [8]:
-
Score 1 Entire area is evenly shaded green, as is the surrounding tissue
-
Score 2 Lesion area shows a mosaic pattern of green, blue, and red
-
Score 3 Central part of the area is blue (stiff), and the peripheral part is green (soft)
-
Score 4 Entire area is blue (stiff)
-
Score 5 Entire area and its surrounding area are blue (stiff)
-
-
The cutoff point of mass strain ratio and shear wave elasticity ratio between the benign and malignant breast lesions was calculated.
Statistical analysis
Statistical analysis was done using IBM SPSS version 20. The mass strain ratio and shear wave elasticity ratio cutoff value was calculated between the benign and malignant groups.
We used MEDCALC to make the receiver operating characteristic (ROC) curve and Youden index to calculate the diagnostic capability of ES, mass SR, and SWER ratio cutoff value for use in the differentiation between the benign and malignant groups that had statistically significant differences between their mean mass strain ratio and shear wave elasticity ratio. The probability (p value) was considered significant when P < 0.05.
The efficacies of the significant parameters were assessed in terms of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy.
Results
Our study was done on 51 patients, with 77 breast masses, 57 of them were malignant and 20 were benign masses. The age ranged from 25 to 75 years (mean age 49.9). All breast lesions were diagnosed pathologically. Demographic data of the patients are summarized in Table 1. Forty-six cases presented with palpable breast mass, two with bleeding per nipple, two with breast pain, and one case was accidentally discovered during the breast screening program.
Surgical and histopathological findings
The benign lesions were 10 fibroadenomas, 1 benign phyllodes tumor, 1 intraductal papillomata, 1 focal adenosis, 2 fibrocystic disease, 2 granulomatous mastitis, 1 foreign body reaction, 1 mass of fibrosis and 1 mass of infected duct ectasia.
While the malignant lesions were 48 invasive duct carcinoma, 1 invasive micropapillary carcinoma, 3 ductal carcinoma in situ, 2 undifferentiated carcinoma, 1 lobular carcinoma, 1 mixed invasive ductal and lobular carcinoma and one case of lymphoma.
Ultrasound images analysis
Breast lesions were evaluated by the B-mode US regarding:
Size mean ± SD and median length of the masses were 2 ± 1.43 cm and 1.8 cm, respectively, while the width was 2.9 ± 2.04 cm and 2.3 cm, respectively. The longest mass was 7.8 cm, while the widest was 10.2 cm.
Shape 9 lesions had an oval shape, 12 were rounded, 7 lesions were lobular and 49 lesions were irregular. Echogenicity 67 lesions were hypoechoic, 7 lesions were isoechoic, 2 lesions were hyperechoic and one lesion was anechoic. Borders and margins 6 lesions had circumscribed regular margins, 10 lesions had micro-lobulated margins, 5 lesions had angular margins, 22 lesions had indistinct margins and 34 lesions had speculated margins. Posterior acoustic properties 4 lesions showed posterior acoustic enhancement, 41 lesions showed posterior acoustic shadowing, 32 lesions had no posterior features. Type of the lesions 75 were described as a mass lesion and 2 lesions were described as an architecture distortion. Nature of the lesion 69 lesions were solid, 3 lesions were cystic and 5 were mixed solid and cystic. The lesions were categorized according to BI-RADS score as follows BI-RADS 3: 18 lesions, BI-RADS 4: 43 lesions (4a: 13 lesions + 4b: 1 lesion + 4c: 29 lesions) and BI-RADS 5: 16 cases.
→ By US when considering BI-RADS 1, 2, and 3 as benign and BI-RADS 4 and 5 as malignant, by Fisher's Exact Test: the sensitivity, specificity, PPV, NPP, false-positive, false-negative and accuracy was 94.7%, 75%, 91.5%, 83.3%, 25%, 5.3% and 89.6%, respectively (at P < 0.001).
