Abstract
Background
Urethral duplication is a rare urogenital anomaly with varying anatomical orientations leading to diverse clinical presentations. We present a case of urethral duplication in a neonate featuring a large dorsal cystic mass on the penis, an unusual presentation.
Case presentation
A term male neonate with a prenatally diagnosed 10 × 10 cm genitourinary cystic mass was delivered via caesarean section. Examination revealed a large cystic mass extending dorsally from the pubic symphysis over a flattened, elongated penile shaft with a single urethral opening in the glans and undescended testes. A size 6-French feeding tube inserted into the urethra drained the bladder.
Urethral communication with the cystic mass was confirmed via voiding cystourethrogram and cyst enlargement noted during voiding. Cyst fluid analysis indicated a urinary origin. Computed tomography and ultrasound were not diagnostic. Initial imaging revealed a dorsal cystic mass projecting from the pubic symphysis without bladder connection.
Surgical intervention at 3 weeks revealed a Type IIA-2 urethral duplication, with a dorsal hypoplastic urethra communicating with the posterior urethra. Correction included resection of the dorsal urethra, cyst excision, and reconstruction of the penis with the orthotopic ventral urethra and bilateral orchidopexies.
Satisfactory functional and cosmetic outcomes were observed at 2 and 8 months after surgery.
Conclusion
This case highlights the significance of identifying unique urethral duplication presentations. The novel occurrence of Type IIA-2 urethral duplication terminating in a dorsal cystic mass underscores diagnostic complexity, surgical intricacies, and aesthetic considerations associated with such cases.
Similar content being viewed by others
Background
Urethral duplication is an uncommon urogenital anomaly with variations in its anatomical orientation. It predominantly occurs in the sagittal plane, with the coronal plane presentation being less frequent and can be complete or incomplete [1,2,3]. Several classifications exist, with the Effmann classification system being the most widely used [4]. The dorsal urethra is usually hypoplastic, while the ventral urethra functions normally and includes the verumontanum and sphincter [5].
The spectrum of clinical presentations can vary widely, ranging from subtle urinary symptoms to complex anatomical abnormalities that require intricate surgical interventions.
In this report, we present a unique case of urethral duplication in a male neonate, marked by the presence of a sizable cystic mass on the dorsal aspect of the penis.
Case presentation
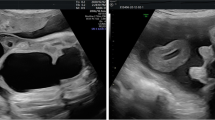
A 3-kg term male neonate was referred on the third day of life with an antenatal diagnosis at 34-week gestation of a sizable genitourinary cystic mass, approximately 10 × 10 cm in size. The prenatal ultrasound indicated that the mass might have originated from the bladder. The rest of the antenatal ultrasound, including amniotic fluid volume, was unremarkable. The neonate was delivered via caesarean section due to the identified foetal anomaly.
Physical examination revealed a large, non-tender cystic mass covered by the skin, located just below the pubic symphysis. The penile shaft displayed elongation and flattening on the ventral aspect of the mass, with a single urethral opening and voiding occurring through a compressed glans. A size 6-French feeding tube was inserted through the visible urethral opening in the glans to catheterize the urethra and drain the bladder (Fig. 1). Both testes were palpable in the inguinal region. No other anomalies were detected, and initial laboratory tests were normal. Ultrasonography confirmed the presence of a cystic mass measuring 12 × 8 × 9 cm, dorsal to the glans and shaft of the penis. Hydronephrosis was absent.
On day 8 of life while waiting for further imaging, the neonate developed signs of sepsis suspected to arise from the cyst as evident from observed erythema on the overlying skin. He was irritable, and laboratory results revealed elevated C-reactive protein and procalcitonin levels, 234 mg/L and 3.26 ug/L, respectively, and a decrease in the white blood count. Odorous straw-coloured fluid was aspirated from the cyst under ultrasound guidance, and a 7-French 20-cm central venous catheter was inserted into the cyst to drain the collection. Fluid analysis confirmed urinary origin and showed elevated urea and creatinine levels (fluid urea and creatinine levels of 27.7 mmol/L and 1109 µmol/L, respectively, in contrast to serum urea levels of 3.2 mmol/L and creatinine levels of 35 µmol/L). Klebsiella pneumonia was cultured from the fluid, leading to intravenous antibiotic treatment with meropenem for 7 days.
