Abstract
Background
Breast cancer is a heterogeneous disease that was explained recently by the presence of multiple molecular subtypes. These subtypes are the luminal A (LA), luminal B (LB), human epidermal growth factor receptor 2 (HER2), and triple-negative (TN). In this study, we aim to investigate whether ultrasound imaging features can play a role in predicting the molecular subtypes of invasive ductal breast cancer (IDC) and to assess whether the nodal metastasis is an independent predictor for each subtype.
Results
The predictive sonographic signs for each subtype are as follows: echogenic halo, posterior shadowing, angular or spiculated margin, and unifocal mass for LA subtype; irregular shape for LB subtype; unifocal mass, abrupt interface of the tumor boundary, and posterior enhancement or no posterior change for HER2 subtype; and circumscribed or lobulated margin, oval or rounded shape, posterior enhancement or no posterior change, abrupt interface of the tumor boundary, and parallel orientation of the mass for TN. By multivariate logistic regression, presence of nodal metastasis is the strongest independent predictor for HER2 subtype, and its absence is the strongest independent predictor for LA subtype.
Conclusions
Certain sonographic signs are predictors for each molecular subtype of IDC. Nodal metastasis is an independent predictor for HER2 subtype when present and for LA subtype when absent.
Similar content being viewed by others
Background
Breast cancer is the most common malignancy in women worldwide [1]. It has been described as a heterogeneous disease that was explained recently by the presence of multiple molecular subtypes [2]. These subtypes are the luminal A (LA), luminal B (LB), human epidermal growth factor receptor 2 (HER2), and triple-negative (TN). They were identified according to the presence of estrogen (ER) or progesterone (PR) receptors and whether or not the HER2 is expressed [3]. Identifying these subtypes has aid significantly in treatment selection and prediction of disease course and progression. Many studies have demonstrated that patients with HER2 and TN tumors have poorer prognosis compared with patients with LA and LB tumors and that those with LA tumors have a better prognosis than those with LB tumors [3]. Although core needle biopsy is the gold standard for pathological assessment, it is invasive and might cause physical and psychological discomfort in patients and may not be an option in certain circumstances. Ultrasound is a simple and noninvasive method for diagnosis of breast cancer, but little is known about its ability to differentiate the molecular subtypes of the breast cancer. Trying to determine specific sonographic features of each subtype that reflect the tumor aggressiveness may allow better organization of the degree of primary care for the patient and probably share in planning for the treatment [4]. Many authors have looked into the mammographic and sonographic features of triple-negative breast cancer because it is the most aggressive subtype [5], and a few studies have focused on ultrasound features of the other subtypes [6, 7] and the relation of the distribution of these subtypes to the lymph node affection [8].
As immunohistochemistry is not available in all institutes, trial to find specific sonographic criteria for each molecular subtype could propose a diagnostic algorithm for each subtype. The purpose of our study is to investigate whether ultrasound can play a valuable role in predicting the molecular subtypes of invasive ductal breast cancer. We also evaluate the possibility of using nodal involvement as an independent predictor of each subtype.
Methods
Patient’s selections
This study was approved by our institutional review board, and informed consent was obtained from all patients. This study was carried out from December 2016 to June 2017. Throughout this period, we retrospectively reviewed the pathological database of our institution and identified 160 patients with invasive ductal carcinoma who has complete ultrasound, histopathological, and immunohistochemistry reports.
Ultrasound analysis
The breast ultrasound was performed using 7–14 MHZ linear array transducer by a trained sonographer with more than 10 years’ experience. The reviewed ultrasound (US) findings were according to the American College of Radiology Breast Imaging Reporting and Data System (ACR BIRADS) lexicon [9]. The following ultrasound features were analyzed: tumor shapes (rounded or oval versus irregular), the tumor margins (circumscribed or lobulated versus angular or spiculated), the posterior acoustic features (posterior enhancement or no posterior change versus posterior shadowing), lesions boundary (abrupt termination versus echogenic halo), echo pattern (hypoechoic or heterogeneous versus isoechoic), and orientation (parallel to the skin versus non parallel). Nodal metastasis was diagnosed as malignant when we found the following features: rounded shape, hypoechoic texture, eccentric or loss of hilum, and non hilar blood flow.
