Abstract
Background
Growing evidence implicates a pro-thrombotic state, caused by ADAMTS13 deficiency, in sepsis-associated organ dysfunction, but pediatric data is limited. Our purpose was to evaluate association of ADAMTS13 with prognosis of pediatric sepsis.
Results
This was prospective observational study, conducted on 70 children with sepsis and 18 healthy controls. Patients were classified upon Pediatric Intensive Care Unit (PICU) admission into sepsis, severe sepsis, and septic shock groups. Serum ADAMTS13 was measured within 24 h of admission. The primary outcome was all-cause PICU mortality. ADAMTS13 was lower among patients than controls [median and interquartile range (IQR): 1.30 (0.88–3.13ng/mL) vs. 6.00 (5.55–6.50 ng/mL); p < 0.001]. ADAMTS13 was lower in both severe sepsis and septic shock than sepsis [median (IQR): 0.90 (0.80–1.75 ng/mL); 1.0 ng/ml (0.90–1.20); and 2.80 (1.00–3.85ng/mL), p = 0.026 and 0.006 respectively]. ADAMTS13 was lower among non-survivors compared with survivors [median (IQR): 0.9 (0.80–1.18 ng/mL) vs. 2.45 (0.98–3.50 ng/mL); p < 0.001]. ADAMTS13 had area under Receiver Operating Characteristic Curve (AUC) of 0.77 for mortality prediction. Lower ADAMTS13 level was associated with mechanical ventilation; vasoactive medications; acute respiratory distress syndrome; and multiple organ dysfunction syndrome. ADAMTS13 correlated with pediatric Sequential Organ Failure Assessment (pSOFA) score (rs = -0.46, p < 0.001); vasoactive infusion days ((rs = -0.48, p < 0.001); and vasoactive-inotropic score on day1 (rs = -0.43, p < 0.001) and day2 ((rs = -0.41; p < 0.001).
Conclusion
In pediatric sepsis, lower ADAMTS13 level is a risk factor for organ dysfunction and mortality, lending theoretical foundations to therapeutic interventions aiming at reversing the pro-thrombotic state in sepsis.
Similar content being viewed by others
Background
Pediatric sepsis is a major health problem with significant morbidity and mortality even in developed countries, where invasive infections, sepsis, and septic shock account for 26.4% of pediatric deaths in intensive care units [1].
Sepsis is commonly complicated by organ dysfunction that may result from coagulation abnormalities. These range from subtle activation of coagulation that can be detected only by molecular assays, to more severe activation of coagulation, with thrombocytopenia and slight elongation of global clotting assay times. In its most extreme form, overt disseminated intravascular coagulation (DIC) with widespread thrombosis and hemorrhage take place [2].
von Willebrand factor (VWF) plays a major role in this process. VWF is a large multimeric glycoprotein that mediates platelet adhesion and aggregation at sites of vascular injury. Upon stimulation, endothelial cells and megakaryocytes release VWF, which is rich in ultralarge (UL) multimers that are hyperactive, and capable of forming very strong bonds with platelet glycoprotein receptor complex. The UL multimers are normally cleaved on the endothelial surface by a VWF-cleaving protease into smaller and less active forms, preventing their entry into the circulation and, avoiding development of thrombotic microangiopathy [3].
This VWF-cleaving enzyme is designated a disintegrin-like and metalloprotease with thrombospondin type 1 motif, 13 (ADAMTS13). It is released principally by hepatic stellate cells and endothelial cells [4, 5].
ADAMTS13 gene mutations or autoantibodies to ADAMTS13 lead to rise of plasma UL-VWF multimers levels, with extensive deposition of VWF and platelet-rich thrombi that cause tissue ischemia and organ dysfunction. This classic presentation is termed thrombotic thrombocytopenic purpura (TTP) that is characterized by thrombocytopenia, microangiopathic hemolytic anemia, renal dysfunction, and neurological abnormalities [6].
Furthermore, ADAMTS13 has been implicated in other clinical conditions, including portal vein thrombosis [7], myocardial infarction [8], and stroke [9].