A computed tomography (CT) scan was performed due to the patient’s septic state, showing a large cystic mass, 10 × 6 cm in size, attached centrally and anteriorly to the groin. The drain was identified within the mass, and no bladder communication was observed.
A few days later, urethral communication with the cystic mass was clinically evident after displacement of both the transurethral feeding tube and drain, coinciding with cyst enlargement noted during voiding. Once antibiotics were completed, a voiding cystourethrogram (VCUG) was performed, revealing a normal-shaped bladder and a dilated posterior urethra with contrast extravasation from the anterior aspect of the posterior urethra into the cystic mass (Fig. 2).
Lateral view of a voiding cystourethrogram depicting the Type IIA-2 urethral duplication in a sagittal plane (long thin arrow, dorsal hypoplastic urethra arising from posterior urethra; short thick arrow, contrast extravasation from the dorsal urethra into the cystic mass; short thin arrow, ventral urethra)
At the age of 3 weeks, the patient underwent surgical intervention. The functional ventral urethra terminated centrally in the glans, flanked by both corpora cavernosum on either side of the corpora spongiosum. Cystoscopy revealed a long, patent urethra with an unobstructive tissue fold between the prostatic and membranous urethra. During cystoscopy, the cyst is filled with fluid; while no immediate communication was evident, it later became apparent upon cyst opening and catheterization of a small-calibre ‘urethral-like’ opening resembling an underdeveloped glans on the cyst’s posterior wall using a 3-French ureteric catheter (Fig. 3A, B).
Intra-operative confirmation of a Type IIA-2 urethral duplication with reconstruction of the penis. A Open dorsal penile cyst (short, thick arrow, dorsal urethra opening on posterior wall of cyst; long thin arrow, ventral urethra opening). B 3-French ureteric catheter in small calibre dorsal urethra communicating with posterior urethra. C Reconstruction of penile shaft after excision of cyst and excess skin
The dorsal hypoplastic urethra, approximately 4 cm in length, coursed along the cyst’s posterior wall and communicated with the anterior wall of the posterior urethra, indicative of a Type IIA-2 complete urethral duplication. Remnants of underdeveloped corpora cavernosa were evident on either side of the dorsal urethra.
The dorsal hypoplastic urethra was secured with a 6–0 monofilament absorbable suture and divided near its entry point into the posterior urethra. Complete excision of the cyst was performed. The ventral urethral corpora cavernosa were rotated dorsally and sutured using 4–0 polyglactin. Orchidopexies were conducted to address bilateral undescended testes located in the inguinal region. Scrotal skin was reconstructed for cosmesis, and excess dorsal skin was excised (Fig. 3C). As part of the postoperative care strategy, a 6-French feeding tube was inserted as a transurethral catheter into the ventral urethra for wound care. The ventral penis measured approximately 8 cm in length at the conclusion of the procedure.
Perioperative antibiotics consisted of three doses of cefazolin. Empiric meropenem was initiated following a post-surgery temperature spike and continued for 7 days. Multidrug-resistant (MDR) Acinetobacter, sensitive to colistin, was isolated from a peripheral blood culture. Despite the positive culture, no changes were made to the antibiotics as the patient remained clinically well. Post-operative analgesia included intravenous fentanyl and paracetamol for 24 h, followed by oral paracetamol for 6 days. Daily paraffin gauze dressings were applied. After 1 week, the transurethral catheter was removed, and the patient voided well and was discharged home (Fig. 4A).
The histology of the urethral fistula showed stratified squamous epithelium and stromal fibrosis. The cystic lesion was lined by keratinizing squamous epithelium, and stromal fibrosis was noted. At the 2-month follow-up, we observed a satisfactory urinary stream and acceptable cosmetic outcomes, even though there was dorsal skin excess and a floppy penis (Fig. 4B). The guardian of the patient was pleased with the results. Further review after 6 months demonstrated a good urinary stream and cosmetic result with spontaneous erections noted by the guardian.
