Abstract
Background
Vitamin D deficiency occurs frequently in cystic fibrosis (CF) and non-CF bronchiectasis patients. Yet, few studies have assessed the impact of vitamin D status on the clinical outcomes in pediatric bronchiectasis. This study is designed to assess vitamin D level and determine its effect on exacerbations, bacterial colonization, and lung function in pediatric patients with CF and non-CF bronchiectasis.
Results
This cross-sectional case-control study assessing vitamin D level was performed in a total of sixty cases under the age of 18 years—forty cases with CF and non-CF bronchiectasis and twenty age- and sex-matched healthy controls. Associations between serum vitamin D and clinical and laboratory parameters were assessed in the patient groups. Vitamin D deficiency was more prevalent among CF and non-CF bronchiectasis patients (75%, 45%) compared to controls (10%) (P < 0.001). In addition, vitamin D deficiency was associated with more frequent and severe pulmonary exacerbations (66.7%, 46.7%) (P=0.033, < 0.001), chronic Pseudomonas infection (80%) (P=0.060) among CF patients, and with lower FEV1 (66%) (P= 0.071) among non-CF bronchiectasis. Moreover, a cutoff value of vitamin D level equal or less than 22.5 ng/ml was accurate in differentiating moderate from mild pulmonary exacerbations in both patients’ groups (AUC=0.809) (p=0.004).
Conclusions
Vitamin D deficiency is not uncommon in both CF and non-CF bronchiectasis. In this population, vitamin D deficiency is associated with more frequent pulmonary exacerbations, chronic Pseudomonas infection, and worse lung function.
Similar content being viewed by others
Background
Bronchiectasis is conventionally used as a descriptive term for an irreversible condition characterized by chronic suppurative airway disease manifested clinically by chronic productive cough and radiologically by bronchial dilation and thick-walled bronchi [1]. Bronchiectasis is classified into bronchiectasis secondary to cystic fibrosis (CF) and bronchiectasis not related to CF, which is called non-cystic fibrosis bronchiectasis [2].
Vitamin D deficiency occurs frequently in patients with CF [3] and non-CF bronchiectasis [4]. In these patients, vitamin D deficiency can arise from multiple causes as pancreatic exocrine insufficiency, absence of outdoor activity, and alterations of vitamin D metabolism [3].
Vitamin D is known to be involved in a wide spectrum of significant immunomodulatory effects as downregulation of pro-inflammatory cytokines and chemokines [2]. It also regulates the secretion of antimicrobial peptides like cathelicidin (LL-37) which has a potent antimicrobial activity against Pseudomonas aeruginosa [4].
Higher vitamin D is associated with better lung function [3]. The mechanism by which vitamin D improves lung function may be through its action on regulating inflammation, inducing antimicrobial peptides, or its action on muscle [5].
We hypothesized that vitamin D deficiency may negatively affect bronchiectasis outcomes in both CF and non-CF bronchiectasis pediatric patients. For this reason, this study was conducted to evaluate the influence of vitamin D status on CF and non-CF bronchiectasis outcomes.
Methods
Patients and settings
This cross-sectional study assessed vitamin D level (25-OHD) in twenty CF and twenty non-CF bronchiectasis children under the age of 18 years during the period between 1 March 2019 and 1 September 2019; the patients were recruited from pediatric chest clinic and chest department, Children’s Teaching Hospital. Also, the study included twenty age- and sex-matched healthy controls without any respiratory problems who were attending the outpatient clinics for routine care (either for vaccination or for following up their anthropometric measurements).
Patients were included if they had (1) confirmed CF diagnosis by positive sweat chloride test equal or above 80 mmol/L using the Nanoduct® Neonatal Sweat Analysis System (Wescor) [6] measured twice and / or had two known CF disease causing mutations [7] and (2) a documented diagnosis of non CF bronchiectasis by clinical history of chronic sputum production with confirmed radiological findings of bronchiectasis by high-resolution computed tomographic (HRCT) lung scanning with a negative sweat test [8, 9]. Control subjects and non-CF bronchetasis patients were not receiving vitamin D supplements before and during the period of the study. The CF patients were taking vitamin D supplement as a part of their multivitamins with a dose not exceeding 2000 IU daily.