Histological analysis and immunohistochemistry
The pathological reports of the surgical specimens were reviewed. Histological grade was classified as 1, 2, or 3 according to the Elston and Ellis grading system [10]. In our study, grades 1 and 2 were considered low grade, whereas grade 3 was considered high grade. The hormone receptor (HR) status, ER, PR, HER2, and Ki-67 were determined through surgical specimen. ER and PR statuses were defined as positive when more than 1% of the tumor cells showed positive nuclear staining for either ER or PR, respectively. HER2 status was graded as 0, 1+, 2+, and 3+. It was considered negative when the grade was 0 or 1+, positive when it was 3+, or borderline when it was 2+. Fluorescence in situ hybridization (FISH) was performed on all HER2(2+) tumors to make a final determination on status. In our study, the Ki-67 index was scored as high when 14% or more of the tumor cells were immunohistostained and low when less than 14% of the tumor cells were immunohistostained in accordance with the St. Gallen International Expert Consensus guidelines. Molecular subtypes were categorized according to the St. Gallen Consensus 2011 [11] (Table 1).
Statistical analysis
All analyses were performed with the use of statistical software (SPSS version 19). The clinico-pathologic data and sonographic features were tabulated for all patients. We correlated the clinico-pathologic data and ultrasound features of the breast mass with the molecular subtypes using chi square test. We considered the TN patients as the reference group. Any sonographic feature with a significant statistical difference was analyzed using univariate and multivariate logistic regression analyses to identify the significant predictive sonographic signs (SS) and the independent predictors of each molecular subtype. The odds ratio (OR) and 95% confidence interval (CI) were calculated for each significant factor. The statistical difference was considered significant when the P value was < 0.05.
Results
The mean age of our enrolled 160 patients was 52 ± 12.73 years (range, 23–86 years). The mean tumor size was 3.1 ± 1.61 cm (ranges, 1–9 cm). LA was the most common subtype which represented 51.2% of our patients (n = 82) (Table 2). LA and LB subtypes represented most of the low grade tumors (69% LA and 20% LB), while most of the high grade tumors were TN (44%) and HER2 (36%) subtypes.
From Table 3, there was no statistical significant difference among the four molecular subtypes regarding the patient age, tumor size, or the tumor echogenicity. Angular or spiculated margin, echogenic halo, and posterior shadowing occurred more frequently in LA subtype than in LB subtype (74.4%, 61.5%/76.8%, 53.8% and 63.4%, 38.5%, respectively). Non parallel orientation was more likely to be seen in LB subtype than in LA subtype (92.3% and 85.4%, respectively) (Figs. 1 and 2). Irregular shape was seen more frequently in LB followed by HER2 (76.8% and 61.5%, respectively). Nodal metastasis was highly characteristic for both LB and HER2 subtypes (84.6% and 76%, respectively) (Figs. 1 and 3), while it was rarely associated with LA (28%) and TN (23.1 %) subtypes (Figs. 2 and 4). Circumscribed or lobulated margin, posterior enhancement or no posterior changes, and abrupt interface of the tumor boundary from the surrounding breast parenchyma were highly characteristic for TN and HER2 (92.3%, 84.6%/92.3%, 53.8% and 84.6%, 76.9%, respectively). Calcification more commonly appeared in HER2 subtype (61.5%). It was noteworthy that TN subtype was more likely to have oval or rounded shape (92.3%) and parallel orientation (38.5%) than the LB, HER2, and LA subtypes.