Ultrasound elastography images analysis
Elastography score ES 2: 11 lesions, ES 3: 9 lesions, ES 4: 48, and ES 5: 9 lesions. ROC curve and Youden index were used to elicit the best cut-off in differentiating benign and malignant mass groups using UE (ultrasound elastography); this cut-off value was > 3, with sensitivity, specificity, PPV, NPP, false-positive, false-negative, and accuracy was 94.7%, 85%, 94.7%, 85%, 15%, 5.3%, and 90.9%, respectively, and AUC = 0.926 (significant at P < 0.001). ROC curve and Youden index were used to compare sensitivity and specificity between the conventional US versus elasticity score and the difference in diagnostic performance was found to be statistically insignificant (P value = 0.2108).
ROC curve and Youden index were used to elicit the best cut-off in differentiating benign and malignant mass groups using mass strain ratio; this cut-off value was > 4.6 with sensitivity, specificity, PPV, NPP, false-positive, false-negative and accuracy was 96.5%, 80%, 93.2%, 88.9%, 20%, 3.5%, and 92.2%, respectively, and AUC = 0.860. The mean mass strain ratio for malignant lesions is 10.1 ± 3.7 SD and for solid benign lesions 4.7 ± 4.3 SD (p value 0.001). They were compared by one way ANOVA test and showed a statistically significant difference in mass strain ratio (p value = 0.000).
ROC curve and Youden index were used to compare sensitivity and specificity between the grayscale US alone versus mass strain ratio, and the difference in diagnostic performance was found to be statistically insignificant (P = 0.8644).
ROC curve and Youden index were used to elicit the best cut-off in differentiating benign and malignant mass groups using shear wave elasticity ratio; this cut off value was > 4.9, with sensitivity, specificity, PPV, NPP, false-positive, false-negative and accuracy was 91.2%, 80%, 92.9%, 76.2%, 20%, 8.8%, and 93.5%, respectively, and AUC = 0.890. The mean shear wave elasticity ratio for malignant lesions is 10.6 ± 5.4 SD and for benign (solid and cystic) lesions 3.6 ± 4.2 SD. They were compared by the Mann–Whitney test and showed a statistically significant difference in shear wave elasticity ratio (p value = 0.000).
ROC curve and Youden index were used to compare sensitivity and specificity between the conventional US versus shear wave elasticity ratio and found that the difference in diagnostic performance was not significant (P = 0.3735). ROC curve and Youden index were used to compare sensitivity and specificity between the elasticity score, strain ratio, and shear wave elasticity ratio and the difference in diagnostic performance was found to be statistically insignificant.
Discussion
Strain elastography mainly provides qualitative information, although strain ratios may be calculated by comparing a lesion to the surrounding normal tissue giving semiquantitative analysis. Strain ratios have been correlated with the benignity or malignancy characteristics of lesions where lower ratios are seen with benign lesions in comparison to malignant lesions [14, 15]. As the SR ratio of > 3 is generally considered suspicious for malignancy [16]. Determining the universal exact cut-off between the benign and malignant breast lesions is one of the greatest challenges in performing strain ratio elastography and shear wave elastography. As in our study, previously published studies have varied cut-offs determined through ROC curves.
Our results elicit the best cut-off in differentiating benign from malignant breast lesions using ES is > 3, with sensitivity and specificity 94.7% and 85%, respectively (at P < 0.001), and there is a significant difference in the diagnostic performance of elasticity scores for differentiation between benign and malignant breast lesions. These results are comparable to those obtained by Mutala et al. [17], Fleury et al. [18], Itoh et al. [8] and Schaefer et al. [14]. Our experience in this study is similar to that reported by Mutala et al. [17] that the size of the breast did not affect the diagnostic performance of the ES, SR, or SWER in differentiation between benign and malignant lesions, even if the mass which was on the larger side of the scale. Fleury et al. [18] found that the USE positive predictive value, specificity, and diagnostic accuracy of the scores were 76.5, 95.9, and 94.7%, respectively. They concluded that classification by elastography can be used as an important tool combined with B mode evaluation for differentiating benign and malignant lesions of the breast.
