Abstract
Objective
Early identification of sickle renovascular changes via renal Doppler sonography among sickle cell disease patients to help in early diagnosis and interventions to prevent progression to end-stage renal disease.
Methods
Forty-five SCD children were included along with 45 healthy control children. Renal Doppler sonography (PI and RI) was performed on all subjects.
Laboratory investigations were done: Hb electrophoresis, complete blood picture with blood indices, reticulocyte count, liver enzymes (ALT and AST), HCV serology, serum ferritin, and lactate dehydrogenase (LDH). Urine analysis and albumin/creatinine ratio in urine were done for all patients as well.
Results
The study group consisted of 45 SCD patients, 27 (60%) males with a mean age of 12 years (± 3 years). By performing renal Doppler sonography, it was found that all study groups had significantly higher Doppler indices (resistivity index and pulsatility index) compared to the control group.
Results of renal Doppler sonography revealed that the main renal pulsatility index was positively correlated with the main renal resistance index (r = 0.454, p = 0.002).
Conclusion
Doppler indices (resistance index and pulsatility index) were of value to assess reno-vascular changes in SCD, Thus, renal Doppler indices could be an early technique in the assessment of sickle renovascular changes, so treatment can be started at an early stage before progressive affection of renal function.
Similar content being viewed by others
Background
Sickle cell disease is a multisystemic autosomal recessive disorder, characterized by chronic hemolytic anemia, painful attacks of vaso-occlusive crises, and then organ damage [1].
Sickle nephropathy (SN) is known as a group of renal complications (including hematuria, hyposthenuria, renal papillary necrosis, proteinuria, renal tubular disorders, acute and chronic kidney injury, sickle cell glomerulopathy, and renal medullary carcinoma) among sickle cell disease patients increasing disease burden. Renal affection is more common in sickle cell disease patients than in trait or combined hemoglobinopathies (HbS/β, HbS/β + , Hb/SC) [1].
Patients with SCD must do regular yearly assessments and screening for sickle cell nephropathy. Prolonged hyperfiltration results in renal injury and proteinuria thus glomerulosclerosis and kidney damage [2].
Early detection of sickle nephropathy by renal vascular Doppler could enable earlier detection and intervention, thus slowing down disease progression to end-stage kidney disease [3].
Methods
This study was conducted on 45 patients (60% males and 40% females) with SCD (sickle SS or sickle β-thalassemia) following up at the hematology outpatient clinic, diagnosis of SCD based on Hb electrophoresis or HPLC. The patients presented to New Children’s Hospital, Faculty of Medicine, Cairo University, during the period of the study (conducted from January 2020 to August 2021). Patients’ ages ranged from 6 to 18 years with no sex preference and with normal urea and creatinine values. Forty-five age- and sex-matched subjects were enrolled as a control group. Informed consent was obtained from the patients or their guardians before enrollment in the study. All SCD patients were in a steady state of the disease at the time of recruitment. The study protocol was approved by the institutional research ethics committees at the Faculty of Medicine, Cairo University (MD-40–2020).
Recruitment of patients
SCD patients were recruited from the pediatric hematology SCD clinic of New Children’s Hospital, Cairo University. All the patients who consented to participate in the study were scheduled for renal Doppler sonography scans in the radiology department based on convenience. Renal Doppler sonographic findings, demographic data, anthropometric measurements, and their records/files were revised for evidence of laboratory investigations such as Hb electrophoresis, complete blood picture with blood indices, reticulocyte count, liver enzymes (ALT and AST), HCV serology, serum ferritin, and lactate dehydrogenase (LDH).
Estimated glomerular filtration rate (eGFR) measurement
We used the CKD-EPI equation for the measurement of GFR (age-related creatinine irrespective of the height of the patients) [4].
eGFR (estimated glomerular filtration rate) = mL/min/1.73 m.2
Scr (serum creatinine) = mg/dL.
K = 0.7 (females) or 0.9 (males).