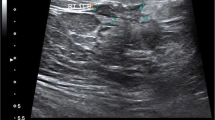
a Ultrasound of the right breast and b right axilla in 40 years old women shows a a unifocal mass with irregular shape, spiculated margin, echogenic halo with non-parallel orientation, and posterior shadowing; it measures 15 × 9 mm. b Multiple malignant featuring right axillary lymph nodes. The mass proved pathologically to be low grade invasive ductal carcinoma with malignant infiltration of the LN and luminal B subtype
a Ultrasound of the right breast and b right axilla in 55 years old women shows a a unifocal mass with irregular shape, spiculated margin, echogenic halo, parallel orientation, and posterior shadowing; it measures 10 × 20 mm. b Benign featuring right axillary lymph nodes. The mass proved pathologically to be low grade invasive ductal carcinoma with no nodal infiltration and luminal A subtype
a Ultrasound of the left breast and b left axilla in 50 years old women shows a breast mass with irregular shape, lobulated margin, calcification, and abrupt interface of the tumor boundary with no posterior change. b Multiple malignant featuring left axillary lymph nodes. The masses proved to be high grade invasive ductal carcinoma with ductal carcinoma in situ component and malignant infiltration of the lymph nodes and HER2 subtype pathologically
a Ultrasound of right breast and b right axilla in 56 years old women shows a mass with oval shape, circumscribed margin, parallel orientation, and abrupt interface of the tumor boundary with no posterior changes. b Benign featuring right axillary lymph nodes. The mass proved to be high grade invasive ductal carcinoma with no nodal infiltration and TN subtype pathologically
By univariate logistic regression analysis (Table 4), the predictive sonographic signs of the LA subtype were echogenic halo (OR = 7.46), posterior shadowing (OR = 6.72), angular or spiculated margin (OR = 4.65), absence of nodal metastasis (OR = 4.10), and presence of unifocal mass (OR = 4.03). The predictive SS of LB subtype were nodal metastasis (OR = 9.54) and irregularity of the tumor shape (OR = 3.65). The predictive SS of HER2 were nodal metastasis (OR = 5.43), presence of unifocal mass (OR = 5.35), abrupt interface of the tumor boundary (OR = 5.09), posterior enhancement or no posterior change (OR = 5.03), and presence of calcification (OR = 3.06). The predictive SS of TN were circumscribed or lobulated margin (OR = 23.73), oval or rounded shape of the tumor (OR = 18.92), posterior enhancement or no posterior change (OR = 11.65), abrupt interface of the tumor boundary (OR = 8.95), parallel orientation of the mass (OR = 3.56), and absence of nodal metastasis (OR = 3.14).
Using multivariate logistic regression (Table 4), we found that absence of nodal metastasis was the strongest independent predictor for LA subtype (OR = 5.40), but it is among the important predictors for the TN subtype (OR = 2.09). The presence of nodal metastasis was the strongest independent predictor for HER2 subtype (OR = 4.17), but it is among the important predictors for LB subtype (OR = 2.54).
Discussion
The development of molecular biology of the breast cancer allowed the management to be personalized according to the molecular subtype. So identification of the breast cancer subtypes by ultrasonographic features could be of pivotal interest to the radiologist as it has a direct effect on the decision of the clinicians. As the axillary lymph node metastasis remains the most important prognostic factor in breast cancer and it has a considerable impact on the outcome of the patient and on the course of the disease, detection of the relationship of nodal metastasis to the molecular subtypes of the breast cancer is of great value [12].
Our data demonstrated that the main predictive SS of the LA subtype are spiculated or angular margin, post-acoustic shadowing, echogenic halo, absence of nodal metastasis, and unifocal mass. Similar data were described by Zhang et al. [13]. Two other studies done by Kim et al. and Ko et al. [14, 15] proved that echogenic halo and post-acoustic shadowing are more commonly seen in ER-positive/PR-negative/HER2-negative breast cancers that represent the LA subtype in our study. Similar to our results, Wiechmann et al. [16] reported that the LA subtype is less likely to be associated with multicentric or multifocal breast cancer and nodal metastasis than HER2 and LB subtypes, so when a breast mass encounters these sonographic features, the radiologist would be reminded that it has a minor chance to be LA subtype.