Subjects were excluded if (1) CF patients had liver disease based on Debray’s criteria [10], or renal disease based on estimated creatinine clearance using the Schwartz formula [11], because these might affect the metabolism of vitamin D (details of these are available in the (supplementary data); (2) CF patients had pancreatic sufficiency; (3) the CF patients were receiving vitamin D supplements exceeding 2000 IU per day; (4) the studied patients were receiving steroid therapy in the last 6 weeks; (5) the studied patients had chronic lung diseases other than CF and non CF bronchiectasis; (6) the studied patients had any other systemic illness; or (7) non CF bronchiectasis patients and controls were taking any vitamin D supplements during the last three months prior to the study.
Ethics approval and consent to participate
The study was approved by the Research Ethical Committee, Faculty of Medicine, Institutional University, Children’s Hospital. Informed written consents were obtained from the patients’ guardians prior to the inclusion in the study.
Measurements
At enrollment, from each patient, a detailed history was undertaken, focusing on respiratory symptoms including type of cough, expectoration, dyspnea, respiratory distress, hemoptysis, and cyanosis, besides, symptoms suggestive of pulmonary exacerbations over the last 12 months. A full dietetic history with special emphasis on dietary vitamin D intake was calculated from the 24-h dietary recall for 3 days for both the patients and the controls. The amount of vitamin D obtained from each food focusing on foods with significant vitamin D content was calculated and was summed to obtain the mean of the total amount of dietary vitamin D intake per day, and its percentage of the recommended dietary allowances (RDAs) [12] for vitamin D per day was calculated in the studied patients and controls. In addition, history of vitamin D supplement and its dose as well as any additional supplements, total daily dose of pancreatic enzyme replacement therapy for CF patients, and history of sunlight exposure were obtained. Adequate sunlight exposure for infants and children was defined as sunshine exposure of extremities at morning 10 a.m. to 2 p.m. for 15–30 min at least three times per week [13, 14]. Socioeconomic status in all participants was also evaluated using El-Gilany score [15].
The forty studied bronchiectasis patients were also assessed by clinical parameters including age, sex, and body mass index (BMI) percentile [16]. Chest auscultation and pulse oximetry were also performed.
Furthermore, the studied bronchiectasis patients were subjected to lower respiratory tract samples cultures, colonization status assessment, and pulmonary function tests (PFTs) for cooperative patients older than 6 years. Serum vitamin D 25(OH) D measurement and serum laboratory inflammatory markers as complete blood count and C-reactive protein were also recorded. For controls, demographic data and anthropometric measures were obtained. Serum vitamin D 25(OH) D was recorded as well.
PFTs were done using standardized spirometry, which was performed and interpreted according to the American Thoracic Society Guidelines [17] for both CF and non-CF bronchiectasis patients.
A pulmonary exacerbation was defined based on Fuchs criteria [18] for the CF patients and British Thoracic Society bronchiectasis guidelines [19] for non-CF bronchiectasis patients which were applied on the studied patients, diagnosed and revised by at least two pulmonologists during examination.
Frequent exacerbations were defined as more than three exacerbations per year [20, 21].
A mild-to-moderate exacerbation was further defined as 1 to 2 signs or symptoms present or that the symptom severity is mild. A moderate-to-severe exacerbation was defined as more than 3 new findings or 1 to 2 severe findings (e.g., oxygen desaturation, new crackles) or a mild-to-moderate exacerbation unresponsive to oral or inhaled antibiotics [22].
Chronic lung colonization was defined by at least two out of the three cultures positive for the same organism with at least 1-month intervals in the absence of signs of infection within the last 6 months prior to the study [23]. Chronic lung infection by Pseudomonas aeruginosa was defined when Pseudomonas cultures were positive in more than 50% of months in a 12-month period prior to the study [24].
Organisms were detected in the lower respiratory tract samples (either induced, expectorated sputum, or bronchial lavage), using standard clinical microbiological protocols [25].