Semi-Quantitative UE analysis was performed using an automatically calculated strain ratio. The best strain ratio cut-off value allows significant differentiation between benign and malignant breast entities was > 4.6 (AUC = 0.860) with sensitivity, specificity and accuracy were 96.5%, 80% and 92.2% (p value < 0.001). Our study results were comparable to the SR cut off value as reported by Mutala et al. [17] and Liu et al. [19], Kumm and Szabunio [20], higher SR cut off value was obtained by Ueno et al. [21], and lower SR cut-off value was reported by Gheonea, et al. [22]. Mutala et al. [17] reported the best SR cutoff value (4.2) with a sensitivity of 0.93 and specificity of 0.96. For the strain score ROC curve, a value of three or greater was considered positive with a sensitivity of 0.86 and specificity of 0.96. Liu et al. [19] reported SR cut-off point of 4.15 (AUC = 0.891) which allowed significant differentiation of malignant and benign lesions with a sensitivity of 92.2%, a specificity of 72.5%, and an accuracy of 86.1%, in a retrospective study they enrolled. In the Ueno et al. [21] study the diagnostic performance of the fat to lesion strain ratio was evaluated, the ROI for the reference was placed in the superficial fat tissue adjacent to the skin layer, the reported SR cutoff point of 4.8 with a sensitivity of 76.6%, specificity of 76.8%, and accuracy of 76.7%. (AUC = 0.818). This SR cutoff value for differentiating benign and malignant masses was higher than that detected in our study and also in other studies. So the selection of the reference ROI is important to obtain the correct SR indicating the real stiffness of the tissue [21]. The reference fat tissues located at the same depth as the target lesion would be the most adequate reference point. This was in agreement with Cho et al. [13].
The false positives in our study encountered were fibroadenoma with macro-calcifications inside, infected duct ectasia, chronic granulomatous mastitis with chronic breast abscess lesions. This can be explained by the fact that these lesions can lead to increased stiffness (Figs. 1, 2, 3).
A 45-year-old lady, presented with a left breast mass. a Ultrasound strain elastography of the mass demonstrates elastography score E 3 with a strain ratio of 12.3 denoting malignancy. b Shear wave elastography shows the elasticity ratio of 13.1, Pathologic findings revealed invasive duct carcinoma grade II
A 40-year-old lady, presented with a right breast mass. a Ultrasound strain elastography of the lesion demonstrates elastography score E 3 with a strain ratio of 3. b Shear wave elastography shows the elasticity ratio of 4, pathologic findings revealed benign proliferative breast disease mostly fibroadenoma
A 55-year-old lady, presented with a right breast mass. a Ultrasound strain elastography of the lesion demonstrates elastography score E 4 with a strain ratio of 15 denoting malignancy. b Shear wave elastography shows an elasticity ratio of 9.9. Pathologic findings revealed invasive duct carcinoma grade II
Quantitative UE analysis was performed using an automatically calculated shear wave elasticity ratio. In our study, the best shear wave elasticity ratio cut-off value that allows significant differentiation between benign and malignant mass groups was > 4.9 (AUC = 0.890), with sensitivity, specificity was 91.2%, 80%, and accuracy was 93.5% at p < 0.001. Little published data about the direct comparison of diagnostic performance between strain and shear wave elastography in the same patients exists and few previous studies have compared strain and elasticity ratios, as determined by strain and shear wave elastography of benign and malignant breast tumors in the same patients [6, 7, 23]. Our results are comparable with SWER cut-off value reported by Kim et al. [24], and lower SWER cut-off value was reported by Au et al. [25].
Kim et al. [24] reported that the cut-off elasticity ratio of shear wave elastography was 4 with sensitivity 65.9%; specificity 89.1%; AUC, 0.810; 95% CI 0.723–0.879).