Discussion
Urethral duplication, a relatively uncommon congenital anomaly of the lower urinary tract, predominantly affects males and can be associated with various genitourinary abnormalities [1,2,3, 5, 6]. Existing literature on this condition primarily comprises retrospective studies with limited patient cohorts, as well as case series and reports.
Urethral duplications are categorized based on position and completeness. The Effmann classification system defines three types [4]. Our patient displayed an unusual variant of Effmann Type IIA-2: a dorsal hypoplastic urethra originating from the posterior urethra and terminating in a cystic mass dorsal to the ventral urethra, instead of the expected second external opening described in the classification. The frequency of Type IIA-2 differs between studies ranging between 17 - 50% [1,2,3, 7]. Guglielmetti et al. and Wani et al. reported it as the most common type in their cohorts of 19 and 20 patients respectively (26% and 50%, respectively) [1, 3].
The age of diagnosis and the clinical presentation of urethral duplication in males can exhibit significant variability [6]. Patients may remain asymptomatic or manifest symptoms such as urinary tract infections, urethral discharge, urinary tract obstructions, incontinence, bifid or double urine streams, or neonatal urinary sepsis [1, 6, 8, 9]. Notably, an antenatal diagnosis of a large cystic mass located dorsal to the penis represents a unique presentation of Type IIA-2 duplication, with no prior cases reported in the literature to our knowledge. This atypical presentation contributed to a slight delay in diagnosis following birth. Only a limited number of case reports in the literature describe Type IIA-2 duplications presenting with cystic masses in infancy, such as a 2 days old with a dorsal preputial cyst and stenosis of the anterior section of the orthotopic ventral urethra [9] and a 3 months old with urethral duplication resembling a perineal mass [10].
The ventral, orthotopic urethra and corpora cavernosa on either side were unusually elongated and flattened. Stretched penile length disparities exist between different populations as outlined in many studies [11,12,13]. However, in our case, extreme stretching of the penis most likely occurred secondary to the progressive enlargement of the dorsal cyst with urine prenatally.
Diagnosis is usually made through a combination of clinical evaluation, imaging such as ultrasonography, VCUG and retrograde urethrography, and cystourethroscopy. Magnetic resonance urography (MRU) or excretory urography is useful if looking for associated genitourinary anomalies [8] and to delineate anatomy prior to surgery [14]. In our patient, due to the atypical presentation and new onset sepsis, a CT scan, which is more accessible than an MRU in our hospital, was performed prior to a VCUG. While the CT scan did not help identify the communication in the posterior urethra, it did confirm the presence of the corpora cavernosa on both sides of the ventral urethra, which were clinically flattened and challenging to assess prior to surgery.
The management of urethral duplication depends on several factors, including the type of duplication, clinical presentation, and associated anomalies. Conservative management may be appropriate for asymptomatic cases [3]. Surgical approaches can range from simple excision of the accessory urethra to more complex reconstructive procedures involving urethral reconstruction that needs to be staged [1]. In most cases of Type IIA-2 duplications, surgery usually involves a complete, simple excision of the duplicated hypoplastic urethra [1, 15] given the normal calibre and orthotopic position of the ventral urethra. A more intricate approach is seldom necessary, as described by Ramareddy et al. in a patient who had an associated congenital ventral urethra stenosis, requiring urinary diversion procedures and urethral dilations [9]. Histological examination of the fistula revealed stratified squamous epithelium consistent with the terminal urethral portion [16].
Surgical reconstruction posed a challenge in our patient due to excessive dorsal skin surrounding the cyst, increased penile length, and laterally displaced corpora cavernosa. While short-term outcomes and guardian satisfaction are mentioned, the report lacks long-term follow-up data, which could offer insights into the durability of the surgical intervention, potential complications, and functional outcomes over time. Further evaluation will need to address cosmetic concerns and erectile function.
Conclusion
Type IIA-2 urethral duplication presenting as a large cystic dorsal penile mass is an exceptionally rare occurrence. This report highlights the clinical evaluation, diagnostic methods, surgical intervention, and postoperative outcomes in managing this challenging presentation.
Availability of data and materials
Not applicable.