Assessment of serum vitamin D levels
At enrollment, 3 ml of venous blood were withdrawn from each child under aseptic conditions on gel vacutainers. Serum was separated by centrifugation at 3500 rpm for 15 min and stored at −20°C till used for assessment of the level of 25(OH)D. Levels of 25(OH)D were assessed using enzyme linked immunoassay Kit supplied by Calbiotech, USA, guided by the manufacturer’s instructions [26]. Briefly, 10 μl of each standard, control, and sample was added to the corresponding wells; then, 200μl of biotinylated conjugate was added. The plate was then incubated for 90 min at room temperature followed by washing three times and the addition of 200 ul of Streptavidin-HRP. The plate was then incubated at room temperature for 30 min followed by washing of the wells 3 times. Then, 200 μl of TMB substrate were added to each well followed by incubation at room temperature for 30 min away from light, after which 50 μl of the stop solution were added and the optical density was read at 450nm. A standard curve was plotted using the standards’ readings, and concentrations of the samples were deduced from the curve.
The studied cases were classified into three groups according to the serum 25(OH)D status: vitamin D-deficient group which had a serum 25(OH)D < 20 ng/ml, vitamin D insufficient group with serum 25(OH)D 20–29.9 ng/ml, and vitamin D sufficient group with serum 25(OH)D ≥ 30 ng/ml [23].
Statistical analysis
Data were analyzed in the form of mean ± standard deviation (± SD), median, and interquartile range (IQR) or frequencies (number of cases) and percentages. One-way ANOVA was used to compare parametric quantitative data while Kruskal-Wallis test was used to compare non-parametric quantitative data and whenever significant a post hoc test was used. Chi-square test was using to compare categorical data and to study associations. P values less than 0.05 was considered statistically significant and P less than 0.01 was considered highly significant. All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) [27].
Sample size was calculated using PASS 11.0. The total sample of 60 subjects achieves 83% power to detect differences among the serum vitamin D level means versus the alternative of equal means using an F test with a 0.01000 significance level. The size of the variation in the means is represented by their standard deviation which is 2.49. The common standard deviation within a group is assumed to be 4.70.
Results
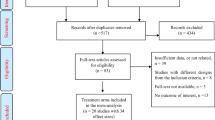
The present study was conducted on sixty participants under the age of 18 years. The subjects were classified into to 3 groups: group I non-cystic fibrosis bronchiectasis patients (no=20), group II cystic fibrosis patients (n=20), and group III age- and sex-matched healthy controls without any respiratory problems (n= 20). Demographics, anthropometric data, and serum vitamin D concentrations were obtained from the three studied groups. The etiology of non-CF bronchiectasis in the patients of group I is summarized in (Table S3).
Demographic data (Table 1)
The mean age of CF patients (4.80±3.85) was significantly lower than the mean age of non-CF bronchiectasis patients (9.55±4.05) (P=0.002). Also, positive consanguinity and family history of bronchiectasis were significantly higher among CF patients (75%, 60%) than controls (35%, 0%) (P= 0.036) (< 0.001).
Comparison of vitamin D level in the studied cases
The mean serum vitamin D level was significantly lower among the CF group (20.15±11.66) and the non-CF bronchiectasis group (20.85±7.21) compared to the control group (35.15±12.84) (P < 0.001). Moreover, deficient vitamin D level was significantly more prevalent among CF patients (75%) and non-CF bronchiectasis patients (45%) compared to controls (10%), while vitamin D insufficiency was more frequent among non-CF bronchiectasis (40%) compared to CF patients (20%) and controls (35%) (p < 0.001). In addition, daily dietary intake of vitamin D was significantly lower among the CF patients (313.80±65.66) than among the controls (357.25±40.54) (P=0.063). Meanwhile, the dietary intake of vitamin D in non-CF bronchiectasis patients was (338.50±62.17). Regarding sun exposure, it was adequate only in 15% of CF patients, 30.0% of non-CF bronchiectasis group, and 40% of the control group. Vitamin D concentrations among the studied groups are presented in Table 2.