α = − 0.241 (females) or − 0.302 (males).
min = indicates the minimum of Scr/K or 1.
max = indicates the maximum of Scr/K or 1.
Renal dysfunction in this study was defined as the presence of either high or low eGFR
-
Hyperfiltration (high eGFR) was defined as an eGFR of more than 140 ml/min/1.73 m2 and low GFR (chronic renal failure) was as an eGFR of less than 80 ml/min/1.73 m2 [5].
Laboratory examination: urine analysis and A/C ratio
Urine samples were collected aseptically midstream samples, voided directly into a sterile container. Centrifuge to remove particulate matter, assay immediately or aliquot, and store at ≤ − 20℃. Repeated freeze–thaw cycles were avoided.
-
The presence of microalbuminuria was considered when albumin excretion was in the range of 30–300 mg/dl and or gross albuminuria when it was more than 300 mg/dl [6].
Renal Doppler sonography (equipment and scanning technique)
We assessed renal Doppler indices (resistance index and pulsatility index) among patients with SCD, and these indices were valuable in assessing reno-vascular changes in SCD.
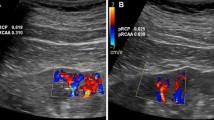
Doppler sonography was performed on an aplio400 Toshiba scanner with a color flow Doppler facility, using a 5-MHz convex probe. The patients were examined in the supine position. Doppler indices assessed were PI, RI, systolic/diastolic ratio (S/D), peak systolic velocity, and end-diastolic velocity for all the patients (Fig. 1).
Doppler indices
The resistance index (RI) is the ratio between the difference of systolic and diastolic velocities with systolic velocity.
-
RI: PSV-DV/PSV
-
PSV: peak systolic velocity.
-
DV: diastolic velocity.
-
PSV: peak systolic velocity.
Normal RI: 0.61 (± 0.05) [7].
The pulsatility index (PI) is the ratio between the difference of systolic and diastolic velocities with average velocity.
-
PI: PSV-DV/AV
-
PSV: peak systolic velocity
-
DV: diastolic velocity
-
AV: average velocity
Normal PI: 1.03 (± 0.18) [7] (Fig. 2).
Statistical analysis
Data was coded and entered using the statistical package SPSS version 20. Data was summarized using mean and standard deviation for quantitative variables and frequencies (number of cases) and relative frequencies (percentages) for categorical variables. Comparisons between groups were done using an unpaired t test in normally distributed quantitative variables while the non-parametric Mann–Whitney test was used for non-normally distributed quantitative variables. Pearson and Spearman’s correlation (r) were used to correlate different parameters. The results were considered statistically significant and highly statistically significant when the significant probability (P value) was < 0.05 and < 0.001, respectively. The diagnostic value of Doppler indices (RI, PI) in predicting possible renal insult was examined using receiver-operating characteristic (ROC) curve analysis.
Results
The study group consisted of 27 (60%) males and 18 (40%) females with an M/F ratio of 1.5. Thirty-eight patients (84.4%) had a history of positive consanguinity, 8 patients (17.8%) had an affected relative in the family, and 25 patients (55.5%) had an affected sibling. The mean age of the enrolled study group was 12 ± 3 years old. The mean age of diagnosis of the disease among the enrolled cases was 1.39 ± 1.04 years. All clinical data of the studied SCD patients are illustrated in Table 1.
Almost all cases (97.8%) were on hydroxyurea (with a dose ranging from 15 to 37 mg/kg/day), and 37.8% were receiving iron chelator (Deferasirox).
Laboratory findings of our patients revealed that the mean HB value was 9 ± 1.42 g/dl ranging from 7 to 12.3 g/dl and patients’ baseline HB S % was 67.06% ± 14.28, mean BUN was 10.39 ± 4.29 mg/dl, the mean creatinine was 0.48 ± 0.13 mg/dl ranging from 0.27 to 0.9 mg/dl, the mean total serum bilirubin was 1.99 ± 1.09 mg/dl, the mean direct serum bilirubin was 0.34 ± 0.19 mg/dl, the mean serum albumin was 4.21 ± 0.64 g/dl, and the mean serum ferritin level was 1107.76 ± 778.97 ng/ml while mean LDH was 659.04 ± 1610.47 U/L.
