Abstract
Background
We performed routine spinal fluid drainage for patients who underwent TEVAR for thoracic aortic pathology together with left subclavian artery coverage, which was needed for achievement of a safe proximal sealing zone. We assessed the occurrence of spinal cord ischemia as well the rate of occurrence of other complications such as stroke, and upper limb ischemia.
Results
This was a case series study done between July 2014 and April 2020, in them all the left subclavian artery was covered to ensure a proximal safe seal zone. Routine spinal fluid drainage was done, keeping the spinal fluid pressure < 10–15 mmHg with catheter in place for 48 h. Data was obtained from twenty-three patients who underwent TEVAR for thoracic aortic dissection (73.91%), thoracic aortic aneurysm (21.74%), or ulcer (4.35%). Planning was based upon multi-slice computed tomographic angiography and covering the left subclavian was mandatory to achieve a proximal sealing zone. Technical success was achieved in 100% of cases. 4.35% of patients had three endograft, 56.52% had two endografts, 39.13% had one endograft. All patients lost their radial pulsations immediately after implantation, 8.70% developed post implantation syndrome(fever) that was managed conservatively, 4.35% developed stroke related to the anterior circulation, 4.35% developed signs of spinal cord ischemia. During the follow up, one patient died within 6 h after the procedure due to extensive myocardial infarction (patient was scheduled for CABG after our procedure). 17.40% developed upper limb symptoms that were tolerable and were managed conservatively.
Conclusion
By adopting routine spinal cord drainage and pressure monitoring, we can consider not to revascularize the left subclavian artery prior to TEVAR if it will be covered.
Similar content being viewed by others
Background
Since Dake et al. demonstrated their preliminary results for treating thoracic aortic aneurysms using endovascular stent graft, TEVAR became an essential treatment modality for treating thoracic aortic dissections and aneurysms [1]. the introduction of this technique has widely replaced open technique and increased the target population to be treated especially ruptured cases [2]. During this procedure it is mandatory to keep perfusion of the innominate artery and the left common carotid artery while maintain antegrade flow of the left subclavian is still controversial [3,4,5]. In order to have a safe sealing zone the left subclavian artery is covered in almost 40% of cases [6,7,8]. In 2007, Buth et al. reported a fourfold increase in the incidence of postoperative paraplegia with intentional coverage of the left subclavian artery [9]. Also Chung et al. showed an increased risk of stroke by 2.17 fold [10]. But up till now there was no consensus regarding revascularization of the left subclavian artery, other investigators concluded that it is safe to cover the left subclavian artery without revascularization such as, Lee et al., who concluded that it is safe to cover the left subclavian artery without revascularization in patients having bilateral patent vertebrobasilar junctions [11]. Another notion by Maldonado et al., who noted that revascularization of the left subclavian artery carried a higher incidence of cerebrovascular accidents [12]. But what about CSF drainage during TEVAR?. Drainage of CSF has been widely practiced during open thoracic aortic repair, as well it has been accepted and recommended during open repair of thoracic aortic pathology [13, 14].
On the contrary, the role of CSF drainage in TEVAR is less defined due to the lack of randomized studies or high quality observational studies [15, 16].
Although the endovascular option for management of thoracic aortic pathology eliminates the need for aortic cross clamping needed in open surgery but the risk of spinal cord ischemia remains [9, 17].
The reported incidence of spinal cord ischemia and associated complications in TEVAR ranges from 1 to 10% [18,19,20].
There is a wide range of varying protocols as regard the employment of CSF drainage during TEVAR procedures with no formal guidelines as regard placement of a CSF drain or not [21,22,23].
However, to date, no consensus has been reached regarding the timing, indication, and use of CSF drainage in patients undergoing endovascular repair of the thoracic and thoracoabdominal aorta [24].
The aim of our study was to record the rate of spinal cord ischemia, stroke, and upper limb ischemia in patients with routine spinal fluid drainage and monitoring in whom there is a necessity to cover the left subclavian artery during deployment of the stent graft in TEVAR procedures.
Methods
Between July 2014 till April 2020, twenty-three (23) patients have had thoracic aortic lesion that was treated by applying a stent endograft, the anatomic morphologic characteristics of the lesion (the dissection flap was starting at the origin of the left subclavian artery and/or retrograde extension of the dissection plane beyond the safe sealing zone) made it necessary to cover the left subclavian artery to achieve a safe sealing zone (presence of 2 cm of healthy aortic wall for proximal sealing). All patients were refereed due to suspicion of thoracic aortic pathology and were treated on elective basis.
