Abstract
Background
Musculoskeletal ultrasound can be now considered a complement to physical examination in rheumatoid arthritis. This study evaluates the role of musculoskeletal ultrasound in assessment of rheumatoid hand function and underlying functional defects and disabilities in order to find out a possibly better tool for assessment.
Results
Hand grip weakness was significantly associated with metacarpophalangeal joints synovitis of ulnar 4 fingers (p = 0.045), wrist joint synovitis (p = 0.009), flexor tendons tenosynovitis of the ulnar 4 fingers (p = 0.001), flexor pollicis longus tendon tenosynovitis (p = 0.013).
Hand function impairment by grip ability test was significantly associated with metacarpophalangeal joints synovitis of ulnar 4 fingers (p = 0.009), wrist joint synovitis (p = 0.004), and flexor tendons tenosynovitis of the ulnar 4 fingers (p = 0.042). Multiple linear regression analysis showed that the most influencing factor affecting grip ability test and hand grip strength was ulnar 4 Flexor tendons tenosynovitis (P = 0.023, P = 0.037) respectively.
Conclusions
Joint synovitis and tenosynovitis that are detected by musculoskeletal ultrasound can be used as an assessment tool for hand function in rheumatoid arthritis, since they are associated with reduced hand grip strength and impaired hand ability.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is a chronic autoimmune disease that primarily affects the lining of the synovial joints causing synovitis which damages cartilage, bone, ligaments, and tendons. In rheumatoid hands, there are reductions in muscle strength and mobility accompanied with deformities. It is associated with functional impairment and disabilities [1, 2]. One of diagnostic criteria for activity in RA is poor hand grip [3]. However, little is known about the relationships between rheumatoid hand function and different pathological findings in the joints, such as inflammation and structural damage. This raises the need for incorporating newer techniques and approaches to assess rheumatoid hand function for more early treatment and prevention of such disabilities [4].
MSUS (musculoskeletal ultrasound) is preferable than physical examination for detecting joint synovitis with analogous sensitivity to magnetic resonance imaging, yet easier to use and cheaper. MSUS can demonstrate synovial fluid effusion and synovial thickening with a greater sensitivity than clinical examination [5]. It was found by one study that ultrasound disease activity score reflects not only disease activity but also the disability status and structural joint damage [6]. It remains to be verified whether MSUS can predict preservation of function and hand functional outcomes in RA patients better than the traditional clinical or serological scores [7].
The aim of this study was to find out the value of musculoskeletal ultrasound as a tool for assessment of hand function, defect, and disability in rheumatoid hand and whether it can be used in early detection of such disability.
Methods
Thirty RA patients were included in a monocentric cross-sectional study of 1.5 years’ duration. Patients were diagnosed according to the American College of Rheumatology (ACR) 1987 criteria for RA diagnosis [8], with at least 6-month disease duration. Patients with previous hand surgery, nerve injury, fracture or dislocation of wrist and hand bones, hand infections, visual, auditory, or cognitive problems were excluded from our study. Full history taking and thorough clinical examination with special attention to the musculoskeletal system including joints and tendons was performed for all patients. Disease activity was assessed using the disease activity score in 28 joints (DAS28) with erythrocyte sedimentation rate (ESR). Hand dysfunction was assessed using the grip ability test (GAT) which is a performance-based test of hand function specifically developed for subjects with RA. It consists of three items which represents different grip types used in daily life [9]. Grip strength was measured by modified Sphygmomanometer Test [10].
Laboratory investigations were done that included ESR by Westergren method; serum C- reactive protein (CRP) by nephelometry; rheumatoid factor (RF) by latex fixation test; and anti-cyclic citrullinated antibody (anti-CCP) by ELISA.