The mean GFR (CKD-EPI) was 165.87 ± 26.18 ml/min/1.73m2. Thirteen percent of our patients reached more than 200 ml/min/1.73m2 and 1 patient reached up to 245 ml/min/1.73m2 denoting hyperfiltration (Illustrated in Supplementary figure 1).
Urine analysis and urinary A/C ratio done to our patients revealed that the mean urine specific gravity among the cases was 1018.13 ± 4.73, and the mean A/C ratio was 28.27 ± 26.14 mg/g.
The results of renal Doppler sonography done on our studied cases revealed that the mean renal artery PI among the cases was 1.48 ± 0.48, and the mean renal artery RI among the cases was 0.69 ± 0.06. The mean interlobar artery PI among the cases was 1.29 ± 0.58, and the mean interlobar artery RI was 0.63 ± 0.06 (Table 2).
The main renal pulsatility index was positively correlated with the main renal resistance index and vice versa (r = 0.454, p = 0.002). Otherwise, renal Doppler indices did not show a statistically significant correlation with the other studied variables (Supplementary Tables 1 and 2).
Upon reviewing the patients that had both vascular insult (affected RI and PI) and tubular affection (microalbuminuria), they were 15 patients, aged above 8 years with monthly severe vaso-occlusive crises and transfusion-dependant every 1–2 months and 4 of these patients had another complication (CNS vasculopathy with high transcranial Doppler).
Cases and controls were compared regarding renal Doppler sonographic indices, and it was found that there was a statistically significant difference (with p value < 0.001) between the studied groups regarding the main renal artery PI (mean 1.48 ± 0.48), main renal artery RI (mean 0.69 ± 0.06), interlobar artery PI (mean 1.29 ± 0.58), and interlobar artery RI (mean 0.63 ± 0.06) as the patients had a higher mean value of all indices compared to the control group; control group main renal artery PI (mean 0.99 ± 0.12), main renal artery RI (mean 0.60 ± 0.03), interlobar artery PI (mean 0.99 ± 0.11), and interlobar artery RI (mean 0.59 ± 0.04) (Supplementary figures 2, 3, 4, and 5).
According to ROC curve analysis results, the main renal pulsatility index may be used as a reliable tool in predicting renovascular changes in sickle cell disease, showing that at the cutoff (1.45), the sensitivity was 95.6%, the specificity was 73.3%, the predictive value for positive was 79.2%, and the predictive value for negative was 94.3% (Supplementary figure 6).
According to ROC curve analysis results, the main renal resistance index may be used as a reliable tool in predicting renovascular changes in sickle cell disease showing that at the cutoff (0.615), the sensitivity was 88.9%, the specificity was 66.7%, the predictive value for positive was 72.7%, and the predictive value for negative was 85.7% (Supplementary figure 7).
Discussion
Sickle cell nephropathy is a major complication of sickle cell disease. It presents as glomerulopathy, proteinuria, hematuria, tubular defects, and eventually end-stage renal disease (ESRD) [3]. Sickle nephropathy is still a challenging complication among sickle cell disease patients that needs early diagnosis and early intervention before disease progression [3].
Patients with SCD with albuminuria are liable to develop hypertension [8], asymptomatic bacteriuria [9], acute chest syndrome [10], stroke, pulmonary hypertension, ESRD, and death. Unfortunately, SCN cannot be detected at subclinical stages and GFR only decreases at late stages [11].
This study was a case–control study conducted on 45 sickle cell disease patients, 23 patients with Hb SS and 22 patients with sickle beta thalassemia, compared to 45 normal children as a control group. When we analyzed renal function tests (BUN, creatinine) among SCD enrolled patients, it was found that mean creatinine among cases was 0.48 ± 0.13 mg/dl, denoting hyperfiltration this result matched with Thompson et al. [12] found that serum creatinine was lower in patients with sickle cell disease but still in normal ranges. The low serum creatinine may be due to tubular dysfunction in SCD patients and increased tubular secretion of creatinine [13, 14].