In sepsis, secondary deficiency of ADAMTTS-13, with thrombotic microangiopathy, has been reported as well [10,11,12]. The mechanisms underlying ADAMTS13 deficiency in sepsis include first massive release of ULVWF multimers during inflammation leads to ADAMTS13 consumption. Second, cleavage of ADAMTS13 by thrombin, plasmin, and neutrophil elastase. Third, inhibition of ADAMTS13 synthesis and activity by pro-inflammatory cytokines like interleukin-6, and interferon-γ. Forth, competitive inhibition of ADAMTS13 binding to VWF by thrombospondin-1. Fifth, decreased ADAMTS13 production by stellate cells due to liver injury in sepsis [2, 13, 14].
Nevertheless, previous pediatric studies were few, small, and inconclusive in terms of ADAMTS13 association with prognosis. We, therefore, conducted the present study for further elucidation of the role ADAMTS13 plays in pediatric sepsis.
Methods
Study design and ethical approval
This was a prospective observational study, conducted from August 2019 to April 2021 in a 10-bed Pediatric Intensive Care Unit (PICU) belonging to Menoufia University Hospital, Menoufia, Egypt. Menoufia University Faculty of Medicine Research Ethics Committee approved the study protocol, and informed consents were obtained from parents.
Patients and data collection
Patients aged 1 month–16 years with sepsis were eligible for enrollment in the study. Another group of age and sex-matched healthy children served as controls. The sample size was calculated based on a previous pediatric study that similarly evaluated ADAMTS13 in sepsis, severe sepsis, septic shock, and healthy controls [11]. We utilized a sample size calculation spreadsheet, assuming a power of 80%, an alpha error of 5%, and 95% confidence interval. Exclusion criteria included failure to obtain blood sample for ADAMTS13 measurement within 24 h of PICU admission; preexistent bleeding or thrombotic disorders; anti-platelet or anticoagulant therapy; and chronic liver disease.
On admission, “sepsis” diagnosis was based on the international pediatric sepsis consensus conference guidelines [15], which require two systemic inflammatory response syndrome (SIRS) criteria, along with proven or suspected infection. According to these guidelines, "severe sepsis" is defined as sepsis associated with acute respiratory distress syndrome (ARDS), cardiovascular system dysfunction, or two other organ system dysfunctions. "Septic shock" is defined as sepsis plus cardiovascular organ dysfunction. Accordingly, septic shock is subtype of severe sepsis. However, for the purpose of the current study, we considered septic shock a separate entity.
Pediatric Index of Mortality-2 (PIM2) was calculated within one hour of PICU admission to predict mortality [16]. The pediatric Sequential Organ Failure Assessment (pSOFA) score was used for quantifying organ dysfunction at the end of the first 24 h [17]. Multiple organ dysfunction syndrome (MODS) was defined as simultaneous dysfunction of ≥ two organs systems, and was assessed at the end of 1st and 3rd days.
Vasoactive-inotropic score [18] was calculated at the end of 1st and 2nd days as follows: [Dopamine dose (µg/kg/min)] + [Dobutamine (µg/kg/min)] + [Epinephrine (µg/kg/min) × 100] + [Norepinephrine(µg/kg/min) × 100] + [Phenylephrine(µg/kg/min) × 10] + [Vasopressin (U/kg/min) × 10,000] + [Milrinone (µg/kg/min) × 10].
Septic shock was managed according to American College of Critical Care Medicine guidelines [19].
Baseline laboratory tests, like complete blood count, serum creatinine, and liver function tests, were obtained on admission. Microbiological cultures were taken on admission from blood, urine, cerebrospinal fluid (CSF), and pleural fluid as clinically indicated. Diagnostic tests for viral agents were not consistently performed. Other laboratory and radiological investigations were requested as clinically indicated.
