Abstract
Background
Volumetric MR neuroimaging can visualize the pattern of hippocampal subfield atrophic changes in AD. This can be used as a biomarker in early diagnosis of AD and allow early treatment to improve memory, behavioral symptoms, and delay the cognitive deterioration. The aim of this work is to assess the role of the volumetric study of different hippocampal subfields as a post-processing technique of structural MR imaging in patients with Alzheimer’s disease of different severity of cognitive functions. The regional ethics committee approved the study and written informed consent was obtained from all participants. In the duration from 2016 to 2018, a cross-sectional study was conducted on 30 patients (17 males and 13 females) and 15 healthy elderly controls (9 males and 6 females) referred to the Radiodiagnosis Department from the Neuropsychiatry Department. Patients were diagnosed with AD by clinical examination and using the Mini Mental State Examination (MMSE) and the Clinical Dementia Rating scale (CDR) as a measure of general cognitive performance.
Results
CA1 and subiculum subfields were significantly reduced in size in patients with Alzheimer’s disease in relation to the age-matched control group (P < 0.05). This finding was positively correlated with the MMSE score and negatively correlated with CDR clinical tests. No significant atrophy was found among other hippocampal subfields in the patients’ group.
Conclusion
This study proposed a new approach to detect atrophy in hippocampal subfields, using MR volumetric study of high-resolution T1 images, that can be used as a biomarker in the diagnosis of AD patients and differentiating them from elderly control subjects which is important in early diagnosis of AD and hence the proper treatment to improve the prognosis of the cognitive function.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly population. It is progressively deteriorating different cognitive and behavioral domains causing serious effects on the quality of life of patients and their family members, and eventually, AD contributes to death. The diagnosis of AD usually involves clinical assessments, cognitive tests, and brain imaging. Early and accurate diagnosis of AD can help to delay the decline of the cognitive functions of the patients for months or years and also help in early initiation of treatment [1].
AD is characterized pathologically by the presence of abundant extracellular amyloid neuritic plaques and intracellular neurofibrillary tangles (NFTs) [1,2,3], the hippocampus and entorhinal cortex were found to be the first sites of pathological changes [4], and these changes are present years or even decades before the onset of the clinical manifestations of the disease.
The most widely used clinical criteria for the diagnosis of AD are the National Institute of Neurological and Communicative Disorders and Stroke Alzheimer’s Disease and Related Disorder Association (NINDS-ADRDA) criteria and Mini Mental Status Examination (MMSE). Developments in magnetic resonance imaging (MRI) have greatly improved the understanding of AD. MRI enables us to visualize structural, metabolic, and functional changes. Medial temporal lobe atrophy can be associated with the disease [5].
Unfortunately, this has been found to have limited utility both in early disease and in the differentiation of AD from other causes of dementia and from normal aging [6]. In other words, by conventional MRI means, atrophy of the hippocampus and entorhinal cortex can be assessed using simple validated scales [7]; however, there is an overlap with age-related atrophy cases that makes the distinction of AD from normal aging more problematic in the elderly to achieve an accurate and reliable assessment using a human rater [8].
Volumetric analyses of brain structures have become increasingly common for diagnostic purposes and for identifying disease progression. It has been demonstrated that atrophy of the medial temporal lobe (MTL) memory structures, where the pathological changes of AD in terms of neurofibrillary tangles and neuronal loss first occur [2], can be detected even in subjects with mild cognitive impairment (MCI) [9, 10].
Accurate regional segmentation on MR images is necessary to obtain the volumetric measurements for various structures as the conventional approach based on signal intensity alone is not sufficient to distinguish between different structures as observed and characterized by Fischl et al. [11].
The hippocampus has a critical role in declarative and episodic memory, as well as a focus of structural change in normal aging [12] and diseases such as epilepsy and Alzheimer’s disease (AD) [13].
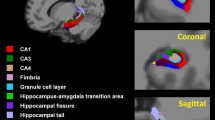
The hippocampal formation consists of a number of distinct, interacting subfields (Figs. 1 and 2). Despite its internal complexity, limited MRI resolution previously forced researchers to model the hippocampus as a single, homogeneous structure in neuroimaging studies of aging and AD [14].