Our study showed higher GFR among SCD patients, and this denotes hyperfiltration (mean GFR 165.87 ± 26.18). On the contrary, another previous study [15] revealed that 11.6% of enrolled patients had eGFR < 90 mL/min/1.73 m2. Also, another study including 112 pediatric SCD patients [16] found that the mean eGFR was 249 ± 56 mL/min/1.73 m2, thus also denoting hyperfiltration.
The main pathophysiology in SCD is vaso-occlusion by sickled erythrocytes, so Doppler assessment of renal arteries among SCD patients is important. Doppler indices (RI, PI) enable the evaluation of renal vascular resistance at different sites and help in the detection of severity and site of vascular narrowing.
In our study, we assessed renal vascular affection among SCD patients, and it was found that renal Doppler indices (RI, PI) among SCD patients were significantly higher than the control group (p value < 0.001 and < 0.001, respectively), denoting renal vascular affection among SCD patients. This goes in agreement with a previous study done in 2017 [17] on the effect of sickle cell disease on arterial renal flow using Doppler sonography indices, which showed that RI and PI were increased in SCD cases more than controls, and this study was performed on 115 Sudanese SCD patients versus 100 normal control group. Similar findings were found in another study [18] where both PI and RI were significantly higher in 42 SCD patients compared with the control group, but it also showed that arterial renal Doppler indices were significantly higher in patients with homozygous SCD other than in patients with sickle β-thalassemia but this was not found in our study, there was no significant difference between patients with Hb SS and patients with sickle beta-thalassemia [18].
Thus, renal vascular Doppler indices (PI and RI) and other non-invasive laboratory parameters such as micro-albumin to creatinine ratio can be used as screening methods for early assessment of renal vascular changes among children with SCD to detect sickle nephropathy at a preclinical stage before disease progression.
Conclusion
The study showed that renal Doppler indices (assessing vascular injury) could be an early technique in the assessment of sickle reno-vascular changes so treatment can be started at an early stage before progressive affection of renal function.
Availability of data and materials
All data used during the current study are available from the corresponding author upon reasonable request.
References
Aeddula NR, Bardhan M, Baradhi KM. Sickle cell nephropathy. (Updated 17 Sep 2022).
McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM (2007) Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 29(3):140–144
Hariri E, Mansour A, El Alam A, Daaboul Y, Korjian S, Aoun BS (2018) Sickle cell nephropathy: an update on pathophysiology, diagnosis, and treatment. Int Urol Nephrol 50(6):1075–1083
Inker LA, Schmid CH, Tighiouart H et al (2012) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367(1):20–29
Eckardt KU, Bansal N, Coresh J, Evans M, Grams ME, Herzog CA, James MT, Heerspink HJ, Pollock CA, Stevens PE, Tamura MK (2018) Improving the prognosis of patients with severely decreased glomerular filtration rate (CKD G4+): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 93(6):1281–1292
Niss O, Lane A, Asnani MR, Yee ME, Raj A, Creary S, Fitzhugh C, Bodas P, Saraf SL, Sarnaik S, Devarajan P (2020) Progression of albuminuria in patients with sickle cell anemia: a multicenter, longitudinal study. Blood Adv 4(7):1501–1511
Tublin ME, Bude RO, Platt JF (2003) The resistive index in renal Doppler sonography: where do we stand? Am J Roentgenol 180(4):885–892
Bolarinwa RA, Akinlade KS, Kuti MA, Olawale OO, Akinola NO (2012) Renal disease in adult Nigerians with sickle cell anemia: a report of the prevalence, clinical features and risk factors. Saudi J Kidney Dis Transpl 23(1):171
Iwalokun BA, Iwalokun SO, Hodonu SO, Aina OA, Agomo PU (2012) Evaluation of microalbuminuria in relation to asymptomatic bacteruria in Nigerian patients with sickle cell anemia. Saudi J Kidney Dis Transpl 23(6):1320