Blood samples for serum ADAMTS13 antigen measurement were obtained from controls and from patients within 24 h of PICU admission, using Human ADAMTS13 SunRed ELISA kits (Catalogue No: 201–12-5457), supplied by Shanghai SunRed Biological Technology Co., Ltd (Shanghai, China) according to manufacturer's instructions. Only a single ADAMTS13 measurement was performed for each of the study participants. Briefly, 2 ml of blood were withdrawn from each patient in a plain tube. Blood was allowed to clot at room temperature for 10–20 min. The clot was removed by centrifugation at 2,000–3,000 rpm for 20 min, then the sample was kept at -80°C until later testing. The assay range is 0.08–20 ng/mL. Intra-assay Coefficient of Variability (CV) is < 10%. Inter-assay CV is < 12%.
Patients were monitored until discharge from PICU. The primary outcome was “all-cause PICU mortality”. Secondary outcomes included mechanical ventilation use, vasoactive medication use, ventilator-free days, and vasoactive-inotropic score.
Statistical methods
Categorical variables were presented as number (percentage). Continuous variables were summarized by median and interquartile range (IQR). Mann–Whitney U test was used to determine whether there is significant difference in median between two groups. For more than two groups, we used Kruskal–Wallis test and pairwise comparisons by Dunn's procedure. Chi-square test, or Fisher-exact test, was used to evaluate associations between categorical variables. Spearman's rank correlation coefficient was calculated to evaluate the relationship between continuous variables. All tests were two-sided, and a difference was considered statistically significant if p-value was < 0.05.
Potential mortality risk factors that were significantly different between survivors and non-survivors in the 2 × 2 table were included in univariate binary logistic regression analysis. Calibration of ADAMTS13 was evaluated by the Hosmer–Lemeshow goodness-of-fit test which is used to determine whether there is agreement between the estimated and the true risk of an outcome. A p-value of 0.05 or more in the goodness-of-fit test implies that the model is well calibrated.
Receiver Operating Characteristic (ROC) curve analysis was utilized to evaluate performance of the admission quantitative variables (found to be associated with mortality through logistic regression analysis and 2 × 2 table) in prediction of mortality. The area under the ROC curve (AUC) represents whether a certain variable is able to discriminate survivors from non-survivors. The best cutoff was chosen by calculating Youden index. Survival analysis was performed by the Kaplan–Meier survival curve. The log-rank test was used to test the null hypothesis of no difference in survival between patients with high, and those with low, ADAMTS13 level.
Statistical calculations were conducted by IBM SPSS (statistical package for social science) version 23.
Results
Characteristics of the study population
Seventy patients and eighteen age and sex-matched controls were recruited. Regarding the control group, 8 (44.4%) were males; the median age and weight were 42 months (IQR 7.5–54) and 16.6 kg (IQR 8.6–18.5), respectively.
Table 1 demonstrates patients' characteristics. 41 patients (58.6%) were identified with sepsis; 14 (20%) had severe sepsis; and 15 (21.4%) had septic shock. Noteworthy, the classifications “sepsis”, ‘severe sepsis”, and “septic shock” were made on admission. Later, some patients with “sepsis” or “severe sepsis” deteriorated and developed septic shock.
24 patients died, giving an overall mortality rate of 34.3%. The number of non-survivors was 6 (14.6%) in the sepsis; 7 (50%) in the severe sepsis; and 11 (73.3%) in the septic shock groups.
The source of infection was respiratory in 41 patients (58.6%); central nervous system in 11 patients (15.7%); renal in 3 patients (4.3%); gastrointestinal in 3 patients (4.3%); and cardiac in one patient (1.4%). In 11 patients (15.7%), sepsis was without focus.
Blood culture was positive in 17 patients (24.3%). Isolated organisms included Staphylococcus aureus (4 patients), E-coli (3 patients), and Klebsiella pneumoniae (4 patients), Pseudomonas (2 patients), Enterococcus (one patient), Coagulase negative staphylococcus (2 patients), and Candida albicans (one patient). Cultures from other body fluids were negative.
ADAMTS13 in patients and controls
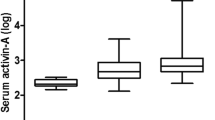
Median ADAMTS13 level was lower among patients compared with healthy controls [1.30 (IQR: 0.88–3.13 ng/ml) versus 6.00 (IQR: 5.55–6.50 ng/mL), p < 0.001] (Fig. 1).