All patients were subjected to careful history taking, examination, routine preoperative laboratory investigations including echocardiogram, cardiological and anesthesiologic assessment. We excluded patients with history of CABG using the internal mammary artery to avoid occurrence of myocardial infarction due to decreased flow in this internal mammary due to blockage of the left subclavian artery. Informed consent was obtained from patients for data collection and for the procedure as is standard according to the local institutional review board’s approval (IRB 00006379).
Pre-interventional imaging and landing zones
The decision of intervention was based upon a multi-slice contrast enhanced computed tomography with three-dimensional reconstruction. The scans of patients were studied using the OsiriX software (www.osirix-viewer.com) to identify the proximal and distal landing zones necessary to cover the whole pathology, the diameter and length of the aortic segment needed to be covered and the diameter of both common femoral arteries to determine the best access site for the delivery system. Endografts were oversized by 10–20% according to the pathology needed to be treated.
Proximal landing zone was identified using “TEVAR reporting standards” made by “Society for Vascular Surgery Ad Hoc Committee” [25].
Pre-interventional spinal drainage
We adopted a routine spinal drainage protocol. A spinal catheter was inserted, and initially 10 ml were drawn slowly upon insertion of the catheter, another 10 ml were drawn slowly upon deployment of the stent graft. We always kept CSF Pressure < 10–15 mmHg.
Procedure
After spinal catheter insertion, all patients were subjected to general endotracheal anesthesia, exposure of the right or left common femoral artery was done surgically according to the planned access site. A 6F sheath was inserted in the common femoral artery through which a hydrophilic 0.035 guide wire was positioned in the ascending aorta, upon which a pig tail supporting catheter was advanced. We exchanged the hydrophilic wire with an extra stiff one (Lunderquist®, Cook Medical).
Another 6F sheath was inserted through a right brachial access, through which a diagnostic catheter is advanced over a hydrophilic wire and positioned in the arch of the aorta for angiography.
The femoral sheath was exchanged with the endograft delivery system (Zenith TX2 or Zeinth Alpha, COOK MEDICAL), the system was advanced to the arch of aorta, angiography was done to identify the proximal sealing zone (zone 2) with origin of the left subclavian arising from it. Deployment of the endograft was done under fluoroscopy to ensure perfect positioning.
Completion angiography was done, and according to the preoperative plan another stent could be applied with attention to the distal seal zone.
Post procedural care and follow up
Patients were awakened checked for signs of stroke, upper limb ischemia, spinal cord ischemia. Then were transferred to intensive care unit for close observation, with the spinal catheter in place to continue monitoring of spinal fluid pressure keeping it < 10–15 mmHg.
The catheter was left in place for 48 h and patients were followed up neurologically for development of weakness of the lower limbs and drainage was done then.
Follow up computed tomographic angiography was done at two month and 6 months for follow up.
Technical success was considered when completion angiography showed precise deployment of the stent graft as planned with complete exclusion of the target pathology (covering of the proximal tear with no type 1a endoleak, exclusion of the aneurysm or ulcer). Freedom from procedure related complications and the need for reintervention was considered as a clinical success.
All procedures were done by skilled vascular surgeons with endovascular capabilities and experience.
Results
This was a case series study with data obtained from 23 patients with demographics shown in Table 1, from the period of July 2014 till April 2020, patients underwent placement of an aortic endograft to treat a thoracic aortic pathology (Table 2) either dissection or aneurysm.
From the five patients with coronary artery disease only one has had CABG not using the internal mammary as a graft.
Exposure of the left common femoral artery was done in 18 patients (78.26%), while the other 5 (21.74%) patients, we did expose the right common femoral artery. The whole procedure time ranged from 50 min to two hours with a mean time of 85 min. Patients were transferred to the intensive care unit post procedural. All procedures were uneventful except for one patient, in whom the proximal sealing zone was not achieved and another endograft was inserted to achieve this goal immediately.
Three endograft stents were used in one patient (4.35%), two endograft stents were used in 13 patients (56.52%), and only one endograft stent was used in the rest of patients (39.13%). The choice of the number of stents were based upon preoperative planning to cover the whole thoracic aortic pathology.
