Abstract
Background
Neonatal sepsis is one of the life-threatening diseases. MicroRNAs are non-coding RNAs that play vital roles in various diseases.
Methods
This study included 50 neonates with sepsis and 60 healthy controls. RNA extraction and assessment of mir-182-5p and miR-590-3p using real-time PCR were done.
Results
Significant downregulation of mir-182-5p and miR-590-3p in neonates with sepsis compared with healthy neonates was observed. Positive correlations were confirmed between the expression levels of miR-182-5p and birth weight (R = 0.355, P = 0.012), RDW (R = 0.476, p = < 0.0001), I/T Neutrophil (R = 0.362, P = 0.012), and a negative correlations were demonstrated between miR-182-5p and each of lyomphocyte count (R = − 0.399, P = 0.004), HCO3 (R = − 0.396, P = 0.004), as well as snap score (R = − 0.321, P = 0.023). Moreover, positive correlations were verified between the expression level of miR-590-3p and I/T Neutrophil (R = 0.420, P = 0.003), RDW (R = 0.359, p = 0.010), CRP (R = 0.285, P = 0.45), and negative correlations were established between the expression level of miR-590-3p and platelets (R = − 0.495, P = < 0.0001), lymphocyte count (R = − 0.365, P = 0.009), and snap score (R = − 0.568, P = < 0.0001).
Conclusion
mir-182-5p and miR-590-3p may be used as new biomarkers for neonatal sepsis suggesting that they could be used in the treatment of neonatal sepsis. Also, a significant negative correlation was noted between expression levels of mir-182-5p and miR-590-3p and snap score.
Similar content being viewed by others
Background
Neonatal sepsis is a bloodstream infection. It is classified as early or late onset. Eighty-five percent of neonates with early-onset sepsis show symptoms within 24 h, 5% within 24–48 h, and a minor proportion within 48–72 h after birth [1].
Numerous risk factors contribute to the progression of neonatal sepsis such as low birth weight and premature birth [2, 3].
Neonatal sepsis is caused by infections with bacteria, viruses, or fungi. Group B streptococcus infection (GBS) and Escherichia coli are the most often found microorganisms linked to early-onset newborn sepsis. E coli has supplanted GBS as the pathogen most frequently related to early-onset infection in low birth weight and preterm neonates [2].
Studies have shown that serial normal complete blood count (CBC) can rule out neonatal sepsis [4]. However, it has been demonstrated that neutropenia is a better indicator of newborn sepsis than neutrophilia [5]. Isolation of the microorganism in blood culture from any sterile place is the gold standard for diagnosing newborn sepsis [6]. Also, it has been demonstrated that serial C-reactive protein(CRP) measurement at 24 to 48 h following the beginning of symptoms increases its sensitivity for neonatal sepsis diagnosis [7, 8].
Since microRNAs (a subclass of non-coding RNAs) have the ability to negatively post-transcriptionally regulate gene expression, it is anticipated that they will play a significant role in illness diagnosis and therapy targets in the future [9].
Recent studies showed altered expression of mir-182-5p and miR-590-3p in sepsis [10,11,12], though, their roles in neonatal sepsis have not been studied yet.
The plan of the current research is to investigate the expression levels of mir-182-5p and miR-590-3p in neonates with sepsis and to assess their expression levels with various clinical and laboratory data.
Methods
The existing study included 50 neonates diagnosed with sepsis (20 males and 30 females) recruited from the neonatal intensive care unit, pediatrics Department, Fayoum University Hospital. Diagnosis of neonatal sepsis was performed consistent with the criteria defined at the 2003 Kunming Neonatal Sepsis Definitions Conference which depends on the clinical manifestations of sepsis and detection of blood pathogens [13].
Additionally, 60 healthy control neonates (21 males and 39 females) who were recruited from outpatients of the Pediatrics Department at Fayoum University Hospital were incorporated into this study.
The following neonates were excluded from the study: (1) neonates with intrauterine growth retardation or perinatal asphyxia or chromosomal anomalies. (2) whose mothers were hypertensive, diabetic, or had any autoimmune or inflammatory illness.
All the parents or legal guardians of enrolled neonates gave signed informed consent. Faculty of Medicine, Fayoum University Local Ethics Committee approved the current study (code number M 653), which is in line with the Declaration of Helsinki.