ADAMTS13 levels in various subgroups. ADAMTS13 median and interquartile range (IQR): all patients: 1.30 ng/ml (0.88 – 3.13); controls: 6.00 (5.55 – 6.50 ng/ml); sepsis: 2.80 (1.00 – 3.85 ng/ml); severe sepsis: 0.90 (0.80 – 1.75 ng/ml); septic shock: 1.00 (0.90 – 1.20 ng/ml). The bold black lines in the boxplot represent the medians. The bottom and top of the box represent the 25th and 75th percentiles respectively (IQR). The lower whisker boundary corresponds to the 25th percentile minus 1.5 times IQR. The upper whisker boundary corresponds to the 75th percentile plus 1.5 times the IQR. Tiny circles represent "outliers" i.e., data values which are 1.5 times IQR larger than the 75th percentile or 1.5 times IQR smaller than the 25 th percentile. Asterisks represent “extreme outliers” i.e., data values greater than 75th percentile plus 3 times IQR
ADAMTS13 had an AUC of 0.99 [95% confidence interval (CI): 0.997–1.000] for discriminating patients from controls (p < 0.001). A cutoff ≤ 4.4 ng/mL had 97.1% sensitivity and 100% specificity (Fig. 2).
ADAMTS13 and disease severity
Figure 1 shows that ADAMTS13 was significantly lower in both severe sepsis and septic shock, compared with sepsis sub-group. No significant difference was found between severe sepsis and septic shock.
Lower ADAMTS13 level was associated with vasoactive medications, mechanical ventilation, ARDS, and MODS on day 1 and 3 (Table 2).
ADAMTS13 was negatively correlated with vasoactive infusion days and vasoactive-inotropic scores on day1 and 2 (Table 5).
ADAMTS13 had an AUC of 0.74 (95% CI: 0.61–0.87, p = 0.002) for predicting MODS on day1. At a cutoff ≤ 1.25, ADAMTS13 had 85% sensitivity and 66% specificity.
ADAMTS13 had an AUC of 0.78 (95% CI: 0.68–0.89, p = 0.001) for predicting MODS on day3. At a cutoff ≤ 1.15, ADAMTS13 had 87.5% sensitivity and 66.7%% specificity.
ADAMTS13 and PICU mortality
Characteristics of survivors and non-survivors are shown in Table 3. Non-survivors had significantly higher frequency of severe sepsis, septic shock, ARDS, mechanical ventilation, vasoactive medication use, hospital-acquired infections, and MODS on day 1 and 3). Non-survivors also had significantly higher vasoactive infusion days, vasoactive-inotropic score (day1 and 2), pSOFA, and PIM2 but significantly lower ventilator-free days and platelet counts.
ADAMTS13 level was significantly lower among non-survivors (median level 0.9 ng/mL) compared with survivors (median levels 2.45 ng/mL); (p < 0.001).
Univariate logistic regression analysis revealed that ADAMTS13 was negatively associated with mortality [odds ratio (OR) and 95% confidence interval (95% CI): 0.38 (0.21–0.69), p = 0.001]. Hosmer–Lemeshow goodness-of-fit test yielded a p-value of 0.28, indicating that ADAMTS13 is well calibrated.
ROC curve analysis (Table 4, Fig. 3) revealed that ADAMTS13 had an AUC of 0.77 (95% CI: 0.66–0.89), while pSOFA had an AUC of 0.81 (95% CI: 0.69–0.92) for mortality prediction. At a cutoff ≤ 1.25 ng/mL, ADAMTS13 had 83.3% sensitivity and 69.6% specificity. After subgrouping patients according to this cutoff, Kaplan–Meier survival curve showed a significant difference in cumulative survival between patients with low ADAMTS13 (≤ 1.25 ng/mL) and those with high ADAMTS13 (p < 0.001) (Fig. 4).