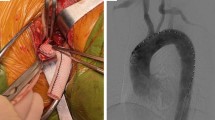
Technical success was achieved in 100% of cases (Fig. 1).
Patients were transferred to the intensive care unit fully conscious, with no signs of spinal cord ischemia. All patients had lost their left radial pulsations, with slight coldness of the left upper limb but with full motor power.
Patients were observed in the intensive care unit for 48 h with the spinal catheter in place keeping the CSF pressure < 10–15 mmHg. As regard the adverse events (Table 3), only one patient developed signs of Spinal cord ischemia after four hours of the procedure then this patient developed severe chest pain then arrested with failure to resuscitate him (the patient died 6 h after the procedure). This patient had a thoracic aortic aneurysm, his preoperative cardiological assessment showed an ejection fraction of 35% with multiple segments of segmental wall motion abnormalities, and his pre-procedural coronary angiography showed multivessel disease for CABG, and cardiac surgery opinion was to postpone the bypass till after treatment of his aneurysm.
Two patients (8.70%) developed post implantation syndrome, fever that was managed conservatively and subsided within two weeks from the procedure.
Neurological assessment of patients was done to assess for development of stroke or not. All patients did not show any sign for development of stroke either related to the anterior or posterior circulation except for one patient who developed left sided hemiparesis three months after the procedure. This patient was investigated and was found to have minute infarcts in the right temporal and parietal lobes. This was a stroke related to the anterior circulation. The patient was managed conservatively, and his symptoms improved.
As regard the upper limb symptoms, immediately after deployment of the endograft, the patients lost their left radial pulsations. After the patient was awakened, his left upper limb was examined for signs of acute ischemia. All patients did not complain of pain, showed good power of movement comparable to the right side, with slight temperature difference. During follow up, left upper limb symptoms were noted in four patients (17.40%) in the form of upper limb claudication or tingling sensation upon elevation of the upper limb. These upper limb symptoms were tolerable, were managed conservatively. Conservative management was in the form of Cilostazol which is an antiplatelet and vasodilator together with Naftidrofuryl which is a vasodilator drug, both are used in case of claudication and chronic limb ischemia.
Follow up contrast enhanced computed tomography was done after two and six months, showed no signs of endoleak (Fig. 2). The origin of the left subclavian from the arch of aorta was not visualized in three patients (3/22) (13.63%), with refilling of the distal part of the left subclavian.
6 months follow up showing the left subclavian covered with the endograft. a patient with two endografts. b Patient with three endograft, the origin of left subclavian was not visualized white arrow, c a patient with a single endograft, d a patient with two endografts with non-visualization of the origin of the left subclavian artery
Discussion
To achieve a successful TEVAR procedure, we must have an adequate proximal seal zone, in one third of the patients this seal zone will not be reached unless we covered the left subclavian artery [8]. But what about the complications related to such maneuver? It is the same with spinal drainage during TEVAR procedures, CSF drainage has proven its efficacy during open surgical repair of thoracic aorta but with TEVAR, it is still under studying. By reviewing literature, we found lack of clear evidence on either spinal fluid drainage or revascularization of the left subclavian artery in the prevention of both spinal cord ischemia and stroke, The Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS) on management of Descending Thoracic Aorta Diseases stated that it should be considered giving it level C evidence, and grade IIa recommendation [26].
In this case series there was a necessity to cover the left subclavian artery to achieve a safe sealing zone. We adopted a policy of routine spinal catheter placement and monitoring of spinal fluid pressure keeping it below 10–15 mmHg. We recorded the occurrence of spinal cord ischemia, stroke, and upper limb ischemia in this case series and we found that 0% of our patients developed acute upper limb ischemia, 4.35% of patients developed stroke related to the anterior circulation 3 months post-procedure, and 4.35% developed signs of spinal cord ischemia.
The rate of spinal cord ischemia during TEVAR, though it is less than open surgery, but it ranges from 3 to 5% [27, 28].
Mazzeffi et al., performed routine spinal fluid drainage in high risk patients undergoing TEVAR and the overall rate of paraplegia was 2% [29].
In the study by Suarez-Pierre et al., which was done over 4287 patients who underwent TEVAR it was found that the rate of spinal cord ischemia was 2.5% in patients who underwent TEVAR without spinal drainage versus 1.5% in patients with spinal drainage [30].