Jiang et al. [17] demonstrated that the absence of echogenic halo was a characteristic sign of the LB subtype using 2D ultrasound, and Yang et al. [18] reported that the absence of retraction phenomena and presence of calcification are predictive factor of LB subtype using 3D ultrasound; both results are inconsistent with our results that found the predictive SS of the LB subtype are the presence of nodal metastasis (OR = 9.54) and irregular tumor shape (OR = 3.65). Such discrepancy may be due to difference in the sample size of LB subtype between their study and ours. Similar to our results, Goldhirsch et al. [19] demonstrated that patients with LB subtype are more likely to associate with nodal metastasis.
As noted in our study that LA subtype followed by LB subtype shows the highest rate of low grade tumors (69% and 20%, respectively) and sonographically tend to have angular or spiculated margin, echogenic halo, and posterior shadowing. This was also described by Irshad et al. [20]. The mechanism of their formation could be attributed to the great desmoplastic reaction which strongly related to the low grade tumor [21]. As the absence of nodal metastasis is the strongest independent predictor of LA subtype (OR = 5.4) Thus, LN status could help to differentiate between these two subtypes that share certain SS as described, so its absence predicts with confidence the LA subtype, while its presence excludes LA subtype and increases the possibility of LB subtype.
In a study done by Seo et al. [22] found that the TN subtype is more likely than the other subtypes to have microlobulated margins, regular shape, and posterior acoustic enhancement and is less likely to have echogenic halo, spiculated margin, and calcifications. Similarly, in our study, the predictive SS of the TN subtype are the circumscribed or lobulated margin, oval or round shape, posterior enhancement or no posterior change, abrupt interface of the tumor boundary, and parallel orientation. Also, the absence of nodal metastases is a predictive SS of TN subtype that agrees with the study done by Yang et al. [23] who reported that the basal subtype (ER- and PR- and HER-2-) which represent the TN subtype in our study is less likely to show nodal involvement than the other subtypes.
The predictive SS of the HER2 subtype in our study are posterior acoustic enhancement or no posterior change, nodal metastases, calcification, unifocal mass, and abrupt interface of the mass boundary. Similar to our results, calcifications and post-acoustic enhancement were shown to be characteristics of the HER2 subtype by Yang et al. [23]. The HER2 subtype was also found to have more calcifications (80%) than the TN subtype (28%) and luminal (A or B) subtypes (41%) by mammography in the study done by Ko et al. [15]; this might be explained by the probability that HER2 breast cancers are more often accompanied by ductal carcinoma in situ. In agreement with our result, Yang et al. [23] observed higher frequency of nodal metastases in the HER2 followed by LB subtype (mostly HER2 positive) than in TN and LA subtype. Wiechmann et al. [16] found a higher frequency of multicentric or multifocal disease in HER2 subtype than other subtypes. On the contrary, our results showed that unifocal mass is one of the predictors SS of HER2 subtype. This could be explained by the big difference between the number of the patients of HER2 subtype in both studies (n = 16 in our study and n = 368 in Wiechman’s study).
This study demonstrated that most of the high grades were TN subtype followed by HER2 subtype (44% and 36%, respectively) and sonographically tend to have circumscribed or lobulated margin, posterior enhancement or no posterior change, and abrupt interface of the tumor boundary. This was also described by Kim et al. [24]. The mechanism of their formation attributed to the high mitotic rates and increased cellularity with less stromal and desmoplastic reaction.
Among the previous, predictive SS of HER2 subtype nodal metastasis are the strongest independent predictor of HER2 subtype (OR = 4.17). As TN and HER2 subtypes share certain SS, thus presence of nodal metastasis strongly predicts the HER2 subtype, and its absence excludes HER2 and predicts with great confidence the TN subtype.
There are some limitations of this study; small sample size in LB subtype may have limited the statistical significance of the data obtained. Using a combination of imaging modalities, including mammography, Doppler, and ultrasonographic elastography, may improve the diagnostic accuracy. There is no correlation between the presence of ductal carcinoma in situ and the molecular subtypes.