PIM2 (AUC: 0.72) and platelet count on admission (AUC: 0.67) were inferior to ADAMTS13 in predicting mortality. ADAMTS13 was negatively correlated with pSOFA and PIM2 scores (Table 5).
As regards other variables, platelet count was negatively associated with mortality [OR: 0.996 (95% CI 0.993–1), p = 0.033]. The variables found to be positively associated with mortality [and their OR (95% CI)] included mechanical ventilation [28.8 (7.0–118.1), p < 0.001]; ARDS [9.1 (1.7–48), p = 0.010]; hospital-acquired infections [6.7 (2.1–21.6), p = 0.002], MODS on day1 [9.3 (2.9–30.4), p < 0.001]; MODS on day3 [33.4 (6.4–173.5), p < 0.001], PIM2 [1.1 (1.0–1.2), p < 0.001]; and pSOFA [1.46 (1.19–1.8), p < 0.001].
Discussion
Understanding the pathophysiology of sepsis appears to be an unending task. Mounting evidence points to a role of ADAMTS13 deficiency in inducing massive microvascular thrombosis, with consequent tissue ischemia and organ dysfunction in sepsis.
The main message given in present study is that ADAMTS13 could play a role in pediatric sepsis severity, development of organ dysfunctions, and mortality. ADAMTS13 is, therefore, important for better understanding, and potentially reversing, sepsis pathophysiology. Moreover, if its role is established, ADAMTS13 could be utilized for guiding sepsis management so that a patient with lower ADAMTS13 levels would need closer monitoring and more aggressive treatment.
ADAMTS13 between health and sepsis
Consistent with previous pediatric [11, 12] and adult [20] studies, we found that ADAMTS13 level was significantly lower among septic children compared with healthy controls, suggesting a role for ADAMTS13 in sepsis pathophysiology and a potential for being utilized as a diagnostic biomarker for sepsis. However, the latter issue was ignored by previous literature in favor of ADAMTS13 prognostic value.
ADAMTS13 and sepsis severity
We have also shown that ADAMTS13 is associated with illness severity as it was significantly lower among patients with septic shock and those with severe sepsis compared with those having sepsis, although the number of patients in the former two groups was low. In comparison, another pediatric study found that ADAMTS13 level was significantly lower in septic shock compared with both sepsis and severe sepsis [11]. On the other hand, we did not find a significant difference in ADAMTS13 level between severe sepsis and septic shock, while previous adult studies yielded conflicting findings [20, 21].
ADAMTS13 and organ dysfunctions
As expected from its biological action, ADAMTS13, in the present study, was significantly lower among patients with MODS and had negative correlation with the organ dysfunction score, pSOFA. Similarly, other studies demonstrated correlations of ADAMTS13 with other adult and pediatric organ dysfunction scores like Pediatric Logistic Organ Dysfunction (PELOD) score [10, 11, 21].
Of note, correlation of ADAMTS13 with pSOFA score on admission was only moderate in our study, implying that ADAMTS13 is not the only determinant of organ dysfunction. It is also possible that consequences of ADAMTS13 deficiency take some time to develop, as suggested by our observation that admission ADAMTS13 level was slightly more accurate in predicting MODS on day3 than on day1, so ADAMTS13 deficiency could serve as a red flag in sepsis.
Unlike its global association with organ dysfunction, ADAMTS13, in the present study, was specifically associated only with respiratory and cardiovascular dysfunction indicators, including mechanical ventilation and vasoactive medication use; vasoactive infusion days; and vasoactive-inotropic score.
Conversely, ADAMTS13 was not correlated with serum creatinine, while previous adult studies gave conflicting findings [20, 22]. Likewise, we failed to find correlation between ADAMTS13 and alanine aminotransferase (ALT) or serum albumin, although liver is the major source of ADAMTS13 production. This is consistent with a previous adult study [22], and suggests that decreased ADAMTS13 synthesis is less important than other mechanisms causing its deficiency in the setting of sepsis.