MSUS was performed by EULAR certified MSUS teacher of 8 years’ experience in MSUS for all joints and tendons of RA hands using logic p5 R4.0. with (7–12 MHz) linear probes. Using this relatively low-frequency prob has limitation of near field resolution. This study addressed this limitation by optimization to the highest frequency available and using high end machine. Eleven joints including the second to fifth proximal interphalangeal (PIP) joints, the first interphalangeal (IP) joint, the first to fifth metacarpophalangeal (MCP), and wrist joint were examined for synovitis. Eleven tendon compartments including the first to fifth finger flexor tendons and compartment I–VI carpal extensor tendons were performed in longitudinal and transverse planes for tenosynovitis. Detection of synovitis according to EULAR-OMERACT combined scoring system for grading synovitis in RA where grade 0, no grey-scale-detected synovial hypertrophy (SH) and no power Doppler (PD) signal (within the synovium); grade 1, grade 1 SH and ≤ grade 1 PD signal; grade 2, grade 2 SH and ≤ grade 2 PD signal or grade 1 SH and a grade 2 PD signal; and grade 3, grade 3 SH and ≤ grade 3 PD signal or grade 1 or 2 synovial hypertrophy and a grade 3 PD signal (Fig. 1) [11, 12]. The setup parameters for PD were the same all over the study. The Doppler frequency was 5 MHz, pulse repetition frequency was 0.9 kHz, gain was 23, and wall filter was 113 Hz. The power mode does not measure velocity or direction and enables detection of low-speed blood flow, as it can be found in newly formed vessels in inflamed synovial tissue (synovitis and tenosynovitis).
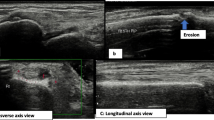
MSUS of some of present study patients. a Dorsal longitudinal scan of a metacarpophalangeal joint. Yellow arrow indicates joint cavity enlargement due to hypoechoic synovial fluid as well as hyperechoic synovial thickening with bulging over the tops of the periarticular bones (grade 2 synovial hypertrophy). b Dorsal longitudinal scan of radiocarpal and midcarpal joints. Yellow arrows indicate joint cavity enlargement due to hypoechoic synovial fluid as well as hyperechoic synovial thickening (grade 2 synovial hypertrophy). c Volar transverse view of middle finger at metacarpal bone showing flexor tendon sheath widening (grade 2 tenosynovitis). d Dorsal longitudinal scan of a wrist joint showing interruption of cortical bone (erosion) measuring 2.9 mm (grade 2). Green arrows indicate distention of extensor carpi-ulnaris sheath involving synovial effusion and synovial hypertrophy (hypoechoic and hyperechoic respectively) (grade 1 tenosynovitis). RT, right; LT, left; RCJ, radio-carpal joint; MCJ, mid-carpal joint; MCP, metacarpal joint; MCP, metacarpophalangeal joint; FDP&S, flexor digitorum profundus and superficialis; ECU, extensor carpi-ulnaris; P, pisiform
Detection of tenosynovitis by semi-quantitative OMERACT four-grade scoring system to score tenosynovitis on B-mode where grade 0, normal; grade 1, minimal; grade 2, moderate; and grade 3, severe, and four-grade semi-quantitative scoring system on Doppler mode where grade 0, no Doppler signal; grade 1, minimal; grade 2, moderate; and grade 3, severe (Fig. 1) [13]. Erosions were observed in two different planes and scored according to semi-quantitative scoring by ultrasound structural total (ScuSST) as follows: grade 0 = absence of erosion, grade 1 = erosion < 2 mm, grade 2 = erosion 2–3 mm or two erosions < 2 mm, and grade 3 erosion > 3 mm or multiple erosions (Fig. 1) [14].
Statistical analysis
The collected data was revised, coded, tabulated, and introduced to a PC using Statistical package for Social Science (SPSS 20). Data was presented and suitable analysis was done according to the type of data obtained for each parameter: mean, standard deviation (±SD), and range for parametric numerical data, while median and interquartile range (IQR) for non-parametric numerical data. Percentage of non-numerical data. Mann-Whitney test was used to assess the statistical significance of the difference of a non-parametric variable between two study groups. Student’s T test was used to assess the statistical significance of the difference between two study group means. Linear regression analysis was used to test and estimate the dependence of a quantitative variable based on its relationship with a set of independent variables. A p value < 0.05 was considered significant.