Blood sample processing
Each participant had a sample of blood collected from their veins. In tubes containing separating gel, samples were collected and left to coagulate for 15 min at room temperature. After that, centrifugation at 4000 × g for 10 min was done. Before analysis, serum samples were divided and kept at − 80 °C.
miRNAs extraction and reverse transcription for synthesis of cDNA
To extract total RNA (including microRNAs) from serum samples, MiRNeasy extraction kit (Qiagen, Valencia, CA, USA) was used consistent with the manufacturer's Catalogue. NanoDrop® (ND)-1000 spectrophotometer (NanoDrop Technologies, Inc. Wilmington, USA) was used to evaluate RNA purity and quantitation.
MicroRNA was reverse-transcribed by means of the miRCURY LNA RT Kit (Qiagen, MD, USA) in a final volume of 10 μl following the manufacturer’s instructions.
mir-182-5p and miR-590-3p detection by RT-qPCR
The reagents of miRCURY LNA miRNA PCR Assays (Qiagen, MD, USA) and miRCURYLNASYBR® Green Master Mix (Qiagen, MD, USA) and cDNA synthesis reaction were used to form a PCR reaction mix for a 10-μl per well reaction volume.
The following procedures were programmed into the real-time cycler (PikoReal 24TM Real-Time PCR System; Thermo Scientific, Finland): initial heat activation at 95 °C for 2 min, then 40 cycles of denaturation at 95 °C for 10 s and combined annealing/extension at 56 °C for 60 s. To evaluate the specificity of the amplified products. melting curve analysis was done between 60 °C and 95 °C. The expression values of mir-182-5p and miR-590-3p were normalized using miR-16-5p as an endogenous reference gene [14, 15]. Catalog no. of miR-182-5p was YP00206070 and its Lot number was 201,803,060,144–2, Catalog no. of miR-590-3p was YP00205448 and its Lot number was 201,705,190,377–1, and Catalog no. of miR-16-5p was YP00205702 and its Lot number was 201,910,040,131–3.
The Eq. 2−ΔΔCt was used to estimate the fold change (FC) of mir-182-5p and miR-590-3p [15, 16]. The FC of the healthy group was assumed as 1.
Statistical methods
The data were compiled and statistical analysis was performed with the aid of the Statistical Package for Social Sciences version 18. For quantitative data, the mean and standard deviation (SD) as well as the median and interquartile range (IQR) were performed. To analyze qualitative data, the chi-squared test was employed. The associations between mir-182-5p and miR-590-3p and laboratory and clinical data were evaluated using Spearman’s correlation. Results were significant at P ≤ 0.05.
Results
Demographic and clinical data of neonates with sepsis and control
Table 1 displays the sociodemographic attribute data. No statistically significant differences were noted between the case and control groups regarding chronological age or sex. Clinical data revealed substantial differences between cases and controls regarding gestational age, Apgar score at 1 min, respiratory rate, heart rate, head circumference, and height (P value < 0.005, each). On the other hand, between the case and control groups, there were no statistically significant differences in either birth weight or weight (at the time of withdrawing blood samples) (Table 1).
Laboratory results of sepsis-affected newborns and the control group
The laboratory findings as presented in Table 2. Significantly elevated levels of TLC, red cell distribution width (RDW), lymphocyte count, I/T Neutrophil as well as CRP were observed in cases with neonatal sepsis compared with control subjects (P value < 0.005, each). However, significantly reduced levels of hemoglobin, PH, CO2, and HCO3 were noted in cases compared with controls (P value < 0.005, each).
Regarding platelet count, no significant differences were shown between cases and controls.
Expression level of miR-182-5P and miR-590-3p in neonates with or without sepsis
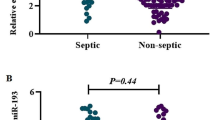
Significant downregulation of miR-182-5P and miR-590-3p serum levels were verified in cases with neonatal sepsis compared to healthy neonates [median (IQR)FC of miR-182-5P in cases was 0.55(0.38–1.97), with a P value of 0.018, while median (IQR)FC of miR-590-3p in cases was 0.44(0.11–1.11) with a P value < 0.0001] (Fig. 1).