Conclusions
Ultrasonographic imaging features, including the tumor shape, margin, boundary, posterior features, multiplicity, orientation, and calcification, are significant predictive sonographic signs of different molecular subtypes. Nodal metastasis is the strongest independent predictor for the HER2 when present and for LA when absent. So nodal status used as a tool in differentiating between each two subtypes has to some extent similar sonographic features; accordingly, it can differentiate firstly between LA and LB subtypes and secondly, between HER2 and TN subtypes. So the sonographic features of the mass and the LN status can expand the scope of the ultrasound in predicting with confidence the molecular subtypes of the breast cancer that are currently beyond the scope of ultrasound BI-RADS. We recommended correlating the BIRADS Ultrasound lexicon with each molecular subtype aiming to change the description of each category of the BIRADS and put the LN status as a separate item in each category of the BIRADS.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SS:
-
Sonographic signs
- IDC:
-
Invasive ductal breast cancer
- LA:
-
Luminal A
- LB:
-
Luminal B
- HER2:
-
Human epidermal growth factor receptor
- TN:
-
Triple negative
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- US:
-
Ultrasound
- HR:
-
Hormonal receptor
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- BIRADS-US:
-
Breast Imaging Reporting and Data System Ultrasound
- ACR:
-
American College of Radiology
- FISH:
-
Fluorescence in situ hybridization
References
Sung JS, Stamler S, Brooks J, Kaplan J, Huang T, Dershaw D, Lee CH, Morris EA, Comstock CE (2016) Breast cancers detected at screening MR imaging and mammography in patients at high risk: method of detection reflects tumor histopathologic results. Radiology 280(3):716–722
Trop I, LeBlanc SM, David J, Lalonde L, Tran-Thanh D, Labelle M, El Khoury MM (2014) Molecular classification of infiltrating breast cancer: toward personalized therapy. Radiographics 34(5):1178–1195
Boisserie-Lacroix M, MacGrogan G, Debled M, Ferron S, Asad-Syed M, McKelvie-Sebileau P, Mathoulin-Pelissier S, Brouste V, Hurtevent-Labrot G (2013) Triple-negative breast cancers: associations between imaging and pathological findings for triple-negative tumors compared with hormone receptor-positive/human epidermal growth factor receptor-2-negative breast cancers. Oncologist 18(7):802–811
Li Z, Tian J, Wang X, Wang W, Wang Z, Zhang L, Jing H, Wu T (2016) Differences in multi-modal ultrasound imaging between triple negative and non-triple negative breast cancer. Ultrasound Med Biol 42(4):882–890
Boisserie-Lacroix M, Mac Grogan G, Debled M, Ferron S, Asad-Syed M, Brouste V, Mathoulin-Pelissier S, Hurtevent-Labrot G (2012) Radiological features of triple-negative breast cancers (73 cases). Diagn Interv Imaging 93(3):183–190
Choi YJ, Seong MH, Choi SH, Kook SH, Kwag HJ, Park YL, Park CH (2011) Ultrasound and clinicopathological characteristics of triple receptor-negative breast cancers. J Breast Cancer 14(2):119–123
Dogan BE, Gonzalez-Angulo AM, Gilcrease M, Dryden MJ, Yang WT (2010) Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. AJR Am J Roentgenol 194:1160–1166
Kojima Y, Tsunoda H (2011) Mammography and ultrasound features of triple-negative breast cancer. Breast Cancer 18:146–151
Krizmanich-Conniff KM, Paramagual C, Patterson SK, Helvie MA, Roubidoux MA, Myles JD, Jiang K, Sabel M (2012) Triple receptor-negative breast cancer: imaging and clinical characteristics. AJR Am J Roentgenol 198:458–464
Wang Y, Ikeda DM, Narasimhan B, Lonqacre TA, Bleicher RJ, Pal S, Jackman RJ, Jeffrey SS (2008) Estrogen receptor-negative invasive breast cancer: Imaging features of tumors with and without human epidermal growth factor receptor type 2 overexpression. Radiology 246:367–375
Wojcinski S, Soliman AA, Schmidt J, Makowski L, Degenhardt F, Hillemanns P (2012) Sonographic features of triple-negative and non-triple-negative breast cancer. J Ultrasound Med 31(10):1531–1541