Results
Descriptive analysis
Thirty RA patients with 60 RA hands were included in the present study. Their mean age was 40.2 ± 11.7 years. Their mean disease duration was 8.3 ± 6.3 years. DAS 28 ESR was calculated and ranged from 2.1 to 6.1 with mean 3.7 ± 1.2. Seven (23.3%) patients were in remission. Nine (30%) patients had experienced low diseases activity. Ten (33.3%) patients showed moderate disease activity. Only 4 (13.3%) patients were in high activity. Correlation between DAS28 ESR score and disease duration showed no statistically significant correlation between the DAS28 ESR score and disease duration, r = 0.239, P > 0.05. Table 1 shows demographic, clinical, and laboratory data.
Joint synovitis was detected according to EULAR-OMERACT combined scoring system for grading synovitis in RA [11, 12]. Synovitis among RA hands was most common in wrist joint (60%) followed by MCP 2 (35%) and MCP 3 (28.4%) joints. Joint erosion was detected semi-quantitatively using ScuSST [14] most common in wrist joint (28.4 %) followed by MCP 3 (15%) and MCP 2 (13.4%) joints. Tenosynovitis was detected by semi-quantitative OMERACT scoring system [13]. The most common affected tendons were extensor carpi-ulnaris (ECU) (23.4%) followed by middle finger flexor tendons (11.7%) and middle finger extensor tendons (10%).
Hand grip strength showed statistically significant negative correlation with DAS28 & ESR (r = − 0.598, p < 0.001, r = − 0.470, P = 0.009) respectively. GAT showed statistically significant correlation with DAS28 and ESR (r = 0.562, p < 0.001, r = 0.499, p = 0.005) respectively. Both hand grip strength and GAT showed no statistically significant correlation with anti-CCP (r = − 0.253, p = 0.282, r = 0.149, p = 0.531) respectively.
Association of ultrasonographic findings with hand function impairment
Comparison between RA hands with and without various ultrasonographic findings as regards GAT was done as shown in Table 2.
Hand function impairment by GAT was significantly associated with MCP joints synovitis of ulnar 4 fingers (p = 0.009), wrist joint synovitis (p = 0.004), and flexor tendons tenosynovitis of the ulnar 4 fingers (p = 0.042). There was no significant association between ulnar 4 fingers extensor tendons tenosynovitis (p = 0.758), flexor pollicis longus (FPL) tenosynovitis (p = 0.396), flexor carpi ulnaris (FCU), ECU, flexor carpi radialis (FCR), extensor carpi radialis longus (ECRL) and extensor carpi radialis brevis (ECRB) tendons tenosynovitis (p = 0.854) and hand function impairment by GAT (Table 2).
Hand grip weakness was significantly associated with metacarpophalangeal joints synovitis of ulnar 4 fingers (p = 0.045), wrist joint synovitis (p = 0.009), flexor tendons tenosynovitis of the ulnar 4 fingers (p = 0.001), flexor pollicis longus tendon tenosynovitis (p = 0.013), and tenosynovitis of EPL, EPB, APL, and FPL (p = 0.000). Hands with metacarpophalangeal joints synovitis of ulnar 4 fingers, wrist joint synovitis, and flexor tendons tenosynovitis of the ulnar 4 fingers were more likely to have weaker hand grip strength than hands without these sonographic findings. There was no significant association between ulnar 4 PIP joints synovitis (p = 0.524); ulnar 4 fingers extensor tendons tenosynovitis (p = 0.808); FCU, ECU, FCR, ECRL, and ECRB tendons tenosynovitis (p = 0.765); and hand grip strength (Table 3).
Influence of significant variables on GAT and hand grip strength
The purpose of this analysis is to know to what extent is the GAT and grip strength were influenced by the significant independent variables. While doing multiple linear regression analysis and entering significant associated variables in univariant analysis, the only factor that affected GAT was flexor tendons tenosynovitis of the ulnar 4 fingers, 95% CI rang 4.9–64.61 (p = 0.023). The only factor that affected grip strength was flexor tendons tenosynovitis of the ulnar 4 fingers, 95% CI range − 62.26 to − 0.5 (p = 0.037) (Table 4).