Vitamin D deficiency and pulmonary exacerbations
Frequent pulmonary exacerbations were significantly higher among vitamin D deficient group (66.7%) than the sufficient (0.0%) and insufficient (0.0%) vitamin D groups in CF bronchiectasis patients (P=0.033). Furthermore, moderate to severe pulmonary exacerbations were more prevalent among vitamin D-deficient patients (93.4%) compared to the other groups (P <0.001) (Table 3). Also, more frequent (66.7%) and moderate to severe pulmonary exacerbations (88.9%) were presented among vitamin D deficient non-CF bronchiectasis patients, although it was statistically non-significant (P= 0.166 and 0.232 respectively) (Table 4).
Vitamin D and colonization status
Among CF patients, bacterial colonization was prevalent among 83% of vitamin D-deficient group, 17% of vitamin D insufficient group, and 0% of the sufficient group, where the most frequently isolated organism was Pseudomonas aeruginosa which was significantly higher among vitamin D-deficient group (80%) than vitamin D insufficient group (20%) and vitamin D sufficient group (0%) (P=0.060) followed by Staphylococcus aureus and MRSA colonization (Table 5).
Among non CF bronchiectasis patients, bacterial colonization was found among 53.3% of vitamin D-deficient group, 26.7% of vitamin D insufficient group, and 20.0% of the sufficient group, where the most predominant organism was Staphylococcus aureus, which was presented among 57.1% of the vitamin D-deficient group, 28.6% of vitamin D insufficient group, and 14.3% of vitamin D sufficient group followed by Pseudomonas aeruginosa and Klebsiella, although the difference was statistically non-significant (p=0.818) (Table 6).
Vitamin D deficiency and its relation to lung function
Among the non-CF bronchiectasis patients, the median FEV1% of predicted was significantly lower among the vitamin D deficient group (66%) with IQR of 56–73% and insufficient group (67%) with IQR of 40–79% than among the vitamin D sufficient group (80.0%) with IQR of 75–88% (P=0.071). Similarly, among CF patients, the median FEV1 was lower among the vitamin D deficient group (69%) with IQR of (60 -70%) and insufficient group (71%) with IQR of (65-75%) than among vitamin D sufficient group (80%), although the difference was not statistically significant (P=0.423). All these data are summarized in Table 7.
Finally, ROC curves of serum vitamin D for diagnosis of CF and non-CF pulmonary exacerbations severity showed that serum vitamin D was 95% accurate in determining exacerbations severity (AUC=0.809, p=0.004). At a cutoff value equal or less than 22.5 ng/ml, serum vitamin D level has shown 79% sensitivity and 75% specificity for differentiating moderate from mild pulmonary exacerbations while at a cutoff value equal or less than 16.5 ng/ml, serum vitamin D level has shown 77% sensitivity and 58% specificity for differentiating severe from moderate pulmonary exacerbations (AUC=0.764, p=0.168) as presented in (Figs. 1 and 2).
Discussion
To our knowledge, this is the one of the fewest pediatric studies that evaluated the serum vitamin D level in both CF and non-CF bronchiectasis patients and determined its relation to the disease outcomes.
The present study showed that the studied subjects’ mean age was 4.80±3.85 in CF patients, 9.55±4.05 in non-CF bronchiectasis, and 5.75±4.23 in controls which was ranged from 1 to 17 years.
Males were predominant in CF bronchiectasis patients (75%), while represented (50.0%) in both non-CF bronchiectasis patients and controls.
The current study showed that vitamin D deficiency and insufficiency were significantly prevalent among CF and non-CF bronchiectasis patients (95%, 85%) respectively and surprisingly among 45% of the healthy control group with the lowest vitamin D levels were among the CF patients’ group (p< 0.001) (Table 2).
Our findings could be explained by low dietary intake of vitamin D especially among the CF patients (313.80±65.66), which presented less 61% of the RDA even in the control group. Also, inadequate sun exposure was prevalent among the studied patients especially among the CF patients (85%).
Similar to our results, recent studies [28, 29] demonstrated that the prevalence of vitamin D insufficiency in the CF population is up to 90% [3]. Also, large CF centers observed that >90% of patients had vitamin D levels <30 ng/mL [30]. In addition, Brodlie and colleagues [31] found that 90% of their pediatric CF population was vitamin D insufficient, and after increasing the supplementation dose, 49% remained insufficient.