Some adopted a selective protocol for spinal fluid drainage keeping it to the high risk group for developing spinal cord ischemia to spare the low risk group the complications of spinal drainage [31, 32]. But the role of this adjunctive procedure is less defined due to the lack of high quality observational evidence or randomized studies [15, 16].
There is a general agreement that patients with focal descending thoracic pathology and can be treated with short endograft (< 15 cm) are at lower risk of spinal cord ischemia [33, 34]. While the EUROSTAR registry showed increased incidence of spinal cord ischemia in patients without revascularization of the left subclavian artery versus patients who underwent prior revascularization (8.4% vs 0%; P = 0.049) [9].
In our study the incidence of spinal cord ischemia was 4.35% with coverage of the left subclavian artery and routine spinal fluid drainage. On the contrary Maldonado et al. reached a conclusion that with covering the left subclavian artery, there is no increased risk of spinal cord ischemia or stroke if a selective revascularization strategy was adopted [12].
As for left subclavian artery coverage, in the beginning it was mandatory to revascularize the left subclavian artery prophylactically if it will be covered during TEVAR procedures [35]. But this did not become a common practice. In the “meta-analysis by Rizvi et al.”, there was a low-quality evidence suggesting that covering the left subclavian artery is associated with significant increase in arm ischemia, vertebrobasilar ischemia, and possibly spinal cord ischemia and anterior circulation stroke [36].
Other studies showed that covering the left subclavian artery during endograft deployment is well tolerated without a revascularization procedure and carries no additional morbidity [37,38,39].
In the series by Antonello et al. for treating traumatic descending thoracic aortic transection, intentional coverage of the left subclavian artery without revascularization, it was found that patients did not develop signs of vertebrobasilar insufficiency, paraplegia, or stroke [40].
Another meta-analysis comparing the outcomes of intentional coverage of the left subclavian artery during TEVAR procedures with or without revascularization, showed nonsignificant trends with revascularization in preventing stroke and spinal cord ischemia [41].
Delafontaine et al. found in his retrospective analysis of the “Nationwide Inpatient Sample Database” that the rates of spinal cord ischemia and upper extremity ischemia were similar between patients who underwent revascularization of the left subclavian artery versus those without while the peri-operative cardiac complications, stroke, pulmonary complications were significantly higher in patients who underwent TEVAR with left subclavian artery revascularization [42].
In is known that the overall incidence of stroke during TEVAR ranges from 3.2 to 6.2% [43]. In our study the rate of stroke was 4.35%, which was related to the anterior circulation and occurred after 3 months of the procedure.
As for upper limb ischemia, in our study 0% of patients had upper lib ischemia. While Youssef et al., concluded that covering the left subclavian artery without revascularization was justifiable in their cohort. The study included 40 patients who underwent TEVAR with complete coverage of the left subclavian during the procedure, only 2.5% developed critical upper limb ischemia that was in need of revascularization by transposition of the left subclavian artery, 2.5% developed anterior spinal artery syndrome, and none developed new neurological symptoms during follow up [44].
In a meta-analysis of 1161 patients of whom 444 underwent revascularization of the left subclavian artery versus 717 who did not, it was found that revascularization was associated with a similar risk of stroke (OR 0.70, 95% CI 0.43–1.14, P = 0.15), spinal cord ischemia (OR 0.56, 95% CI 0.28–1.10, P = 0.09), and mortality (OR 0.87, 95% CI 0.55–1.39, P = 0.56) compared with no revascularization [45].
Varkevisser et al. in their analysis of 2346 cases from the “National Surgical Quality Improvement Program registry” found that left subclavian artery revascularization was not associated with lower incidence of stroke [46].
Conclusions
Revascularization of the left subclavian artery is a procedure that carries an increased rate of morbidity, and it is done to decrease the risk of stroke or spinal cord ischemia. But as we can see from the above results and discussion that the benefit of this procedure is still debatable, as well as the need for routine prophylactic spinal fluid drainage, using either methods have a class c evidence recommendation which is not backed up by randomized controlled studies. The need for these studies is mandatory to end up the debate.
Availability of data and materials
The data sets used and/ or analyzed during this study will be available from the corresponding author on reasonable request.