Alvarez O, Montane B, Lopez G, Wilkinson J, Miller T (2006) Early blood transfusions protect against microalbuminuria in children with sickle cell disease. Pediatr Blood Cancer 47(1):71–76
Belisario AR, da Silva AA, Silva CV et al (2019) Sickle cell disease nephropathy: an update on risk factors and potential biomarkers in pediatric patients. Biomark Med 13(11):965–985
Thompson J, Reid M, Hambleton I, Serjeant GR (2007) Albuminuria and renal function in homozygous sickle cell disease: observations from a cohort study. Arch Intern Med 167(7):701–708
Asnani MR, Lynch ON, Reid ME (2013) Determining glomerular filtration rate in homozygous sickle cell disease: utility of serum creatinine-based estimating equations. PLoS ONE 8(7):e69922
Lima CS, Bottini PV, Garlipp CR, Santos AO, Costa FF, Saad ST (2002) Accuracy of the urinary albumin to creatinine ratio as a predictor of albuminuria in adults with sickle cell disease. J Clin Pathol 55(12):973–975
Yee MM, Jabbar SF, Osunkwo I et al (2011) Chronic kidney disease and albuminuria in children with sickle cell disease. Clin J Am Soc Nephrol 6(11):2628–2633
Brewin J, Tewari S, Hannemann A et al (2017) Early markers of sickle nephropathy in children with sickle cell anemia are associated with red cell cation transport activity
Eltahir MA, Gar-elnabi ME, Omer MA, Abdelgadir O, Abdallah EA (2017) Impact of sickle cell disease in renal arteries blood flow indices using ultrasonography. J Med Imaging 5:9
Saif A, Soliman N, Abdelhamid A (2015) Doppler assessment of renal hemodynamic alterations in homozygous sickle cell disease and sickle Beta-thalassemia. Ultrasonic Imaging 37(3):258–64
Acknowledgements
We would like to acknowledge our patients and their parents who were involved in the study.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors listed have contributed sufficiently to the project. M.E. generated the idea. H.S. performed renal Doppler for the patients. D.E. collected the clinical data and analyzed them and drafted the manuscript. M.E., N.M., Y.S., and M.A. conceived and designed the evaluation and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki for studies including human participants and was approved by the institutional research ethics committees at the Faculty of Medicine, Cairo University (approval code: MD-40–2020). Written informed consent was obtained from the parents. Participant data has been anonymized.
Consent for publication
Written informed consent was obtained from the parents. Participant data have been anonymized.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Comparison of the renal Doppler parameters of the studied SCD patients versus the control normal group (cases versus control group). Table S2. Correlations of renal Doppler findings and different parameters among SCD patients. Figure S1. Estimated GFR among studied 45 SCD patients via CKD-EPI. Figure S2. Showing main renal artery pulsatility index among the 45 SCD patients versus control group. Figure S3. Showing main renal artery resistance index among the 45 SCD patients versus control group. Supplementary Figure 4. Showing intrarenal vessel pulsatility index among the 45 SCD patients versus control group. Supplementary Figure 5. Showing intrarenal vessel resistance index among the 45 SCD patients versus control group. Supplementary Figure 6. Roc curve illustrating validity of main renal artery pulsatility index as a predictor for sickle cell nephropathy. Supplementary Figure 7. ROC curve illustrating validity of main renal artery resistance index as a predictor for sickle cell nephropathy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eltagui, M.H., Seif Eldein, H.M., Elhady, M.A. et al. Renal Doppler sonography as a non-invasive technique for early detection of reno-vascular changes in sickle cell disease in children. Egypt Pediatric Association Gaz 71, 81 (2023). https://doi.org/10.1186/s43054-023-00228-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-023-00228-0