Our results also go parallel with the findings of a case control study conducted on 402 adults with non-CF bronchiectasis by Chalmers et al. [4] which showed that the median serum 25-OHD was 24.7 nmol/l and 50% of patients with bronchiectasis were vitamin D deficient and 43% insufficient, only 7% sufficient and these percentages were significantly higher in comparison with only 12% in control group.
The higher level of vitamin D deficiency in our study may be explained by poor nutritional status with lower mean BMI (P=0.062), lower socioeconomic status with bad housing among vitamin D deficient non CF bronchiectasis patients (P= 0.040) (Table S1) and the prevalence of pancreatic insufficiency (72.2%) and severe CFTR mutations (53.2%) among vitamin D-deficient CF patients (Table S2) and the absence of routine screening of vitamin D level among the studied CF patients, inadequate sun exposure and inadequate dietary intake of vitamin D among the study participants (Table 2).
The higher prevalence of vitamin D deficiency and insufficiency among the normal healthy controls (45%) compared to previous studies [32, 33] conducted on vitamin D level among the healthy subjects should raise the awareness about the importance of adequate sun exposure and routine screening of vitamin D deficiency among the Egyptian children.
Our study has found that vitamin D deficiency among CF patients was significantly linked with more frequent and mostly moderate to severe pulmonary exacerbations compared to vitamin D sufficient and insufficient groups (P=0.033, < 0.001). Similar findings were reported among vitamin D deficient non-CF bronchiectasis patients, although it was statistically non-significant (P= 0.166, 0.232) (Tables 3 and 4).
These finding may indicate a relation between vitamin D deficiency and the bronchiectasis severity as reported by several studies that frequent pulmonary exacerbations are associated with a higher risk of future exacerbations [20] and higher mortality rates [21].
Our results were also in agreement with previous retrospective studies [30, 34, 35] which have indicated that low vitamin D status in children was associated with increased number of pulmonary exacerbations of CF [36].
Similarly, McCauley et al. [34] reported that serum vitamin D level less than or equal to 20 mg/L in CF children is associated with a three times higher rate of pulmonary exacerbations than those with vitamin D levels greater than or equal to 30 mg/L(sufficient). Furthermore, having a higher serum 25-hydroxyvitamin D in children was protective of having a pulmonary exacerbation during adolescence [36].
On the same hand, Chalmers et al. [4] highlighted that vitamin D deficient non-CF bronchiectasis patients have more frequent pulmonary exacerbations and 27.4% of deficient patients had an unscheduled hospitalization for a severe exacerbation compared to 19.7% of insufficient patients and 7.1% of sufficient patients.
The previous findings may raise the possibility of the use of vitamin D therapy as a therapeutic tool for prevention and treatment of pulmonary exacerbations in bronchiectasis patients.
The current study found that a higher percentage of bacterial colonization was found among vitamin D-deficient CF group than vitamin D insufficient and sufficient groups where chronic Pseudomonas infection was significantly higher among vitamin D deficient group (80%) than the other two groups (20%, 0%) (P=0.060) (Table 5). Similar findings were found among non-CF bronchiectasis patients where 75% of chronic pseudomonas infection and 57.1% of Staphylococcus aureus colonization were more prevalent among vitamin D-deficient group (P=0.215, 0.818) (Table 6).
These findings came different with what was reported in the literature that the most commonly isolated organisms in non-CF bronchiectasis children are non-type-able Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, and to a lesser extent Staphylococcus aureus and Pseudomonas aeruginosa [2]. This observation highlights the role of vitamin D deficiency in enhancing the growth of Pseudomonas aeruginosa and Staphylococcus aureus by probably influencing the innate immunity, decreasing the induction of cathelicidin [37].
Our results were consistent with the findings of Simoneau et al. [23] who found that Pseudomonas aeruginosa was a frequent pathogen in the CF patients who were vitamin D-insufficient/deficient as compared to patients with sufficient vitamin D status (29% prevalence compared with 13% prevalence in the vitamin D-sufficient group).