Abbreviations
- CABG:
-
Coronary artery bypass graft
- CSF:
-
Cerebrospinal fluid
- TEVAR:
-
Thoracic endovascular aorta repair
References
Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP (1994) Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 331:1729–1734
Ultee KHJ, Zettervall SL, Soden PA, Buck DB, Deery SE, Shean KE, Verhagen HJM, Schermerhorn ML (2017) The impact of endovascular repair on management and outcome of ruptured thoracic aortic aneurysms. J Vasc Surg 66:343–352
Sepehripour AH, Ahmed K, Vecht JA, Anagnostakou V, Suliman A, Ashrafian H, Darzi A, Athanasiou T (2011) Management of the left subclavian artery during endovascular stent grafting for traumatic aortic injury -a systematic review. Eur J Vasc Endovasc Surg 41:758–69
Cooper DG, Walsh SR, Sadat U, Noorani A, Hayes P, Boyle JR (2009) Neurological complications after left subclavian artery coverage during thoracic endovascular aortic repair: a systematic review and meta-analysis. J Vasc Surg 49:1594–1601
Noor N, Sadat U, Hayes PD, Thompson MM, Boyle JR (2008) Management of the left subclavian artery during endovascular repair of the thoracic aorta. J Endovasc Ther 15:168–176
Kotelis D, Geisbusch P, Hinz U, Hyhlik-Durr A, von TenggKobligk H, Allenberg JR, Bockler D (2009) Short and midterm results after left subclavian artery coverage during endovascular repair of the thoracic aorta. J Vasc Surg 50:1285–1292
Feezor RJ, Martin TD, Hess PJ, Klodell CT, Beaver TM, Huber TS, Seeger JM, Lee WA (2007) Risk factors for perioperative stroke during thoracic endovascular aortic repairs (TEVAR). J Endovasc Ther 14:568–573
Peterson BG, Eskandari MK, Gleason TG, Morasch MD (2006) Utility of left Subclavian artery revascularization in association with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg 43:433–439
Buth J, Harris PL, Hobo R, vanEps R, Cuypers P, Duijm L, Tielbeek X (2007) Neurologic complications associated with endovascular repair of thoracic aortic pathology: incidence and risk factors. A study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg 46:1103–10
Chung J, Kasirajan K, Veeraswamy R, Dodson TF, Salam AA, Chaikof EL, Corriere MA (2011) Left subclavian artery coverage during thoracic endovascular aortic repair and risk of perioperative stroke or death. J Vasc Surg 54:979–984
Lee M, Lee DY, Kim MD, Won JY, Yune YN, Lee TY, Choi D, Ko YG (2013) Selective coverage of the left subclavian artery without revascularization in patients with bilateral patent vertebrobasilar junctions during thoracic endovascular aortic repair. J Vasc Surg 57:1311–1316
Maldonado TS, Dexter D, Rockman CB, Veith FJ, Garg K, Arko F, Bertoni H, Ellozy S, Jordan W, Woo E (2013) Left subclavian artery coverage during thoracic endovascular aortic aneurysm repair does not mandate revascularization. J Vasc Surg 57:116–124
Coselli JS, LeMaire SA, Köksoy C, Schmittling ZC, Curling PE (2002) Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 35:631–639
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DJ et al (2010) ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/ SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol 55:e27-129
Wong CS, Healy D, Canning C, Coffey JC, Boyle JR, Walsh SR (2012) A systematic review of spinal cord injury and cerebrospinal fluid drainage after thoracic aortic endografting. J Vasc Surg 56:1438–1447
Arnaoutakis DJ, Arnaoutakis GJ, Beaulieu RJ, Abularrage CJ, Lum YW, Black JH (2014) Results of adjunctive spinal drainage and/or left subclavian artery bypass in thoracic endovascular aortic repair. Ann Vasc Surg 28:65–73
Ullery BW, Cheung AT, Fairman RM, Jackson BM, Woo EY, Bavaria J, Pochettino A, Wang GJ (2011) Risk factors, outcomes, and clinical manifestations of spinal cord ischemia following thoracic endovascular aortic repair. J Vasc Surg 54(3):677–684
Scott DA, Denton MJ (2016) Spinal cord protection in aortic endovascular surgery. Br J Anaesth 117(Suppl 2):ii26–ii31