Similarly, we found no correlation between ADAMTS13 and platelet count, possibly because thrombocytopenia in sepsis arises not only from platelet consumption but from other mechanisms like immune-mediated platelet destruction, platelet sequestration in liver, drug-induced thrombocytopenia, and hemodilution [23]. However, previous studies divided over the correlation of ADAMTS13 with platelet count; some confirmed it [10, 21, 24], while others did not [20, 22].
The above-mentioned discrepancies among studies might arise from differences in sample size (most studies were small), sepsis etiology, or illness severity. Moreover, ADAMTS13 measurement was inconsistent ("antigen", "activity", or both) although ADAMTS13 activity may decrease without concomitant decrease in antigen due to inactivation by various inhibitors [22]. What is more, ADAMTS13 activity is under genetic influences e.g. a single nucleotide polymorphism in ADAMTS13 gene results in a transition of Pro 475 to Ser, with lower ADAMTS13 activity [20, 25].
ADAMTS13 and mortality
Organ dysfunction portends death, and this was the case in our study where ADAMTS13 was independent predictor of mortality. This finding is consistent with some adult [21] and pediatric [11] studies, although others failed to replicate this association [12, 20, 24]. These studies are generally small and none can provide conclusive evidence alone.
Additionally, ADAMTS13 was negatively correlated with PIM2 score, which is consistent with previous adult [21] and pediatric [10, 11] studies showing correlations with other mortality predictive scores like Pediatric Risk of Mortality.
The ability of ADAMTS13 to predict mortality in our study was fair, with an AUC of 0.77, which was equal to that reported by another study [11]. This moderate performance is plausible; certainly, there is a role for other molecules involved in coagulation and other factors having direct impact on organ function, including hypoxia, acidosis, toxins, electrolyte imbalance, and metabolic abnormalities.
Importantly, it is oversimplification to assume that lower ADAMTS13 activity leads inevitably to clinical consequences. In fact, there is a role for factors causing endothelial injury. In one study, complete lack of ADAMTS13 in mice produced a pro-thrombotic state but was not sufficient alone to cause TTP except after injection of collagen and epinephrine [26]. Other endogenous molecules can enhance or inhibit thrombosis. For instance, during inflammation, high-density lipoprotein, which possesses antithrombotic properties, decreases while low-density lipoprotein, which possesses pro-thrombotic properties, increases [6]. Consequently, thrombosis is the net effect of many endogenous and exogenous factors, rather than ADAMTS13 alone.
Therapeutic prospects
Association of ADAMTS13 with prognosis has therapeutic implications. Recombinant ADAMTS13 could be a future strategy for patients with sepsis and ADAMTS13 deficiency [27]. It is also possible to target ADAMTS13 inhibitors, like IL-6, by such medications as Tocilizumab. Nonetheless, ADAMTS13-based therapies are not entirely experimental; the commonly available antiplatelet agents, particularly aspirin, reduced sepsis-associated mortality in adults according to a recent met-analysis [28]. Of note, antiplatelets not only inhibit thrombus formation but also dampen inflammation triggered by activated platelets [29].
A biological role for therapeutic plasma exchange (TPE) seems particularly plausible in children with sepsis and thrombocytopenia-associated multiple organ failure (TAMOF), in which disseminated microvascular thrombosis occurs. In TAMOF, TPE aims at removing ULVWF and ADAMTS13 inhibitors and restoring ADAMTS13 activity. However, because of limited evidence, the recent surviving sepsis campaign guidelines was not able to make a recommendation for or against TPE in TAMOF [30].
Noteworthy, we found that ADAMTS13 cutoff that discriminated septic from normal children was much higher than that which predicted organ dysfunction or mortality, suggesting that mild ADAMTS13 deficiency is well tolerated and that ADAMTS13-based therapies should be offered only to patients with severe deficiency who are more liable to complications.
Although those therapeutic lines are theoretically appealing, large randomized controlled trials (RCTs) are indispensable. It is prudent not to be too optimistic since the outcome of sepsis is the final product of intricate interplay of many pathways and molecules, with limited contribution of each to the whole scene. This makes it unlikely that benefits from any specific therapy, apart from antimicrobials, will be dramatic.