Four coronal slices for hippocampal head and body with corresponding manual annotations. The slices are ordered from anterior to posterior. Red = CA1, blue = subiculum, dark purple = presubiculum, yellow = parasubiculum, cyan = granule cell layer of the dentate gyrus (GC-DG), light green = hippocampus-amygdala-transition-area (HATA)
Consequent stages during the processing of 3D T1-W images in FreeSurfer software. a Conventional coronal image at the hippocampal body level showing mild widening of the choroid fissure and temporal horns. b Axial image showing removal of non-brain tissue followed by subcortical segmentation where there is the identification and outlining of different brain structures including here the hippocampus as a whole organ. c Axial image showing segmentation of the hippocampus into subfields. d Coronal image showing filling of different hippocampal subfields and annotation in different colors. The table is showing calculation of the hippocampal subfield volume done by quantification of the number of voxels
Even though studies have shown that whole hippocampal volumes derived from segmented MRI scans are powerful biomarkers for AD [11, 15], treating the hippocampus as a single entity disregards potentially useful information about its subfields (Fig. 3); they are also thought to play different roles in memory and learning [16, 17] and to be affected differently by AD and normal aging—as indicated by ex vivo, histological studies [18].
Consequent stages during the processing of 3D T1-W images in FreeSurfer software. a Conventional coronal image at the hippocampal body level showing moderate widening of the choroid fissure and temporal horns. b Axial image showing removal of non-brain tissue followed by subcortical segmentation where there is identification and outlining of different brain structures including here the hippocampus as a whole organ. c Axial image showing segmentation of the hippocampus into subfields. d Coronal image showing filling of different hippocampal subfields and annotation in different colors. The table is showing calculation of the hippocampal subfields volume done by quantification of the number of voxels
Studies have shown that atlas-based volumetric analysis found that even hippocampal subfields have specific changes and neurodegenerative atrophy patterns associated with AD and can distinguish different diagnostic groups.
Methods
This study was conducted on a total of 45 subjects: 15 controls (9 men, 6 women) with mean age 62.4 years (± 6.2), ranging from 56 to 69 years old, and 30 Alzheimer’s disease patients (17 men, 13 women) with mean age 64.5 years (± 3.3), ranging from 58 to 70 years old; this was done in the time period from 2016 to 2018. The patients were referred to the Radiodiagnosis Department and the controls were among their relatives and caregivers. Then approval of Research Ethics Committee (REC) of Tanta University and written consent were obtained from all studied participants. Inclusion criteria were patients who have been diagnosed with Alzheimer’s disease by clinical examination, after fulfilling the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA), Diagnostic and Statistical Manual of Mental Disorders IV (DSM IV) criteria, MMSE score ranged from 9 to 23, and CDR score from 1 to 3. All individuals of the study were subjected to full history taking, thorough clinical examination, and routine laboratory investigations in the form of CBC, liver and kidney function test, fasting and 2-h postprandial blood glucose, ESR, LDH, and CRP to exclude diseases that affect the conscious level and memory function. Exclusion criteria included dementia of other types other than Alzheimer’s disease, systemic illness or organ failure, and patients unable to do MRI who have a cardiac pacemaker or metallic prosthesis.
Image acquisition and post-processing
The images were acquired by using 1.5 Tesla GE closed configuration whole-body scanner using a standard quadrature head coil. The patients were entered head first in supine position. All patients were subjected to the following conventional MRI protocols: sagittal 3D T1-weighted spoiled gradient (SPGR) utilizing the following parameters—a repetition time (TR) of 7.2 ms, an echo time (TE) of 120 ms, a slice thickness of 1.2 mm, and FOV = 256 × 256 mm. A high spatial resolution (isotropic) three-dimensional (3D) T1-weighted spoiled gradient echo (SPGR) of 1.2 mm slice thickness with no spacing was obtained in sagittal plane, with preparation time of 500 ms and an imaging duration of 5–7 min per acquisition; a repetition time 7.2 ms and minimum full echo time; a flip angle of 10° (to partly compensate inhomogeneity in the radiofrequency field); 160 slices, encoding frequency 16 kHz, a field of view of 30 cm, and an acquisition matrix size of 192 × 192 (FH AP RL; FH = foot-to-head; AP = anteriorto-posterior; RL = right-to-left); and frequency DIR S/I, NEX 1.0 with active automated shimming. Phased-array uniformity enhancement (PURE) was applied and surface coil intensity correction (SCIC) was off. This protocol resulted in high-resolution images with high gray-white matter contrast, used for measuring of specific gray matter (hippocampus) region of interest.
MRI data preprocessing
The preprocessed images are lodged into the automated brain segmentation software FreeSurfer, then algorithm initiation and calculation of hyperparameters for subcortical segmentation.