Box plot illustration showing miR-182-5p and miR-590-3p serum expression (fold change) in neonatal sepsis in comparison to levels in healthy neonates. A box plot of the data is used to display the median, higher, and lower quartiles. The miR-182-5p and miR-590-3p expression levels in healthy newborns are shown by the horizontal line. The potential of miR-182-5P and miR-590-3p blood levels as predictive biomarkers for newborn sepsis was assessed using a ROC curve
Associations of miR-182-5p and miR-590-3p with clinical data in cases with neonatal sepsis
The relations between miR-182-5P and clinical parameters in cases are represented in Table 3. It was observed that the expression level of miR-182-5P was significantly reduced in preterm neonates compared with full-term one (P value = 0.032). In addition, miR-182-5P was significantly decreased in feverish cases and those with convulsions compared with normal one (P value < 0.001, each). Also, miR-182-5P was significantly reduced in neonates with bleeding than without bleeding (P value = 0.014).
Regarding miR-590-3p, a significant decrease was noted in late-onset than in early-onset sepsis (P value = 0.034). Furthermore, miR-590-3p was observed to be decreased in neonates without premature rupture of membrane (PROM) than those with PROM (P value < 0.001). Moreover, miR-590-3p was significantly reduced in feverish cases, those with convulsions, and those with apnea compared with normal one (P value < 0.001, each).
Regarding the relation with blood culture, miR-182-5P expression level was noted to be significantly downregulated in positive culture compared with negative culture (P value = 0.030). Also, significant downregulation was noted in cases infected with Klebsiella rather than in other species (P value < 0.001).
Besides, miR-590-3P expression level was noted to be markedly downregulated in gram-positive cultures compared with gram-negative cultures (P value = 0.007). Additionally, miR-590-3P was declined in cases those infected with Klebsiella rather than other species (P value = 0.012).
Correlations between expression level of (miR-182-5p) and (miR-590-3p) with clinical and laboratory data in cases with neonatal sepsis
To assess the correlations of the expression levels of (miR-182-5p) and (miR-590-3p) with the clinical and laboratory variables, Spearman’s analysis was performed (Table 4). Positive correlations were established between the expression levels of miR-182-5p and each of birth wight (R = 0.355, P = 0.012), RDW (R = 0.476, p = < 0.0001), I/T Neutrophil (R = 0.362, P = 0.012), and a negative correlations were demonstrated between miR-182-5p and each of lyomphocyte count (R = − 0.399, P = 0.004), HCO3 (R = − 0.396, P = 0.004) as well as snap score (R = − 0.321, P = 0.023). Moreover, positive correlations were verified between the expression level of miR-590-3p and each of I/T Neutrophil (R = 0.420, P = 0.003), RDW (R = 0.359, p = 0.010), CRP (R = 0.285, P = 0.45), and negative correlations were reported between the expression level of miR-590-3p and platelets (R = − 0.495, P = < 0.0001), lymphocyte count (R = − 0.365, P = 0.009), and snap score (R = − 0.568, P = < 0.0001).
Receiver operating characteristic curve (ROC) analysis
The potential of miR-182-5P and miR-590-3p blood levels as predictive biomarkers for newborn sepsis was assessed using a ROC curve (Fig. 2). For miR-182-5P, the area under the curve (AUC) was 0.620 with sensitivity and specificity of 62% and 100%, respectively, and P < 0.031. Moreover, the AUC for miR-590-3p was 0.700 with sensitivity and specificity of 70% and 100% respectively, and P < 0.0001.
Analysis of the receiver operating characteristic curve (ROC). The area under the curve (AUC) for miR-182-5P was 0.620 with 62% and 100% sensitivity and specificity, respectively, and a P value of 0.031. Additionally, the sensitivity and specificity of miR-590-3p were 70% and 100%, respectively, with a P value of 0.0001 for the AUC
Discussion
Neonatal sepsis is the term used to describe a severe form of morbidity and mortality causing systemic illness that has a viral, bacterial, or fungal source, is accompanied by hemodynamic abnormalities, and results in clinical findings. Clinical signs can range from mild localized or systemic disease to subclinical infection [17, 18].
In the current study, we reported significant differences between cases and controls regarding gestational age, Apgar score at 1 min, respiratory rate, heart rate, head circumference, and height (P value < 0.005, each) which is in agreement with Belachew and Tewabe, 2020 who revealed the relationship between neonates with sepsis and each of low birth weight and preterm state explaining that preterm babies have immature immune systems so it could not fight infection [1]. Also, Gutbir et al. (2020) showed that an Apgar score with a low score had a higher risk of infection [19]. Interestingly, Pawar et al. (2018) exhibited that neonatal infection is associated with low weight and poor head growth [20]
MiRNAs, which are short non-coding RNAs, control a variety of biological activities. There is growing evidence that miRNAs are critical for immune regulation in autoimmune and infectious illnesses [21, 22].