In our study, the ES, SR, and SWE had good diagnostic accuracy in differentiation between benign and malignant breast masses and their difference in diagnostic performance wasn’t statistically significant. This was in agreement with Zhi et al. [26] and Thomas et al. [27]. Semi-quantitatively calculated strain and elasticity ratios offer the potential benefit of more objective measurement methods, which correlate with the tissue’s elasticity characteristics. However, the reported strain or elasticity ratios varied with the study population and the specific elastography machine used [24].
In our study, the difference in diagnostic performance was not significant when comparing conventional US versus ES or SR or SWER and this was similar to some previous studies reporting that the joint use of US and shear wave images showed no statistically significant improvement over the use of US alone [24, 28]. And similar to Mutala, et al. who had reported that semiquantitative ultrasound elastography has good diagnostic accuracy in differentiating benign from malignant breast solid lesions with no statistically significant difference between strain score and strain ratio in sensitivity, specificity, and accuracy [17].
Conclusion and future perspective
ES, SR, and SWER have a high diagnostic performance in differentiating malignant from benign breast lesions with no statistically significant difference between them.
Limitation
The limitation of our study is the relatively small number of patients and wide range of SR and SWER values where some malignant lesions showed low SR, while some benign lesions showed high SR. This caused overlapping in the diagnoses of some benign and malignant breast lesions. However, the use of ES together with SR and also the B-mode US allowed the correct diagnosis.
Availability of data and materials
Available on request with the corresponding author.
Abbreviations
- US:
-
Ultrasound
- UE:
-
Ultrasound elastography
- ES:
-
Elasticity score
- SR:
-
Strain ratio
- SWE:
-
Shear wave elastography
- SWER:
-
Shear wave elastography ratio
- AUC:
-
Area under the receiver operating characteristic curve
- SD:
-
Standard deviation
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- BI-RADS:
-
Breast imaging-reporting and data system
- kPa:
-
Kilo Pascals
- ROI:
-
Region of interest
- ROC:
-
Receiver operating characteristic
- ACR:
-
American College of Radiology
References
Helal M et al (2017) Accuracy of CESM versus conventional mammography and ultrasound in evaluation of BI-RADS 3 and 4 breast lesions with pathological correlation. Egypt J Radiol Nucl Med 48(3):741–750
Zeeneldin AA, Ramadan M, Gaber AA et al (2013) Clinico-pathological features of breast carcinoma in elderly Egyptian patients: a comparison with the non-elderly using population-based data. J Egypt Natl Cancer Inst 25(1):5–11
Garra BS, Ignacio E et al (1997) Elastography of breast lesions: initial clinical results. Radiology 202(1):79–86
Thitaikumar A, Mobbs LM et al (2008) Breast tumor classification using axial shear strain elastography: a feasibility study. Phys Med Biol 53(17):4809
Barr RG (2012) Sonographic breast elastography: a primer. J Ultrasound Med 31(5):773–783
Seo M, Ahn HS et al (2018) Comparison and combination of strain and shear wave elastography of breast masses for differentiation of benign and malignant lesions by quantitative assessment: preliminary study. J Ultrasound Med 37(1):99–109
Youk JH, Son EJ et al (2014) Comparison of strain and shear wave elastography for the differentiation of benign from malignant breast lesions, combined with B-mode ultrasonography: qualitative and quantitative assessments. Ultrasound Med Biol 40(10):2336–2344
Itoh A, Ueno E et al (2006) Breast disease: clinical application of US elastography for diagnosis. Radiology 239(2):341–350
Ning C-P, Jiang S-Q et al (2012) The value of strain ratio in differential diagnosis of thyroid solid nodules. Eur J Radiol 81(2):286–291