Data processing technique
A sequence of steps is then performed by the software as following: removal of the skull and any remaining background noise from T1 images to produce a skull-stripped corrected volume that is used in the segmentation algorithm; spatial normalization process is then done to the volume to correct the displacements, rotations, and scales and bring the person’s volume into Talairach space which is a standard morphological space; identification of the boundary of the gray/white matter interface as well as the pial surface; segmentation of the different brain anatomical structures, where different measures can be made; and the segmentation of the subcortical white matter and deep gray matter volumetric structures, such as the hippocampus. These structures can then be filled in different label colors.
Measurement of the hippocampal subfield volume ROI analysis
Further segmentation of the hippocampus is done and different subfields can be extracted to be labeled differently, and calculation of volume is then measurable and generated for each of the segmented hippocampal subfields (Fig. 4). The conventional images are interpreted by two neuroradiologists and one neurologist who have a medical doctorate degree and are experienced in neuroimaging for more than 10 years duration, and then the final volume measurements are the results of an automated processing which has no human rater involved.
Coronal T1-weighted images through the hippocampus head (nearly the same level) showing hippocampal subfields segmented and annotated in different colors, a in two different healthy control subjects and b in two different Alzheimer’s disease patients, during the processing. Red = CA1, blue = subiculum, dark purple = presubiculum, yellow = parasubiculum, cyan = granule cell layer of the dentate gyrus (GC-DG), light green = hippocampus-amygdala-transition-area (HATA)
Statistical analysis of the data
Data were provided for the computer and analyzed using IBM SPSS software package version 20.0. To assess both the principal impacts of diagnosis (ADversus-control) factors on the hippocampal subfield volume, this work used analysis of covariance with total intracranial volume, education, sex, and age as nuisance variables.
Qualitative data were described using number and percent. Quantitative data were described using range, mean, standard deviation, and median. Comparison of MRI findings with normal values was done using one sample t test. Significant test results are quoted as two-tailed probabilities. The significance of the obtained results was judged at the 5% level. P value < 0.05 was considered significant.
Results
This study included 45 subjects: 15 controls (9 men, 6 women; mean age 62.4 ± 6.2 years) who were proved to be healthy and 30 Alzheimer’s disease patients (17 men, 13 women; mean age 64.5 ± 3.3 years). The demographic data and cognitive scores for control and Alzheimer’s disease cases are shown in Table 1. All patients were diagnosed by clinical examination, conventional MRI, and then hippocampal subfields volumetric measurements after segmentation with FreeSurfer (Fig. 4). The subiculum and CA1 subfield volumes in patients with Alzheimer’s disease were significantly reduced on both sides in relation to age-matched controls (P < 0.05), and there was no significant difference found between both sides (Table 2).
They were found to have a positive correlation with the MMSE score which proved to be statistically significant when compared with normal elderly subjects; correlation coefficient (r) was 0.971, 0.957, 0.98, and 0.987 for right CA1, right subiculum, left CA1, and left subiculum, respectively (Table 3).
Meanwhile, they showed a negative correlation with the CDR score which was also statistically significant when compared with normal elderly subjects; correlation coefficient (r) was − 0.892, − 0.879, − 0.917, and − 0.871 for right CA1, right subiculum, left CA1, and left subiculum, respectively (Table 3).
Other studied subfields that were decreased could not be proved to be statistically significant. There was no significant correlation between group differences in age, sex, or years of education. Sensitivity, specificity, PPV and NPV of CA1, and subiculum volume reduction in AD relative to healthy elderly control were high, and the sensitivity of the study is increased by combination between volumes and the clinical exam scores.
Discussion
Alzheimer’s disease is the most common cause of dementia in elderly people, and it is a progressive disease and its prevalence is predictable to increase as populations continue to age around the world. It is associated with neurofibrillary tangles and neuritic plaques, along with other changes as well as amyloid angiopathy and age-related brain atrophy which may contribute to cognitive impairment [19].
The hippocampus was found to have a special affinity for AD pathology and considered an early site for involvement, so hippocampal atrophy is used to be an imaging marker of AD and included in the diagnostic criteria [20].