On the same side, Chalmers et al. [4] reported that non-CF bronchiectasis patients with vitamin D deficiency (25[OH]D less than 25 n mol/L) had more bacterial colonization and 21.4% of colonized patients had Pseudomonas aeruginosa colonization compared to 10.4% of insufficient patients and 3.6% of sufficient patients.
Our study also revealed that median (FEV1) % of predicted was significantly lower among vitamin D-deficient (66%) and insufficient (67%) non-CF bronchiectasis groups than among the sufficient group (80%) (p=0.071). The same findings were found among the CF patients, although the difference was not statistically significant (P=0.423) (Table 7)
Similar studies [2, 5, 30, 38] showed that higher vitamin D status in children and adults with CF has been associated with better lung function assessed by (FEV1).
On the same hand, Chalmers et al. [4] highlighted that vitamin-D-deficient patients had lower FEV1 % predicted (p=0.002); the median FEV1 was 68.0% (IQR 45.3–84.9%) in the vitamin D-deficient group. The study also demonstrated a more rapid decline of lung function over a 3-year follow-up period among vitamin D-deficient non-CF bronchiectasis patients.
On the contrary, previous studies [29, 39], have studied the prevalence of vitamin D deficiency in CF and examined its association with FEV1 but have found no association between vitamin D deficiency and FEV1.
Our results also showed the sensitivity of serum vitamin D for diagnosis of CF and non-CF bronchiectasis pulmonary exacerbations severity, where at a cutoff value equal or less than 22.5 ng/ml, serum vitamin D level has significantly differentiated moderate from mild pulmonary exacerbations (P= 0.004) (Fig. 1). While at a cutoff value equal or less than 16.5 ng/dl, serum vitamin D level has differentiated severe from moderate bronchiectasis pulmonary exacerbations (Fig. 2).
These findings suggest that serum vitamin D is playing an important role in determining the severity of pulmonary exacerbations which is the most contributing factor of bronchiectasis severity.
Conclusions
Vitamin D deficiency is prevalent among both CF and non-CF bronchiectasis pediatric patients (despite daily supplementation of the vitamin D in CF patients). In this population, vitamin D deficiency is associated with more severe and frequent pulmonary exacerbations, higher rates of chronic Pseudomonas infection, and lower lung function as measured by FEV1.
From these data, routine screening for vitamin D level is better to be done in both CF and non-CF bronchiectasis children. Additionally, further, large, prospective, multi-center randomized clinical trials should be conducted to justify the application of vitamin D supplementation as one of the therapeutic guidelines in all cases of bronchiectasis, not only in CF but also in non-CF bronchiectasis children.
Study limitation
The current study has some limitations. First, the sample size was relatively small. Second, the design was cross-sectional and only diseased cases were registered. Third, only pancreatic insufficient CF patients were included in the study. However, this study is one of the fewest studies that examined and compared the relation between vitamin D status and CF and non-CF bronchiectasis outcomes in pediatric patients
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CF:
-
Cystic fibrosis
- HRCT:
-
High resolution computed tomography
- RDA:
-
Recommended daily allowance
- BMI:
-
Body mass index
- PFTs:
-
Pulmonary function tests
- IQR:
-
Interquartile range
- AUC:
-
Area under the curve
References