Wortmann M, Böckler D, Geisbüsch P (2017) Perioperative cerebrospinal fluid drainage for the prevention of spinal ischemia after endovascular aortic repair. Gefasschirurgie 22(Suppl 2):35–40
Dijkstra ML, Vainas T, Zeebregts CJ, Hooft L, van der Laan MJ (2018) Editor’s choice—Spinal cord ischaemia in endovascular thoracic and thoraco-abdominal aortic repair: review of preventive strategies. Eur J Vasc Endovasc Surg 55(6):829–841
Khan NR, Smalley Z, Nesvick CL, Lee SL, Michael LM (2016) The use of lumbar drains in preventing spinal cord injury following thoracoabdominal aortic aneurysm repair: an updated systematic review and meta-analysis. J Neurosurg Spine 25(3):383–393
Banga PV, Oderich GS, Reis de Souza L, Hofer J, Cazares Gonzalez ML, Pulido JN, Cha S, Gloviczki P (2016) Neuromonitoring, cerebrospinal fluid drainage, and selective use of iliofemoral conduits to minimize risk of spinal cord injury during complex endovascular aortic repair. J Endovasc Ther. 23(1):139–49
Maier S, Shcherbakova M, Beyersdorf F, Benk C, Kari FA, Siepe M, Czerny M, Rylski B (2019) Benefits and risks of prophylactic cerebrospinal fluid catheter and evoked potential monitoring in symptomatic spinal cord ischemia low-risk thoracic endovascular aortic repair. Thorac Cardiovasc Surg 67(5):379–384
Khemlani KH, Schurink GW, Buhre W, Schreiber JU (2021) Cerebrospinal fluid drainage in thoracic and thoracoabdominal endovascular aortic repair: a survey of current clinical practice in European Medical Centers. J Cardiothorac Vasc Anesth S1053-0770(21)00639-X
Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL (2010) Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg 52(4):1022–1033
Riambau V, Böckler D, Brunkwall J, Cao P, Chiesa R, Coppi G, Czerny M, Fraedrich G, Haulon S, Jacobs MJ, Lachat ML, Moll FL, Setacci C, Taylor PR, Thompson M, Trimarchi S, Verhagen HJ, Verhoeven EL, Esvs Guidelines Committee, Kolh P, de Borst GJ, Chakfé N, Debus ES, Hinchliffe RJ, Kakkos S, Koncar I, Lindholt JS, Vega de Ceniga M, Vermassen F, Verzini F, Document Reviewers, Kolh P, Black JH 3rd, Busund R, Björck M, Dake M, Dick F, Eggebrecht H, Evangelista A, Grabenwöger M, Milner R, Naylor AR, Ricco JB, Rousseau H, Schmidli J. Editor's choice—Management of descending thoracic aorta diseases: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53(1):4–52.
Etz CD, Weigang E, Hartert M, Lonn L, Mestres CA, Di Bartolomeo R, Bachet JE, Carrel TP, Grabenwöger M, Schepens MA, Czerny M (2015) Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European Association for Cardio-Thoracic Surgery†. Eur J Cardiothorac Surg 47(6):943–957
Arora H, Kumar PA (2018) Prophylactic cerebrospinal fluid drainage for high-risk thoracic endovascular aortic repair: safe and effective? J Cardiothorac Vasc Anesth 32:890–892
Mazzeffi M, Abuelkasem E, Drucker CB, Kalsi R, Toursavadkohi S, Harris DG, Rock P, Tanaka K, Taylor B, Crawford R (2018) Contemporary single-center experience with prophylactic cerebrospinal fluid drainage for thoracic endovascular aortic repair in patients at high risk for ischemic spinal cord injury. J Cardiothorac Vasc Anesth 32(2):883–889
Suarez-Pierre A, Zhou X, Gonzalez JE, Rizwan M, Fraser CD 3rd, Lui C, Malas MB, Abularrage CJ, Black JH 3rd (2019) Association of preoperative spinal drain placement with spinal cord ischemia among patients undergoing thoracic and thoracoabdominal endovascular aortic repair. J Vasc Surg 70(2):393–403
Patel HJ, Williams DM, Drews JD, Dasika NL, Eliason JL, Passow MC, Deeb GM (2014) A 20-year experience with thoracic endovascular aortic repair. Ann Surg. 260(4):691–6 (discussion 696–7)
Kotelis D, Bianchini C, Kovacs B, Müller T, Bischoff M, Böckler D (2015) Early experience with automatic pressure-controlled cerebrospinal fluid drainage during thoracic endovascular aortic repair. J Endovasc Ther 22(3):368–372