Several biomarkers have been investigated to help in sepsis diagnosis and prognosis, although they have limits in severe and early cases [23, 24]. Serum miRNAs have attracted a lot of attention as indicators recently for the diagnosis and prognosis of sepsis. External cells release miRNAs into the serum, which remain stable in the bloodstream. This is because they are simple to detect [25].
Human miR-182-5p, which is transcribed from the cluster of the miR-183 family and is found at the 7q32 region of chromosome 7, has been widely studied in human malignancies. MiR-182-5p has been described as an oncogene in the majority of common forms of human malignancies, but it also has tumor-suppressive properties in human lung, gastric, and posterior uveal melanoma adenocarcinomas [26, 27].
In the current study, we showed that individuals with neonatal sepsis had markedly lower serum expression levels of miR-1825p compared to healthy controls.
The presenting results are in compliance with a prior study that established that miR-182-5p expression level is reduced by hypoxia, a critical pathologic condition in sepsis [28]. Additionally, miR-182-5p overexpression improved cell survival by protecting human retinal microvascular endothelial cells from hypoxia [28].
Along the lines of current results, Gregory et al. (2018) proved that In vitro miR-182 transfection protects against intracellular bacterial infection. Additionally, they demonstrated that miR-182 overexpression in primary human macrophages could protect against pro-inflammatory and autophagic responses to infection [29].
As well, it was noted that the downregulation of miR-182 contributed to the proliferation of renal cell carcinoma via over-expression of its target gene flotillin 1(FLOT1) [30], which was, on the other hand, proved to play an important role in pediatric sepsis [31].
Besides, According to research, lncRNA cardiac hypertrophy-related factor causes miR-182-5p to be negatively regulated, which raises the level of autophagy-related 7 (ATG7) and accelerates autophagy in myocardial I/R injury. It is interesting to note that ATG7 has been demonstrated to control autophagy, a pathogenic process involved in sepsis-induced acute kidney damage [32].
In this study, we discovered that patients with neonatal sepsis had considerably lower serum expression levels of miR-590-3p than healthy newborns. Some research has studied the function of miR-590-3p in inflammatory responses. Also, by targeting lipoprotein lipase, miR-590 reduced the levels of the pro-inflammatory cytokines monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1b (IL-1b) [33].
Also, sepsis reduced the expression of miR-590-3p, which may lessen the injury to cardiomyocytes caused by lipopolysaccharide (LPS) as demonstrated by Liu et al. Furthermore, in LPS-induced cardiomyocytes, miR-590-3p also decreased the expression of Tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6) [11].
Our results are compatible with a prior study that reported that miR-590-3p was reduced in mice treated with (LPS) and mice injected with ad-miR-590-3p showed reduction of inflammatory responses via reduction of renal TRAF6 expression and nucleic nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-p65, suggesting that Inflammatory responses to septic challenges may be negatively regulated by miR-590-3p, which may cause better survival results [12].
Moreover, miR-590-3p was reported to be reduced in systemic lupus erythematosus (SLE) and lupus mice, according to Huang et al. (2022). Additionally, they demonstrated that 590-3p reduced Th17 cells by inhibiting autophagy activity [34]. Consequently, miR-590-3p may represent a new strategy for the management and treatment of SLE and other autoimmune diseases related to Th17 [34].
However, our result disagreed with Li et al. (2021) who proved that miR-590-3p was elevated in mice with sepsis [35].
Conclusion
To sum up, our study proved that there is a considerable decrease in the level of miR-182-5P and miR-590-3P in neonatal cases when compared to controls highlighting their possible role in the treatment of neonatal sepsis through upregulation of their levels.
Also, a significant negative correlation was noted between expression levels of mir-182-5p and miR-590-3p and snap score suggesting their correlation with the disease activity.
Future studies are necessary to be done to explain the relationship between miR-182-5p and miR-590-3p and different bacterial pathogens. Also, their dysregulation in neonatal sepsis needs to be investigated on a large scale. Finding the role of miR-182-5p and miR-590-3p in bacterial pathogens is essential for the deep understanding and therefore effective treatment of this disease.
Availability of data and materials
The data that support the findings of this study are available upon request due to patients’ confidentiality.