Bastardo CM, Sonnappa S, Stanojevic S, Navarro A, Mondejar PL, Jaffe A, Bush A (2009) A.Non-cystic fibrosis bronchiectasis in childhood: longitudinal growth and lung function. Thorax 64:246–251
Moustaki M, Loukou I, Priftis KN, Douros K (2017) Role of vitamin D in cystic fibrosis and non-cystic fibrosis bronchiectasis. World J Clin Pediatr 6(3):132–142. https://doi.org/10.5409/wjcp.v6.i3.132
Chesdachai S, Tangpricha V (2016) Treatment of vitamin D deficiency in cystic fibrosis. J Steroid Biochem Mol Biol 164:36–39. https://doi.org/10.1016/j.jsbmb.2015.09.013
Chalmers JD, McHugh BJ, Docherty C, Govan JR, Hill AT (2013) Vitamin-D deficiency is associated with chronic bacterial colonization and disease severity in bronchiectasis. Thorax 68:39–47 [PMID: 23076388]. https://doi.org/10.1136/thoraxjnl-2012-202125
Finklea JD, Grossmann RE, Tangpricha V (2011) Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Adv Nutr 2(3):244–253. https://doi.org/10.3945/an.111.000398
Sezer RG, Aydemir G, Akcan AB et al (2013) Nanoduct sweat conductivity measurements in 2664 patients: relationship to age, arterial blood gas, serum electrolyte profiles and clinical diagnosis. J Clin Med Res 5(1):34–41. https://doi.org/10.4021/jocmr1191w
Colombo C, Ellemunter H, Houwen R, Munck A, Taylor C, Wilschanski M (2011) Guidelines for the diagnosis and management of distal intestinal obstruction syndrome in cystic fibrosis patients. J Cyst Fibros 10(2):S24–S28
Brower KS, Del Vecchio MT, Aronoff SC (2014) The etiologies of non-CF bronchiectasis in childhood: a systematic review of 989 subjects. BMC Pediatr 14:4 [PMID: 25492164]. https://doi.org/10.1186/s12887-014-0299-y
Chang AB, Bell SC, Torzillo PJ, King PT, Maguire GP, Byrnes CA, Holland AE, O’Mara P, Grimwood K (2015) Extended voting group. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand Thoracic Society of Australia and New Zealand guidelines. Med J Aust 202:130 [PMID: 25669469]. https://doi.org/10.5694/mjac14.00287
Debray D, Kelly D, Houwen R, Strandvik B, Colombo C (2011) Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 10(Suppl 2):S29–S36. https://doi.org/10.1016/S1569-1993(11)60006-4
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 58:259–263
Ross AC, Taylor CL, Yaktine AL, Del Valle HB. IOM (Institute of Medicine). Dietary reference intakes for calcium and vitamin D. 2011.
Abate A, Murugan R, Gualu T (2016) Assessment of practice and factors affecting sunlight exposure of infants among mothers attending governmental health facilities in debre markos town, East Gojjam, Ethiopia, 2015. Am J Nurs 5(1):30–36. https://doi.org/10.11648/j.ajns.20160501.15
Alshahrani FM, Almalki MH, Aljohani N, Alzahrani A, Alsaleh Y, Holick MF (2013) Vitamin D: Light side and best time of sunshine in Riyadh, Saudi Arabia. Dermato-Endocrinol 5(1):177–180
El-Gilany A, El-Wehady A, El-Wasify M (2012) Updating and validation of the socioeconomic status scale for health research in Egypt. East Mediterr Health J. 18(9):962–968. https://doi.org/10.26719/2012.18.9.962 PMID: 23057390
Shafique S, Akhter N, Stallkamp G, de Pee S, Panagides D, Bloem MW (2007) Trends of under- and overweight among rural and urban poor women indicate the double burden of malnutrition in Bangladesh. Int. J. Epidemiol. 36(2):449–457. https://doi.org/10.1093/ije/dyl306
American Thoracic Society (1995) Standardization of spirometry. Am J Respir Crit Care Med 152:1107–1136
Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstien BJ, Smith AL, Wohl ME (1994) Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. New Engl J Med 331(10):637–642
Pasteur MC, Bilton D, Hill AT (2010). British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 65(suppl 1): i1–58.