Athanasiou A, Tardivon A et al (2010) Breast lesions: quantitative elastography with supersonic shear imaging—preliminary results. Radiology 256(1):297–303
Hyun LS, Chang JM et al (2014) Practice guideline for the performance of breast ultrasound elastography. Ultrasonography 33(1):3
Youk JH, Son EJ et al (2014) Shear-wave elastography for breast masses: local shear wave speed (m/sec) versus Young modulus (kPa). Ultrasonography 33(1):34
Cho N, Moon WK et al (2010) Sonoelastographic strain index for differentiation of benign and malignant nonpalpable breast masses. J Ultrasound Med 29(1):1–7
Schaefer FKW, Heer I et al (2011) Breast ultrasound elastography—results of 193 breast lesions in a prospective study with histopathologic correlation. Eur J Radiol 77(3):450–456
Zhao QL, Ruan LT et al (2012) Diagnosis of solid breast lesions by elastography 5-point score and strain ratio method. Eur J Radiol 81(11):3245–3249
Wang Z, Yang T, Wu Z et al (2010) Correlation between elastography score and strain rate ratio in breast small tumor. J Central South Univ Med Sci 35(9):928–932
Mutala TM, Ndaiga P, Aywak A (2016) Comparison of qualitative and semiquantitative strain elastography in breast lesions for diagnostic accuracy. Cancer Imaging 16(1):1–7
Fleury Ede F, Fleury JC, Piato S, Roveda D Jr (2009) New elastographic classification of breast lesions during and after compression. Diagn Interv Radiol 15(2):96
Liu XJ, Zhu Y, Liu PF et al (2015) Elastography for breast cancer diagnosis: a useful tool for small and BI-RADS 4 lesions. Asian Pac J Cancer Prev 15(24):10739–10743
Kumm TR, Szabunio MM (2010) Elastography for the characterization of breast lesions: initial clinical experience. Cancer Control 17(3):156–161
Ueno E (2007) New quantitative method in breast elastography: fat-lesion ratio (FLR). Radiological Society of North America scientific assembly and annual meeting, 2007
Gheonea IA, Stoica Z et al (2011) Differential diagnosis of breast lesions using ultrasound elastography. Indian J Radiol Imaging 21(4):301
Barr RG, Zhang Z (2015) Shear-wave elastography of the breast: value of a quality measure and comparison with strain elastography. Radiology 275(1):45–53
Kim HJ, Kim SM et al (2018) Comparison of strain and shear wave elastography for qualitative and quantitative assessment of breast masses in the same population. Sci Rep 8(1):1–11
Au F-F, Ghai S et al (2014) Diagnostic performance of quantitative shear wave elastography in the evaluation of solid breast masses: determination of the most discriminatory parameter. Am J Roentgenol 203(3):W328–W336
Zhi H, Xiao X-Y et al (2010) Ultrasonic elastography in breast cancer diagnosis: strain ratio vs 5-point scale. Acad Radiol 17(10):1227–1233
Thomas A, Degenhardt F et al (2010) Significant differentiation of focal breast lesions: calculation of strain ratio in breast sonoelastography. Acad Radiol 17(5):558–563
Berg WA, Mendelson EB et al (2015) Quantitative maximum shear-wave stiffness of breast masses as a predictor of histopathologic severity. Am J Roentgenol 205(2):448–455
Acknowledgements
Not applicable.
Funding
This research was funded by the research unit at South Egypt Cancer Institute.
Author information
Authors and Affiliations
Contributions
RS, HK and OM designed the research. RS performed the research and wrote the manuscript. RS and HK analyzed the collected data. HK, OM, and MK revised data and manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted after approval of the Ethical Committee of South Egypt Cancer Institute (Approval Number 439). Informed written consent was obtained from each participant.
Consent for publication
All patients included in this study gave a written informed consent to publish the data contained in this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shehata, R.M.A., El-Sharkawy, M.A.M., Mahmoud, O.M. et al. Qualitative and quantitative strain and shear wave elastography paradigm in differentiation of breast lesions. Egypt J Radiol Nucl Med 53, 23 (2022). https://doi.org/10.1186/s43055-022-00697-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00697-0