Melissano G, Kahlberg A, Bertoglio L, Chiesa R (2011) Endovascular exclusion of thoracic aortic aneurysms with the 1- and 2-component Zenith TX2 TAA endovascular grafts: analysis of 2-year data from the TX2 pivotal trial. J Endovasc Ther 18:338–349
Feezor RJ, Lee WA (2009) Management of the left subclavian artery during TEVAR. Semin Vasc Surg 22:159–164
Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, Williams D, Cambria RP, Mitchell RS (2005) Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg 41:1–9
Rizvi AZ, Murad MH, Fairman RM, Erwin PJ, Montori VM (2009) The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: a systematic review and meta-analysis. J Vasc Surg 50:1159–1169
Riesenman PJ, Farber MA, Mendes RR, Marston WA, Fulton JJ, Keagy BA (2007) Coverage of the left subclavian artery during thoracic endovascular aortic repair. J Vasc Surg 45:90–4 (discussion 94-5)
Galili O, Fajer S, Eyal A, Karmeli R (2007) Left subclavian artery occlusion by thoracic aortic stent graft: long-term clinical and duplex follow-up. Isr Med Assoc J 9:668–670
Woo E, Carpenter J, Jackson B, Pochettino A, Bavaria J, Szeto W, Fairman RM (2008) Left subclavian artery coverage during thoracic endovascular aortic repair: a single-center experience. J Vasc Surg 48:555–560
Antonello M, Menegolo M, Maturi C, Dall’Antonia A, Lepidi S, Frigo AC, Grego F, Frigatti P (2013) Intentional coverage of the left subclavian artery during endovascular repair of traumatic descending thoracic aortic transection. J Vasc Surg 57:684–90
Sobocinski J, Patterson BO, Karthikesalingam A, Thompson MM (2016) The effect of left subclavian artery coverage in thoracic endovascular aortic repair. Ann Thorac Surg 101:810–817
Delafontaine JL, Hu B, Tan TW, Tang GL, Starnes BW, Virk C, Chow WB, Zhang WW (2019) Outcome comparison of TEVAR with and without left subclavian artery revascularization from analysis of nationwide inpatient sample database. Ann Vasc Surg 58:174–179
Cheng D, Martin J, Shennib H, Dunning J, Muneretto C, Schueler S, Von Segesser L, Sergeant P, Turina M (2010) Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J Am Coll Cardiol. 55(10):986–1001
Youssef A, Ghazy T, Kersting S, Leip JL, Hoffmann RT, Kappert U, Matschke K, Weiss N, Mahlmann A (2018) Management of the left subclavian artery during TEVAR—complications and mid-term follow-up. Vasa 47(5):387–392
Hajibandeh S, Hajibandeh S, Antoniou SA, Torella F, Antoniou GA (2016) Meta-analysis of left subclavian artery coverage with and without revascularization in thoracic endovascular aortic repair. J Endovasc Ther 23(4):634–641
Varkevisser RRB, Swerdlow NJ, de Guerre LEMV, Dansey K, Li C, Liang P, Latz CA, Carvalho Mota MT, Verhagen HJM, Schermerhorn ML (2020) Thoracic endovascular aortic repair with left subclavian artery coverage is associated with a high 30-day stroke incidence with or without concomitant revascularization. J Endovasc Ther 27(5):769–776
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MR was involved in the study design, collection, analysis, and interpretation of data. Also, writing and revising and approving the final paper draft. MI was involved in study design, collection of data. Also, revision and approval of the final paper. KG was involved in data collection and revising and approving the final paper draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We had the approval of the ethics committee for research of general surgery department, faculty of medicine, Ain shams university (IRB 00006379). All patients were consented for sharing the data in a scientific research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rizk, M.A.EM.A.ES., Ismail, M.I.M. & Gohar, K.S. Stroke, spinal cord ischemia and upper limb ischemia in patients undergoing TEVAR with coverage of the left subclavian artery: a case series study. Egypt J Radiol Nucl Med 52, 276 (2021). https://doi.org/10.1186/s43055-021-00654-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-021-00654-3