Discussion
The role of MSUS in early diagnosis and detecting disease activity is well established. Moreover, some investigators find it even better than clinical examination [15, 16]. It has the advantage to assess all structures directly involved in rheumatoid process such as synovium, tendons, and cartilage [17]. An important issue regarding MSUS is its reliability; it is considered a highly operator-depending technique. Its accuracy depends on both acquisition and interpretation of US images. This raises the need for a uniform evaluation of US-detected pathologies [18]. Thus, this study used universal guidelines for pathology definitions and semi-quantitative scoring systems.
It was important to consider pitfalls in PD ultrasound specially because RA patients mostly receive steroids and disease-modifying anti-rheumatic drugs. Consequently, this study used GS in addition to PD to evaluate synovitis and GS only to evaluate tenosynovitis.
PD-detected tenosynovitis had only a small or no additive value to GS tenosynovitis. A reason for this could be that PD performs better from the dorsal side of the joint than from the palmar side [19]. It was also important to consider standardization of gain settings and avoidance of unnecessary probe pressure and ensure complete relaxation of part under evaluation to avoid masking the Doppler activity [20].
This study evaluated associations between hand dysfunction and findings detected by MSUS in patients with or without hand deformity and disability.
Among present study, RA hands’ mean value of handgrip strength was lower than normal population. Moreover, GAT was prolonged among RA hands. Current study results were matching with Bircan et al.’s [21] and Verma and his colleagues’ [4] studies where GAT scores were seen affected in 95 % of the studied RA patients. These findings were in agreement with the findings of Silva et al. [22] who found that the handgrip strength was weak among the patients with RA compared with the controls. This is attributed to the joint damage, pain, and muscle weakness due to disuse and disease progression [23]. These inflammatory markers are reported to have catabolic effects on muscle. TNF-alpha and its soluble receptors are associated with a decline in muscle mass and muscle strength [24].
There was a positive correlation between GAT and CRP as activity marker (p = 0.001). This is in concordance with Westbury and his colleagues [25] who noted that increased high-sensitivity CRP was associated with poorer grip strength. Dedeoglu and his colleagues [26] in their study determined that grip strength was significantly related to disability and impairment, disease activity, and articular damage. A systematic review of twenty articles by Arab Alkabeya and colleagues [27] found out that grip strength and disease activity were identified as the most influential factors on hand function in people with RA. Similar to this study, Taştekin et al. [28] and Dedeoglu et al. [26] reported that grip strength was negatively correlated with the DAS 28 whereas in patients with relatively early RA and relatively lesser deformities, hand strength is affected more by disease activity. This study showed that both GAT and hand grip strength were not associated with levels of anti-CCP antibodies. However, elevated titers of anti-CCP antibodies may contribute to a poor radiological outcome and severity of the disease [29]. Abdel-Nasser and colleagues [30] stated that no relation was identified between anti-CCP and disease activity score based on 28 joints (DAS 28) in RA patients.
Hands with ulnar 4 MCP joints synovitis and wrist joint synovitis were associated with functional impairment as assessed by GAT. These were also significantly associated with weak hand grip strength. This was matching with a study by Závada and his colleagues [15]. They concluded that the articular synovitis scores were significantly correlated with HAQ score while erosions’ score was not correlated with HAQ score. Another study [6] stated that power Doppler ultrasound scores were significantly associated with the functional status.
This may relate to biomechanical factors such as relative increased range of movement of these joints as has been suggested for the reason of a higher prevalence of bone erosion and synovitis [31]. A stable wrist is needed to perform power grip. Biomechanically, a stable wrist prevents the dissipation of finger flexion and extensor forces as the tendons move over the carpus. Synovitis may cause biomechanical instability [32].