Visual rating methods used in the assessment of the volume of the medial temporal lobe and the size of the surrounding CSF spaces showed obviously that they are lacking the accuracy in anatomical segmentation, as a result has low sensitivity and specificity for distinguishing AD from normal subjects with senile changes. In other words, there were dementia subjects with minimal MTA score who have only increased width of the choroid fissure without the temporal horn dilatation and were found to have already established regionally pronounced hippocampal atrophy by volumetric means. It was noted also that cases showing some degree of temporal horn dilatation in conventional imaging could have normal volumetric studies without any significant atrophy [21].
This study aimed to identify a specific regional atrophy pattern characteristic for Alzheimer’s disease in comparison to normal age-matched control, and this could enable us to better understand the course of the disease and help in its early effective treatment. FreeSurfer software was used for automated hippocampal segmentation using the preprocessed images, after initiation of the segmentation algorithm and calculation of hyperparameters and then using the output to calculate their volumes. Volumes were normalized by the intracranial volume and the gray matter volume [22].
One of the advantages of FreeSurfer software is that it is applicable for images produced by any MRI machine. Patients were classified according to MMSE and CDR clinical examinations into mild, moderate, and severe groups. The mean age of each group was 62.4, 65.7, and 66 years for mild, moderate, and severe groups, respectively. The number of years of education was included in the criteria, and their means for the three groups were 12.2, 11.2, and 12.5 years, respectively. The patients also had mean number of years since first diagnosed as AD: 3.5, 4.7, and 6.25 years for the three groups, respectively. They had presented variable medial temporal lobe atrophy-MTA scores and white matter lesion FAZKEAS scale.
After the segmentation process of the hippocampal subfields, it was found that both subiculum and CA1 on both sides had significantly reduced volumes in AD patients relative to the control group. This finding is matching with other previous studies as Kerchner et al. [23] , Zhao et al. [24], and Trujillo-Estrada et al. [25] which suggested the involvement of CA1 and subiculum subfields in particular.
These findings were correlated with the MMSE and CDR scores. All results showed significant P value and correlation. Correlation to age was determined by Pearson’s correlation coefficient. The other subfields showed no significant volume differences between AD and controls.
This work is also matching with recent studies; Hett et al. [26] have presented in their study that CA1 and subiculum are the subfields which show the most significant atrophy in AD. Also, Zhao et al. [24] has found that significant atrophy is seen in CA1, subiculum, presubiculum, molecular layer, and fimbria subfields among subjective mild cognitive decline, amnestic mild cognitive impairment, and Alzheimer’s groups; they in turn had suggested that this could be used as a biomarker in early stages of AD. Using the same image analysis approach, Hanseeuw et al. [27] showed some difference as their study reported significant volume losses in the subiculum and CA2-3 subfields in a small group of 15 amnestic MCI subjects and 15 healthy controls.
Mueller et al. [28] evaluated correlations between subfield volumes and two different memory scores based on the California Verbal Learning Test in a mixed group including cognitively normal healthy controls with a subjective memory complaint and patients with cognitive impairment, and it was found that atrophy of CA subfields appear to be related to associative memory dysfunction observed in patients with cognitive impairment. In a similar way, analysis was run on 490 individuals (including controls, MCI, and AD patients), and the authors did not find any specific correlates in healthy controls, while regions corresponding to CA1 and subiculum were associated with delayed recall performances in patients [29].
An automated hippocampal shape-analysis method by using a pattern-recognition algorithm revealed a positive correlation between CERAD delayed recall scores and hippocampal deformation in the CA1 and subiculum [30].
The earlier postmortem pathologic studies showed that degeneration of the CA1 and subiculum found to be more severe as compared with other hippocampal components in early stages of AD, and these findings are correlated with this study. Human autopsy studies suggest that volume loss of subiculum and CA1 is related to the number of neurofibrillary tangles in these areas, neuronal loss, loss of dendritic arbor, or afferent innervation. Previous studies suggested that marked degeneration of the perforant path, that is providing input from layer III of entorhinal cortex to CA1 and subiculum, was a characteristic feature of AD [29].