Zheng FY, Lu Q, Huang B, Yan LX, Wang X, Yuan W, Wang W (2017) Imaging features of automated breast volume scanner: correlation with molecular subtypes of breast cancer. Eur J Radiol 86:267–275
Zhang L, Li J, Xiao Y, Cui H, Du G, Wang Y, Li Z, Wu T, Li X, Tian J (2015) Identifying ultrasound and clinical features of breast cancer molecular subtypes by ensemble decision. Sci Rep 5:11085
Kim EK, Ko KH, Oh KK, Kwak JY, You JK, Kim MJ, Park BW (2008) Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol 190:1209–1215
Ko ES, Lee BH, Kim HA, Noh WC, Kim MS, Lee SA (2010) Triple-negative breast cancer: correlation between imaging and pathological findings. Eur Radiol 20(5):1111–1117
Wiechmann L, Sampson M, Stempel M, Jacks LM, Patil SM, King T, Morrow M (2009) Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol 16(10):2705–2710
Jiang J, Chen YQ, Xu YZ, Chen ML, Zhu YK, Guan WB, Wang XJ (2014) Correlation between three-dimensional ultrasound features and pathologicalprognostic factors in breast cancer. Eur Radiol 24:1186–1196
Yang Q, Liu HY, Liu D, Song YQ (2015) Ultrasonographic features of triple-negative breast cancer: a comparison with other breast cancer subtypes. Asian Pac J Cancer Prev 16(8):3229–3232
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1747
Irshad A, Leddy R, Pisano E, Baker N, Lewis M, Ackerman S, Campbell A (2013) Assessing the role of ultrasound in predicting the biological behavior of breast cancer. AJR Am J Roentgenol 200(2):284–290
Tamaki K, Sasano H, Ishida T, Ishida K, Miyashita M, Takeda M, Amari M, Harada-Shoji N, Kawai M, Tamaki THN, Ohuchi N (2010) The correlation between ultrasonographic findings and pathologic features in breast disorders. Jpn J Clin Oncol 40(10):905–912
Seo BK, Pisano ED, Kuzimak CM, Koomen M, Pavic D, Lee Y, Cole EB, Lee J (2006) Correlation of HER-2/neu overexpression with mammography and age distribution in primary breast carcinomas. Acad Radiol 13(10):1211–1218
Yang WT, Dryden M, Broglio K, Glicrease M, Dawood S, Dempsey PJ, Valero V, Hortobagy G, Atchley D, Arun B (2008) Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Res Treat 111(3):405–410
Kim MY, Choi N (2013) Mammographic and ultrasonographic features of triple-negative breast cancer: a comparison with other breast cancer subtypes. Acta Radiol 54(8):889–894
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not for the profit sectors.
Guarantor
The scientific guarantor of this publication is Dr. Lamiaa M.R Khalaf.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
Corresponding author: LMRK. Dr. Lamiaa was responsible for the study design, revision and interpretation of the sonographic images for all the patients who enrolled in this study, analysis and interpretation of the data, statistical analysis, editing, drafting and submission of the manuscript, and guarantor of integrity of the entire study. Co-author: RAH was responsible for diagnosis of all the pathological specimen after surgery or biopsy and writing the pathological part of the manuscript. All authors have approved and read the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors whose names are listed on the title page and shared in the Manuscript entitled “Role of Ultrasound in predicting the molecular subtypes of Invasive Breast Ductal Carcinoma,” certified that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria, educational grants, participation in speakers, membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony; or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethics approval and consent to participate
This prospective study was approved by the Research Ethics Committee of the Faculty of Medicine at Assiut University in Egypt (its number is 17100016). Written informed consent was obtained from each patient after receiving information about the details of the study.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalaf, L.M.R., Herdan, R.A. Role of ultrasound in predicting the molecular subtypes of invasive breast ductal carcinoma. Egypt J Radiol Nucl Med 51, 138 (2020). https://doi.org/10.1186/s43055-020-00240-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-020-00240-z