Tendons play an important role in the function of the hand. This study therefore assessed tenosynovitis as well as articular synovitis. Flexor tendons tenosynovitis of the ulnar 4 fingers and tenosynovitis of EPL, EPB, APL, and FPL were associated with functional impairment as assessed by GAT. Tenosynovitis of the mentioned tendons was also significantly associated with weak hand grip strength. This study also observed that the association between hand function impairment as assessed by GAT and the tenosynovitis of flexor pollicis longus alone was weak. This result may be due to the fact that the frequency of FPL tenosynovitis was low in this study (only 3 hands). It did not reach statistical significance. However, all hands with FPL tenosynovitis had hand function impairment as assessed by GAT. Erol et al. [33] found negative correlation between grip strength with tenosynovitis score by MRI. The gripping and wrist actions share several muscles; flexor digitorum profundus (FDP) and flexor pollicis longus (FPL) contribute to wrist flexion and grip-force production [33, 34]. A study by Nishino and coworkers [35] indicated that the US scores of combined articular synovitis and tenosynovitis scores correlated better with hand dysfunction than either individual score.
This study has few limitations. The sample size was small. In addition, due to the cross-sectional design of this study, findings of this study cannot be used to predict future hand dysfunction. Further investigations in greater detail with larger numbers of patients are needed.
Conclusions
Joint synovitis and tenosynovitis that are detected by musculoskeletal ultrasound can be used as an assessment tool for hand function in rheumatoid arthritis, since they are associated with reduced hand grip strength and impaired hand ability.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RA:
-
Rheumatoid arthritis
- MSUS:
-
Musculoskeletal ultrasound
- ACR:
-
American College of Rheumatology
- DAS28:
-
Disease activity score in 28 joints
- ESR:
-
Erythrocyte sedimentation rate
- GAT:
-
Grip ability test
- CRP:
-
C-reactive protein
- RF:
-
Rheumatoid factor
- Anti-CCP:
-
Anti-cyclic citrullinated antibody
- PIP:
-
Proximal interphalangeal
- MCP:
-
Metacarpophalangeal
- IP:
-
Interphalangeal
- ECU:
-
Extensor carpi-ulnaris
- APL:
-
Abductor pollicis longus
- EPL:
-
Extensor pollicis longus
- EPB:
-
Extensor pollicis brevis
- FPL:
-
Flexor pollicis longus
- FCU:
-
Flexor carpi ulnaris
- FCR:
-
Flexor carpi radialis
- ECRL:
-
Extensor carpi radialis longus
- ECRB:
-
Extensor carpi radialis brevis
References
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J (2018) Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 6:1–14. https://doi.org/10.1038/s41413-018-0016-9
Croia C, Bursi R, Sutera D, Petrelli F, Alunno A, Puxeddu I (2019) One year in review 2019: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 37(3):347–357
Doko I, Bajić Ž, Dubravić A, Qorolli M, Grazio S (2019) Hand grip endurance moderating the effect of grip force on functional ability and disease activity in rheumatoid arthritis patients: a cross-sectional study. Rheumatol Int 39:647–656. https://doi.org/10.1007/s00296-019-04250-7
Verma C, Parikh R, Nadkar M, Mehta A (2017) Correlation of functional ability of the hand with upper limb function and quality of life in patients with rheumatoid arthritis. J Assoc Physicians India 65:20–24
Szkudlarek M, Wakefield RJ, Backhaus M, Terslev L (2012) The discriminatory capacity of ultrasound in rheumatoid arthritis: active vs inactive, early vs advanced, and more. Rheumatology 51:vii6–vii9. https://doi.org/10.1093/rheumatology/kes334
El-Serougy EM, Eesa NN, El-Azizi HM, Badawi HA (2019) Power Doppler ultrasound in the evaluation of hand joints in rheumatoid arthritis patients in clinical remission: Association with composite index scores and functional status. Egypt Rheumatol 41:7–10. https://doi.org/10.1016/j.ejr.2018.02.001