References
Belachew A, Tewabe T (2020) Neonatal sepsis and its association with birth weight and gestational age among admitted neonates in Ethiopia: systematic review and meta-analysis. BMC Pediatr 20:1–7
Shane AL, Sánchez PJ, Stoll BJ (2017) Neonatal sepsis The lancet 390(10104):1770–1780
Satar M, Arısoy AE, Çelik İH (2018) Türk Neonatoloji Derneği yenidoğan enfeksiyonları tanı ve tedavi rehberi. Türk Pediatri Arşivi 53(1):88–100
Murphy K, Weiner J (2012) Use of leukocyte counts in evaluation of early-onset neonatal sepsis. Pediatr Infect Dis J 31(1):16–19
Philip AG, Hewitt JR (1980) Early diagnosis of neonatal sepsis. Pediatrics 65(5):1036–1041
Brilli RJ, Goldstein B (2005) Pediatric sepsis definitions: past, present, and future. Pediatr Crit Care Med 6(3):S6-8
Hofer N, Zacharias E, Müller W, Resch B (2012) An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology 102(1):25–36
Pourcyrous M, Bada HS, Korones SB, Baselski V, Wong SP (1993) Significance of serial C-reactive protein responses in neonatal infection and other disorders. Pediatrics 92(3):431–435
Ardekani AM, Naeini MM (2010) The role of microRNAs in human diseases. Avicenna J Med Biotechnol 2(4):161
Zhang X, Dong S (2021) Circ_0091702 relieves lipopolysaccharide (LPS)-induced cell injury by regulating the miR-182/PDE7A axis in sepsis. Biosci Biotechnol Biochem 85(9):1962–1970
Liu L, Liu F, Sun Z, Peng Z, You T, Yu Z (2020) LncRNA NEAT1 promotes apoptosis and inflammation in LPS-induced sepsis models by targeting miR-590-3p. Exp Ther Med 20(4):3290–3300
Ma J, Li YT, Zhang SX, Fu SZ, Ye XZ (2019) MiR-590-3p attenuates acute kidney injury by inhibiting tumor necrosis factor receptor-associated factor 6 in septic mice. Inflammation 42:637–649
Chiesa C, Panero A, Osborn JF, Simonetti AF, Pacifico L (2004) Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin Chem 50(2):279–287
Solayman MH, Langaee T, Patel A, El-Wakeel L, El-Hamamsy M, Badary O, Johnson JA (2016) Identification of suitable endogenous normalizers for qRT-PCR analysis of plasma microRNA expression in essential hypertension. Mol Biotechnol 58:179–187
Abdelaleem OO, Mohammed SR, El Sayed HS, Hussein SK, Ali DY, Abdelwahed MY, Gaber SN, Hemeda NF, El-Hmid RG (2022) Serum miR-34a-5p and miR-199a-3p as new biomarkers of neonatal sepsis. PLoS ONE 17(1):e0262339
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–8
Kimberlin DW, Whitley RJ, Wan W, Powell DA, Storch G, Ahmed A, Palmer A, Sánchez PJ, Jacobs RF, Bradley JS, Robinson JL (2011) Oral acyclovir suppression and neurodevelopment after neonatal herpes. N Engl J Med 365(14):1284–1292
Dong Y, Speer CP (2015) Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed 100(3):F257–F263
Gutbir Y, Wainstock T, Sheiner E, Segal I, Sergienko R, Landau D, Walfisch A (2020) Low Apgar score in term newborns and long-term infectious morbidity: a population-based cohort study with up to 18 years of follow-up. Eur J Pediatr 179(6):959–971. https://doi.org/10.1007/s00431-020-03593-9. (Epub 2020 Feb 3 PMID: 32016603)
Pawar SJ, Oleti T, Bharathi S, Tipparaju S, Mustafa E (2018) Growth and neurodevelopmental outcome in preterm LBW infants with sepsis in india: a prospective cohort. Int J Pediatr 21(2018):5735632. https://doi.org/10.1155/2018/5735632.PMID:29681952;PMCID:PMC5841091
Desvignes L, Wolf AJ, Ernst JD (2012) Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J Immunol 188(12):6205–6215
Khan AA, Penny LA, Yuzefpolskiy Y, Sarkar S, Kalia V (2013) MicroRNA-17∼ 92 regulates effector and memory CD8 T-cell fates by modulating proliferation in response to infections. Blood 121(22):4473–4483