Strengths and limitations
Overall, the present study adds a new evidence to the limited pediatric literature on the role ADAMTS13 plays in sepsis. Notwithstanding, it has some limitations: the sample size was small. However, prior sample size calculation predicted that a large patient or control group is not needed to demonstrate significant differences. Additionally, we did not compare ADAMTS13 between septic and non-septic critically ill children. Furthermore, we did not repeat ADAMTS13 measurement serially. Undoubtedly, analyzing ADAMTS13 in the subset of patients with sepsis who deteriorated to severe sepsis or septic shock would have shed more light on the role of ADAMTS13 but this was not possible for financial reasons and this can be the subject of future studies. Finally, the mortality rate in our study was high, which could be explained by the limited resources that precluded taking some necessary diagnostic and therapeutic measures. So, some of the current findings might not hold in other settings.
Conclusion
ADAMTS13 is associated with mortality and illness severity, including the presence of severe sepsis and septic shock on admission; development of multiple organ dysfunction syndrome; and requirement for mechanical ventilation and vasoactive medications. We have not shown that these associations are causal. However, they raise the question of whether therapeutic interventions targeting restoration of ADAMTS13 activity or antagonizing the consequences of its deficiency can improve sepsis prognosis.
Availability of data and materials
Not applicable.
Abbreviations
- ADAMTS13:
-
A disintegrin-like and metalloprotease with thrombospondin type 1 motif,13
- ALT:
-
Alanine aminotransferase
- ARDS:
-
Acute respiratory distress syndrome
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CSF:
-
Cerebrospinal fluid
- CV:
-
Coefficient of Variability
- DIC:
-
Disseminated intravascular coagulation
- ELISA:
-
Enzyme-linked immunosorbent assay
- IQR:
-
Interquartile range
- MODS:
-
Multiple organ dysfunction syndrome
- PELOD:
-
Pediatric Logistic Organ Dysfunction
- PICU:
-
Pediatric Intensive Care Unit
- PIM2:
-
Pediatric Index of Mortality-2
- pSOFA:
-
Pediatric Sequential Organ Failure Assessment score
- RCT:
-
Randomized controlled trial
- ROC curve:
-
Receiver Operating Characteristic curve
- SIRS:
-
Systemic inflammatory response syndrome
- TAMOF:
-
Thrombocytopenia-associated multiple organ failure
- TPE:
-
Therapeutic plasma exchange
- UL:
-
Ultralarge
- ULVWF multimers:
-
Ultralarge von Willebrand factor
- VWF:
-
Von Willebrand factor
References
Schlapbach LJ, Straney L, Alexander J et al (2015) Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. Lancet Infect Dis 15:46–54
Levi M, Scully M, Singer M (2018) The role of ADAMTS-13 in the coagulopathy of sepsis. J Thromb Haemost 16:646–651
Bernardo A, Ball C, Nolasco L et al (2004) Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood 104:100–106
Uemura M, Tatsumi K, Matsumoto M et al (2005) Localization of ADAMTS13 to the stellate cells of human liver. Blood 106:922–924
Shelat SG, Ai J, Zheng XL (2005) Molecular biology of ADAMTS13 and diagnostic utility of ADAMTS13 proteolytic activity and inhibitor assays. Semin Thromb Hemost 31:659–672
Chen J, Chung DW (2018) Inflammation, von Willebrand factor, and ADAMTS13. Blood 132:141–147
Mikuła T, Kozłowska J, Stańczak W et al (2018) Serum ADAMTS-13 Levels as an Indicator of Portal Vein Thrombosis. Gastroenterol Res Pract 2018:3287491
Maino A, Siegerink B, Lotta LA et al (2015) Plasma ADAMTS-13 levels and the risk of myocardial infarction: an individual patient data meta-analysis. J Thromb Haemost 13:1396–1404
Beltrami-Moreira M, DeSancho MT (2022) Delayed diagnosis of congenital thrombotic thrombocytopenic purpura in a patient with recurrent strokes. J Thromb Thrombolysis 53:734–738
Nguyen TC, Liu A, Liu L et al (2007) Acquired ADAMTS-13 deficiency in pediatric patients with severe sepsis. Haematologica 92:121–124