However, CA3 and the DG, in contrast to subiculum and CA1, are not affected by the formation of plaques, tangles, and neuronal loss until a later stage in AD [31]. Mueller et al. [28] found that atrophy of CA subfields, as grouped together in a single region, appear to be related to associative memory dysfunction observed in patients with cognitive impairment and correlate with incapability of the patients to benefit from semantic processing during encoding new information. In a similar way, analysis was run on 490 individuals (including controls, MCI, and AD patients from the Alzheimer’s disease neuroimaging initiative), and the authors did not find any specific correlates in healthy controls, while regions corresponding to CA1 and subiculum were associated with delayed recall performances in patients. A reliable result appears to determine the role of CA1 atrophy in memory dysfunction in MCI and AD [29]. The results of this study reveal that sensitivity, specificity, PPV and NPV of CA1, and subiculum volume reduction in AD in relation to healthy elderly control were high, and the sensitivity of the study is increased by combination between volumes and the clinical exam scores. Limitations of this study are firstly that it is not possible to separate the temporal relation between subfield volume changes and the clinical symptoms because of its design as a cross-sectional study, and secondly, the relatively small number of studied subjects. Future longitudinal study and larger sample size combined with other biomarkers will be needed to determine which hippocampal subfields show the earliest atrophy in the disease process and to confirm the findings.
Conclusion
This work has proposed a new approach to better capture Alzheimer’s disease. Reduced CA1 and subiculum volumes is seen in patients with Alzheimer’s disease. Further studies are needed to integrate these findings in the routine workup in evaluating patients with AD.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AD:
-
Alzheimer’s disease
- MMSE:
-
Mini Mental State Examination
- CDR:
-
Clinical Dementia Rating scale
- NFTs:
-
Neurofibrillary tangles
- NINDS-ADRDA:
-
National Institute of Neurological and Communicative Disorders and Stroke Alzheimer’s Disease and Related Disorder Association
- MTL:
-
Medial temporal lobe
- MTA score:
-
Medial temporal lobe atrophy score
- CA1:
-
Cornu ammonis
- DG:
-
Dente gyrus
- fMRl:
-
Functional magnetic resonance imaging
- MCI:
-
Mild cognitive impairment
- REC:
-
Research Ethics Committee
- DSM IV:
-
Diagnostic and Statistical Manual of Mental Disorders IV
- SPGR:
-
Sagittal 3D T1-weighted spoiled gradient
- PURE:
-
Phased-array uniformity enhancement
- SCIC:
-
Surface coil intensity correction
- PPV and NPV:
-
Positive and negative predictive values
References
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegeneration 14(1):32
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica 82(4):239–259
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM et al (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Neurology 41(4):479
O’Brien JT, Metcalfe S, Swann A, Hobson J, Jobst K, Ballard C et al (2000) Medial temporal lobe width on CT scanning in Alzheimer disease: comparison with vascular dementia, depression and dementia with Lewy bodies. Dement Geriatr Cogn Disord 11(2):114–118
Jobst KA, Smith AD, Szatmari M, Molyneux A, Esiri ME, King E (1992) Detection in life of confirmed Alzheimer’s disease using a simple measurement of medial temporal lobe atrophy by computed tomography. Lancet 340(8829):1179–1183
Dekeyzer S, De Kock I, Nikoubashman O, Vanden Bossche S, Van Eetvelde R, De Groote J et al (2017) “Unforgettable” - a pictorial essay on anatomy and pathology of the hippocampus. Insights Imaging 8(2):199–212
Goos JD, Kester MI, Barkhof F, Klein M, Blankenstein MA, Scheltens P et al (2009) Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke 40(11):3455–3460
de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S et al (2004) MRI and CSF studies in the early diagnosis of Alzheimer’s disease. J Intern Med 256(3):205–223
De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A et al (2001) Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging 22(4):529–539
Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K et al (2007) Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain 130(4):1159–1166
Fischl B, Salat D, Busa E, Albert M, Dieterich M, Haselgrove C et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3):341–355
Frisoni GB, Ganzola R, Canu E, Rüb U, Pizzini FB, Alessandrini F et al (2008) Mapping local hippocampal changes in Alzheimer’s disease and normal ageing with MRI at 3 Tesla. Brain 131(12):3266–3276
Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL et al (2006) 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer’s disease. Brain 129(11):2867–2873
Boccardi M, Ganzola R, Bocchetta M, Pievani M, Redolfi A, Bartzokis G et al (2011) Survey of protocols for the manual segmentation of the hippocampus: preparatory steps towards a joint EADC-ADNI harmonized protocol. J Alzheimers Dis 26(3):61–75