Fatmi A, Rebiahi SA, Chabni N, Zerrouki H, Azzaoui H, Elhabiri Y, Benmansour S, Ibáñez-Cabellos JS, Smahi MC, Aribi M, García-Giménez JL (2020) miRNA-23b as a biomarker of culture-positive neonatal sepsis. Mol Med 26:1–9
Wang D, Han L (2021) Downregulation of miR-1184 serves as a diagnostic biomarker in neonatal sepsis and regulates LPS-induced inflammatory response by inhibiting IL-16 in monocytes. Exp Ther Med 21(4):1
Shen X, Zhang J, Huang Y, Tong J, Zhang L, Zhang Z, Yu W, Qiu Y (2020) Accuracy of circulating microRNAs in diagnosis of sepsis: a systematic review and meta-analysis. J Intensive Care 8(1):1
Yan D, Dong XD, Chen X, Yao S, Wang L, Wang J, Wang C, Hu DN, Qu J, Tu L (2012) Role of microRNA-182 in posterior uveal melanoma: regulation of tumor development through MITF, BCL2 and cyclin D2. PLoS One 7(7):e40967
Kong WQ, Bai R, Liu T, Cai CL, Liu M, Li X, Tang H (2012) MicroRNA-182 targets cAMP-responsive element-binding protein 1 and suppresses cell growth in human gastric adenocarcinoma. FEBS J 279(7):1252–1260
Li C, Lie H, Sun W (2022) Inhibitory effect of miR-182-5p on retinal neovascularization by targeting angiogenin and BDNF. Mol Med Rep 25(2):1–9
Gregory DJ, Kramnik I, Kobzik L (2018) Protection of macrophages from intracellular pathogens by miR-182-5p mimic—a gene expression meta-analysis approach. FEBS J 285(2):244–260
Xu X, Wu J, Li S, Hu Z, Xu X, Zhu Y, Liang Z, Wang X, Lin Y, Mao Y, Chen H (2014) Downregulation of microRNA-182-5p contributes to renal cell carcinoma proliferation via activating the AKT/FOXO3a signaling pathway. Mol Cancer 13(1):1–1
Zhang X, Cui Y, Ding X, Liu S, Han B, Duan X, Zhang H, Sun T (2021) Analysis of mRNA-lncRNA and mRNA-lncRNA-pathway co-expression networks based on WGCNA in developing pediatric sepsis. Bioengineered 12(1):1457–1470
Mo Y, Wu H, Zheng X, Xu L, Liu L, Liu Z (2021) LncRNA CHRF aggravates myocardial ischemia/reperfusion injury by enhancing autophagy via modulation of the miR-182-5p/ATG7 pathway. J Biochem Mol Toxicol 35(4):e22709
He PP, Ouyang XP, Tang YY, Liao L, Wang ZB, Lv YC, Tian GP, Zhao GJ, Huang L, Yao F, Xie W (2014) MicroRNA-590 attenuates lipid accumulation and pro-inflammatory cytokine secretion by targeting lipoprotein lipase gene in human THP-1 macrophages. Biochimie 106:81–90
Huang J, Xu X, Wang X, Yang J, Xue M, Yang Y, Zhang R, Yang X, Yang J (2022) MicroRNA-590-3p inhibits T helper 17 cells and ameliorates inflammation in lupus mice. Immunology 165(2):260–273
Li Y, Huang Y, Lin Z, Huang X (2021) microRNA-590–3p regulates inflammation and organ dysfunction in sepsis mouse model via the Syap1-mediated TGF-β/Smad signaling pathway
Acknowledgements
We thank all the medical and paramedical staff who helped in the achievement of this work.
Funding
This research did not obtain any grant from funding agencies.
Author information
Authors and Affiliations
Contributions
S.M.H., O.O.A., and Y.A. performed the biochemical assay. R.G.A. performed the patient examination. Y.A., R.G.A, and O.O.A. were the major contributors to interpreting the data and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the parents or legal guardians of enrolled neonates gave signed informed consent. Faculty of Medicine, Fayoum University Local Ethics Committee approved the current study (code number M 653), which is in line with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamdy, S.M., Othman, Y.A., Abdelaleem, O.O. et al. Expression of serum microRNAs, mir-182-5p, and miR-590-3p and its clinical significance in neonatal sepsis. Egypt Pediatric Association Gaz 72, 17 (2024). https://doi.org/10.1186/s43054-024-00260-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-024-00260-8