Deodhar AA, Coşkun ÖK (2019) Hand function and imaging outcomes. Hand Funct. Springer, pp 315–330. https://doi.org/10.1007/978-3-030-17000-4_22
Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum Off J Am Coll Rheumatol 31:315–324
Dellhag B, Burckhardt CS (1995) Predictors of hand function in patients with rheumatoid arthritis. Arthritis Rheum Off J Am Coll Rheumatol 8:16–20
Martins JC, Aguiar LT, Lara EM, Teixeira-Salmela LF, Faria CDCM (2015) Assessment of grip strength with the modified sphygmomanometer test: association between upper limb global strength and motor function. Brazilian J Phys Ther. https://doi.org/10.1590/bjpt-rbf.2014.0118
D’Agostino M-A, Terslev L, Aegerter P, Backhaus M, Balint P, Bruyn GA et al (2017) Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce—Part 1: definition and development of a standardised, consensus-based scoring system. RMD Open 3. https://doi.org/10.1136/rmdopen-2016-000428
Terslev L, Naredo E, Aegerter P, Wakefield RJ, Backhaus M, Balint P et al (2017) Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce-Part 2: reliability and application to multiple joints of a standardised consensus-based scoring system. RMD Open 3. https://doi.org/10.1136/rmdopen-2016-000427
Naredo E, D’Agostino MA, Wakefield RJ, Möller I, Balint PV, Filippucci E et al (2013) Reliability of a consensus-based ultrasound score for tenosynovitis in rheumatoid arthritis. Ann Rheum Dis 72:1328–1334. https://doi.org/10.1136/annrheumdis-2012-202092
Sommier J, Michel-Batot C, Sauliere N, Sauliere N, Rat A, Hoenen-Clavert V et al (2006) Erosion and joint space narrowing in RA: proposition for a new semiquantitative score (ScuSST: Scoring by ultrasound structural total). Arthritis Rheum 54:S139–S139
Závada J, Hánová P, Hurvnáková J, Szczuková L, Uher M, Forejtová Š et al (2017) The relationship between synovitis quantified by an ultrasound 7-joint inflammation score and physical disability in rheumatoid arthritis--a cohort study. Arthritis Res Ther 19:5. https://doi.org/10.1186/s13075-016-1208-6
Kamel SR, Sadek HA, Mohamed FA, Osman HM (2018) Role of ultrasound disease activity score in assessing inflammatory disease activity in rheumatoid arthritis patients. Egypt Rheumatol 40:1–5. https://doi.org/10.1016/j.ejr.2017.04.002
Poole JL (2019) Hand function in rheumatoid arthritis. Hand Funct., Springer, pp 73–82. https://doi.org/10.1007/978-3-030-17000-4_5
Naredo E, Montoro M, Janţă I (2015) Rheumatoid arthritis. In: El Miedany Y (ed) Musculoskeletal Ultrasonography in Rheumatic Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-15723-8_3
Ohrndorf S, Boer AC, Boeters DM, Robin M, Burmester GR, Kortekaas MC, van der Helm-van AH (2019) Do musculoskeletal ultrasound and magnetic resonance imaging identify synovitis and tenosynovitis at the same joints and tendons? A comparative study in early inflammatory arthritis and clinically suspect arthralgia. Arthritis Res Ther 21(1):59
Smith E, Azzopardi C, Thaker S, Botchu R, Gupta H (2020) Power Doppler in musculoskeletal ultrasound: uses, pitfalls and principles to overcome its shortcomings. J Ultrasound:1–6
Bircan Ç, Gündüz NE, Tekgül A, Çetin P, Önen F, Kizil R et al (2014) Grip ability test in rheumatoid arthritis patients: Relationship with disease activity and hand-specific self-report questionnaires. Arch Rheumatol 29:160–166. https://doi.org/10.5606/tjr.2014.4590
Silva GS da, Lourenço M de A, Assis MR de. (2018) Hand strength in patients with RA correlates strongly with function but not with activity of disease. Adv Rheumatol 58:20. doi.https://doi.org/10.1186/s42358-018-0020-1
Higgins SC, Adams J, Hughes R (2018) Measuring hand grip strength in rheumatoid arthritis. Rheumatol Int 38:707–714. https://doi.org/10.1007/s00296-018-4024-2
Schaap LA, Pluijm SMF, Deeg DJH, Harris TB, Kritchevsky SB, Newman AB et al (2009) Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol Ser A Biomed Sci Med Sci 64:1183–1189. https://doi.org/10.1093/gerona/glp097