Lin JJ, Chan OW, Hsiao HJ et al (2016) Decreased ADAMTS 13 Activity is associated with disease severity and outcome in pediatric severe sepsis. Medicine (Baltimore) 95:e3374
Bongers TN, Emonts M, de Maat MP et al (2010) Reduced ADAMTS13 in children with severe meningococcal sepsis is associated with severity and outcome. Thromb Haemost 103:1181–1187
Zeineddin A, Dong JF, Wu F et al (2021) Role of Von Willebrand factor after injury: It may do more than we think. Shock 55:717–722
Hugenholtz GC, Adelmeijer J, Meijers JC et al (2013) An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology 58:752–761
Goldstein B, Giroir B, Randolph A (2005) International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6:2–8
Slater A, Shann F, Pearson G (2003) PIM2: a revised versionof the Paediatric index of mortality. Intensive Care Med 29:278–285
Matics TJ, Sanchez-Pinto LN (2017) Adaptation and Validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr 171:e172352
Nguyen HV, Havalad V, Aponte-Patel L et al (2013) Temporary biventricular pacing decreases the vasoactive-inotropic score after cardiac surgery: a substudy of a randomized clinical trial. J Thorac Cardiovasc Surg 146:296–301
Davis AL, Carcillo JA, Aneja RK et al (2017) American college of critical care medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med 45:1061–1093
Kremer Hovinga JA, Zeerleder S, Kessler P et al (2007) ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost 5:2284–2290
Martin K, Borgel D, Lerolle N et al (2007) Decreased ADAMTS-13 (A disintegrin-like and metalloprotease with thrombospondin type 1 repeats) is associated with a poor prognosis in sepsis-induced organ failure. Crit Care Med 35:2375–2382
Ono T, Mimuro J, Madoiwa S et al (2006) Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood 107:528–534
Ghimire S, Ravi S, Budhathoki R et al (2021) Current understanding and future implications of sepsis-induced thrombocytopenia. Eur J Haematol 106:301–305
Karim F, Adil SN, Afaq B et al (2013) Deficiency of ADAMTS-13 in pediatric patients with severe sepsis and impact on in-hospital mortality. BMC Pediatr 13:44
Kokame K, Matsumoto M, Soejima K et al (2002) Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci USA 99:11902–11907
Banno F, Kokame K, Okuda T et al (2006) Complete deficiency in ADAMTS13 is prothrombotic, but it alone is not sufficient to cause thrombotic thrombocytopenic purpura. Blood 107:3161–3166
Levy B, Lacolley P, Regnault V (2007) ADAMTS-13 (A disintegrin-like and metalloprotease with thrombospondin) and endothelial dysfunction in sepsis: marker or culprit? Crit Care Med 35:2453–2454
Ouyang Y, Wang Y, Liu B et al (2019) Effects of antiplatelet therapy on the mortality rate of patients with sepsis: a meta-analysis. J Crit Care 50:162–168
Nicolai L, Gaertner F, Massberg S (2019) Platelets in host defense: experimental and clinical insights. Trends Immunol 40:922–938
Weiss SL, Peters MJ, Alhazzani W et al (2020) Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med 21:e52–e106
Acknowledgements
None.
Funding
No funding or grants were received for conducting this study.
Author information
Authors and Affiliations
Contributions
MSE: designed the study, evaluated the patients, analyzed the results, and wrote the manuscript; MFE recruited the patients and analyzed the results; SME: Performed the laboratory analyses and analyzed the results. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval was given by the ethical committee of Faculty of Medicine, Menoufia University: approval number: 19919PEDI47.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Mekkawy, M.S., El-Deeb, S.M. & El-Hanafy, M.F. ADAMTS13 in pediatric sepsis: a prognostic biomarker with potential therapeutic implications. Egypt Pediatric Association Gaz 71, 71 (2023). https://doi.org/10.1186/s43054-023-00219-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-023-00219-1