den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM (2006) Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry 63(1):57–62
Kesner RP (2013) An analysis of the dentate gyrus function. Behav Brain Res 254:1–7
Reagh ZM, Watabe J, Ly M, Murray E, Yassa MA (2014) Dissociated signals in human dentate gyrus and CA3 predict different facets of recognition memory. J Neurosci 34(40):13301–13313
Braak E, Braak H (1997) Alzheimer’s disease: transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon’s horn. Acta Neuropathol 93:323–325
Pradier C, Sakarovitch C, Le Duff F, Layese R, Metelkina A, Anthony S et al (2014) The mini mental state examination at the time of Alzheimer’s disease and related disorders diagnosis, according to age, education, gender and place of residence: a cross-sectional study among the French National Alzheimer database. PloS One 9(8):e103630
Mufson EJ, Mahady L, Waters D, Counts SE, Perez SE, DeKosky ST et al (2015) Hippocampal plasticity during the progression of Alzheimer’s disease. Neuroscience 309:51–67
Todd MT, Nystrom LE, Cohen JD (2013) Confounds in multivariate pattern analysis: theory and rule representation case study. Neuro Image 77:157–165
Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M et al (2015) A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115:117–137
Kerchner GA, Deutsch GK, Zeineh M, Dougherty RF, Saranathan M, Rutt BK (2012) Hippocampal CA1 apical neuropil atrophy and memory performance in Alzheimer’s disease. Neuroimage 63(1):194–202
Zhao W, Wang X, Yin C, He M, Li S, Han Y (2019) Trajectories of the hippocampal subfields atrophy in the Alzheimer’s disease: a structural imaging study. Front Neuroinform 13:13
Trujillo-Estrada L, Dávila JC, Sánchez-Mejias E, SanchezVaro R, Gomez-Arboledasa A, Vizueteb M et al (2014) Early neuronal loss and axonal/presynaptic damage is associated with accelerated amyloid-β accumulation in aβpp/ps1 Alzheimer’s disease mice subiculum. J Alzheimers Dis 42(2):521–541
Kilian Hett, Vinh-Thong Ta, José Manjón, Pierrick Coupé (2018) Graph of hippocampal subfields grading for Alzheimer’s disease prediction. Machine Learning in Medical Imaging hal-01859257
Hanseeuw BJ, Van Leemput K, Kavec M, Grandin C, Seron X, Ivanoiu (2011) A Mild cognitive impairment: differential atrophy in the hippocampal subfields. AJNR Am J Neuroradiol 32(9):1658–1661
Mueller SG, Chao LL, Berman B, Weiner MW (2011) Evidence for functional specialization of hippocampal subfields detected by MR subfield volumetry on high resolution images at 4 T. Neuroimage 56(3):851–857
Apostolova LG, Thompson PM, Green AE, Hwang KS, Zoumalan C, Jack CR Jr et al (2010) 3D comparison of low, intermediate, and advanced hippocampal atrophy in MCI. Hum Brain Mapp 31(5):786–797
Costafreda SG, Dinov ID, Tu Z, Shi Y, Liu CY, Kloszewska I et al (2011) Automated hippocampal shape analysis predicts the onset of dementia in mild cognitive impairment. Neuroimage 56:212–219
Lindberg O, Walterfang M, Looi JC, Malykhin N, Ostberg P, Zandbelt B et al (2012) Hippocampal shape analysis in Alzheimer’s disease and frontotemporal lobar degeneration subtypes. J Alzheimers Dis 30(2):355–365
Acknowledgements
Not applicable
Funding
This study had no funding from any resource.
Author information
Authors and Affiliations
Contributions
1. S. S.: Data collection and analysis, reviewing literature, share in statistical analysis, manuscript editing, and preparation of figures and tables. 2. M. A.: Suggest and develop the research idea, reviewing literature, data collection and analysis, perform statistical analysis, write and revise the manuscript. 3.A. A.: Data collection and analysis, reviewing literature, share in statistical analysis, manuscript editing and preparation of figures and tables. 4. M. D.: Data collection and analysis, reviewing literature, share in statistical analysis, manuscript editing, and preparation figures and tables. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the Faculty of Medicine at Tanat University in Egypt in December 2017. All patients included in this study gave written informed consent to participate in this research.
Please note that reference number is not available.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study. If the patient was unconscious when consent for publication was requested, written informed consent for the publication of this data was given by their legal guardian. I confirm that the content of the manuscript has not been published or submitted for publication elsewhere.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saad, S.H.S., Alashwah, M.M.A., Alsafa, A.A. et al. The role of brain structural magnetic resonance imaging in the assessment of hippocampal subfields in Alzheimer’s disease. Egypt J Radiol Nucl Med 51, 53 (2020). https://doi.org/10.1186/s43055-020-00164-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-020-00164-8