Abbreviations
- CT:
-
Computed tomography
- VCUG:
-
Voiding cystourethrogram
- MRU:
-
Magnetic resonance urography
References
Guglielmetti LC, Delcont M, Walker J et al (2020) Urethral duplication-epidemiology, diagnosis, and treatment in a case series of 19 patients. J Pediatr Urol. 16(3):385.e1-385.e9. https://doi.org/10.1016/j.jpurol.2020.02.010. (Epub 2020 Feb 24).
Mane SB, Obaidah A, Dhende NP et al (2009) Urethral duplication in children: our experience of eight cases. J Pediatr Urol 5(5):363–7. https://doi.org/10.1016/j.jpurol.2009.01.006. (Epub 2009 Feb 23).
Wani SA, Munianjana NB, Jadhav V et al (2019) Urethral duplication in children: experience of twenty cases. J Indian Assoc Pediatr Surg 24(4):275–280. https://doi.org/10.4103/jiaps.JIAPS_164_18.
Effmann EL, Lebowitz RL, Colodny AH (1976) Duplication of the urethra. Radiology 119(1):179–85. https://doi.org/10.1148/119.1.179.
Levin TL, Han B, Little BP (2007) Congenital anomalies of the male urethra. Pediatr Radiol 37(9):851–62. https://doi.org/10.1007/s00247-007-0495-0. quiz 945. (Epub 2007 May 22).
Nnabugwu II, Onoh WC, Ukekwe FI et al (2022) Urethral duplication associated with complex chordee: a narrative review of literature and report of a case. Afr J Urol 28:42. https://doi.org/10.1186/s12301-022-00311-9.
Kang SK, Kim J, Lee YS et al (2020) Urethral duplication in male children: a study of 12 cases. J Pediatr Surg 55(10):2216–2220. https://doi.org/10.1016/j.jpedsurg.2019.12.012. (Epub 2020 Jan 29).
Lopes RI, Am G et al (2017) Urethral duplication type influences on the complications rate and number of surgical procedures. Int Braz J Urol 43(6):1144–1151. https://doi.org/10.1590/S1677-5538.IBJU.2016.0269.
Ramareddy RS, Alladi A, Siddappa OS (2012) Urethral duplication: experience of four cases. J Indian Assoc Pediatr Surg. 17(3):111–5. https://doi.org/10.4103/0971-9261.98127.
Feins NR, Cranley WR (1982) Urethral duplication with the dorsal urethra presenting as a perineal mass. J Pediatric Surg 17(6):743–744. https://doi.org/10.1016/S0022-3468(82)80438-7.
Chikani Ugo N, Chinawa Josephat M, Ikefuna Anthony N et al (2015) Stretched penile length of healthy term neonates: normative values among Igbo babies in southeastern Nigeria. J Trop Pediatrics 61(1):69–73. https://doi.org/10.1093/tropej/fmu064.
Wang CH, Line WD (2006) Penile length of normal boy in Taiwan. Acta Paediatr Taiwan 47:293–296.
Vasudevan G, Manivarmane BBV et al (1995) Genital standards for South Indian male newborns. Indian J Pediatr 62(5):593–596. https://doi.org/10.1007/BF02761887.
Bhadury S, Parashari UC, Singh R, Kohli N (2009) MRI in congenital duplication of urethra. Indian J Radiol Imaging 19(3):232–234. https://doi.org/10.4103/0971-3026.54884.
AbouZeid AA, Safoury HS, Mohammad SA et al (2015) The double urethra: revisiting the surgical classification. Ther Adv Urol. 7(2):76–84. https://doi.org/10.1177/1756287214561760.
Stoddard N, Leslie SW (2023) Histology, male urethra. [Updated 2023 May 1]. In: StatPearls. StatPearls Publishing, Treasure Island. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542238/.
Acknowledgements
Not applicable
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
SG wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the guardian for publication of this case report and accompanying images.
Competing interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Govender, S. Urethral duplication with a large cystic dorsal penile mass in a newborn: a case report. Egypt Pediatric Association Gaz 72, 57 (2024). https://doi.org/10.1186/s43054-024-00303-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-024-00303-0