Westbury LD, Fuggle NR, Syddall HE, Duggal NA, Shaw SC, Maslin K et al (2018) Relationships between markers of inflammation and muscle mass, strength and function: findings from the Hertfordshire Cohort Study. Calcif Tissue Int 102:287–295. https://doi.org/10.1007/s00198-018-4503-z
Dedeoglu M, Gafuroglu U, Yilmaz O, Bodur H (2013) The relationship between hand grip and pinch strengths and disease activity, articular damage, pain, and disability in patients with rheumatoid arthritis: romatoid artritli hastalarda elle kavrama ve tutma guclerinin hastalik aktivitesi, eklem hasari, Agr. Turkish J Rheumatol 28:69–78
Arab Alkabeya H, Hughes A-M, Adams J (2019) Factors associated with hand and upper arm functional disability in people with rheumatoid arthritis: a systematic review. Arthritis Care Res 71:1473–1481. https://doi.org/10.1002/acr.23784
Tastekin N, Uzunca K, Birtane M, Kabayel DD, Ozturk G (2006) The relationship of range of motion and grip strength of the hand with disease activity, hand functions and disability in patients with rheumatoid arthritis. Rheumatism 21:13–17
Soliman AF, Egaila SE-S, Ali AI, Azab NI, Al-Gohary HH (2016) HLA-DRB1 alleles in Egyptian rheumatoid arthritis patients: Relations to anti-cyclic citrullinated peptide antibodies, disease activity and severity. Egypt Rheumatol 38:269–275. https://doi.org/10.1016/j.ejr.2016.03.007
Abdel-Nasser AM, Mahmoud MH, El Mansoury TM, Osman AM (2008) Anti-CCP2 is an adjunct to, not a surrogate for, rheumatoid factor in the diagnosis of rheumatoid arthritis: Diagnostic utility of anti-CCP2 antibodies in Egyptian patients with rheumatoid arthritis. Scand J Rheumatol 37:329–336. https://doi.org/10.1080/03009740802116208
Wakefield RJ, O’connor PJ, Conaghan PG, McGonagle D, Hensor EMA, Gibbon WW et al (2007) Finger tendon disease in untreated early rheumatoid arthritis: a comparison of ultrasound and magnetic resonance imaging. Arthritis Care Res 57:1158–1164. https://doi.org/10.1002/art.23016
Duncan SFM, Saracevic CE, Kakinoki R (2013) Biomechanics of the hand. Hand Clin 29:483–492. https://doi.org/10.1016/j.hcl.2013.08.003
Erol AM, Ceceli E, Ramadan US, Borman P (2016) Effect of rheumatoid arthritis on strength, dexterity, coordination and functional status of the hand: relationship with magnetic resonance imaging findings. Acta Reumatol Port 41
Kahle W, Leonhardt H, Platzer W, Palmer E, Platzer W. (2004) Color atlas and textbook of human anatomy. Vol. 1, Locomotor system. Thieme.
Nishino A, Kawashiri S, Shimizu T, Umeda M, Fukui S, Koga T et al (2017) Assessment of both articular synovitis and tenosynovitis by ultrasound is useful for evaluations of hand dysfunction in early rheumatoid arthritis patients. Mod Rheumatol 27:1–4. https://doi.org/10.1080/14397595.2016.1253813
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AS analyzed and interpreted the patient data and was a major contributor in writing the manuscript. MZ has made substantial contributions to the design of the study. DA substantively revised it. RE contributed to the patient selection and statistical analysis. NA performed the ultrasound examination for all patients included in the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
A written consent was taken from all patients who participated in this study according to the ethics committee recommendations after approval from the ethical committee of Ain Shams University (Faculty of Medicine) for the study. Committee’s reference number is 64/2018.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study.
Competing interests
The authors declare that they have no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Safar, A.H., Zaki, M.G., Al-Zifzaf, D.S. et al. Value of musculoskeletal ultrasound in assessment of rheumatoid hand function. Egypt J Radiol Nucl Med 51, 207 (2020). https://doi.org/10.1186/s43055-020-00327-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-020-00327-7