Chalmers JD, Aliberti S, Filonenko A et al (2018) Characterization of the ‘frequent exacerbator phenotype’ in bronchiectasis. Am J Respir Crit Care Med 197:1410–1420 Google Scholar
Martinez-Garcia MÁ, Athanazio R, Gramblicka G et al (2019) Prognostic value of frequent exacerbations in bronchiectasis: the relationship with disease severity. Arch Bronconeumol. 55:81–87 Google Scholar 1700629
Borowitz D, Robinson K, Rosenfeld M, Davis SD, Sabadosa KA, Spear SL et al (2009) Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatrics 155:S73–S93
Simoneau T, Bazzaz O, Sawicki GS, Gordon C (2014) Vitamin D status in children with cystic fibrosis. Associations with inflammation and bacterial colonization. Ann Am Thorac Soc 11:205–210 [PMID:24423241]. https://doi.org/10.1513/annalsats.201306-171bc
Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM (2003) Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2(1):29–34
Gleckman R, DeVita J, Hibert D et al (1988) Sputum gram stain assessment in community-acquired bacteremic pneumonia. J ClinMicrobiol. 26:846–849
Holick MF (2009) Vitamin D status: measurement, interpretation and clinical application. Ann Epidemoil. 19(2):73–78
Torky H, Hegab M, Kamel M, Eldin MS, Hussein HE (2017) The Egyptian formula a new formula for prediction of fetal birth weight using liver volume measured by three-dimensional ultrasound (VOCALSystem). MOJ Womens Health 4(2):00078. https://doi.org/10.15406/mojwh.2017.04.00078
Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V (2008) Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol 69(3):374–381 [PubMed: 18284636]
Rovner AJ, Stallings VA, Schall JI, Leonard MB, Zemel BS (2007) Vitamin D insufficiency in children, adolescents, and young adults with cystic fibrosis despite routine oral supplementation. Am J ClinNutr. 86(6):1694–1699 [PubMed: 18065588]
Wani WA, Nazir M, Bhat JI, Ahmad QI, Charoo BA, Ali SW (2019) Vitamin D status correlates with the markers of cystic fibrosis-related pulmonary disease. Pediatrics Neonatol 60(2):210–215. https://doi.org/10.1016/j.pedneo.2018.07.001 LicenseCC BY-NC-ND
Brodlie M, Orchard WA, Reeks GA, Pattman S, McCabe H, O’Brien CJ, Thomas MF, Spencer DA (2012) Vitamin D in children with cystic fibrosis. Arch Dis Child. 97:982–984
Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ (2004) Hypovitaminosis D in healthy adolescents. Arch Pediatr Adolesc Med 158:531–537
Weng FL, Shults J, Leonard M, Stallings V, Zemel B (2007) Risk factors for vitamin D deficiency in otherwise healthy children. Am J Clin Nutr. 86:150–158
McCauley LA, Thomas W, Laguna TA, Regelmann WE, Moran A, Polgreen LE (2014) Vitamin D deficiency is associated with pulmonary exacerbations in children with cystic fibrosis. Ann Am ThoracSoc. 11:198–204
Vanstone MB, Egan ME, Zhang JH, Carpenter TO (2015) Association between serum 25-hydroxyvitamin D level and pulmonary exacerbations in cystic fibrosis. Pediatric Pulmonol. 50(5):441–446 [PubMed: 25657016]
Tangpricha V, Lukemire J, Chen Y, Binongo J, Judd S, Michalski E, Lee M, Walker S, Ziegler T, Tirouvanziam R et al (2019) Vitamin D for the Immune System in Cystic Fibrosis (DISC): a double-blind, multicenter, randomized, placebo-controlled clinical trial. Am J Clin Nutr. 109(3):544–553. https://doi.org/10.1093/ajcn/nqy291
Gallo RL, Hooper LV (2012) Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12:503–516 [PMID: 22728527]. https://doi.org/10.1038/nri3228
Pincikova T, Nilsson K, Moen IE, Karpati F, Fluge G, Hollsing A, Knudsen PK, Lindblad A, Mared L, Pressler T, Hjelte L (2011) Scandinavian Cystic Fibrosis Study Consortium. Inverse relationbetween vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur J Clin Nutr 65(1):102–109
Green D, Carson K, Leonard A, Davis JE, Rosenstein B, Zeitlin P, Mogayzel P Jr (2008) Current treatment recommendations for correcting vitamin D deficiency in pediatric patients with cystic fibrosis are inadequate. J Pediatr. 153:554–559
Acknowledgements
The authors deeply appreciated the help of our patients to complete this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HA performed the study design and wrote the manuscript. TD shared in the study design and approved the manuscript. DM did the laboratory work, data interpretation, and paper drafting. MM did the patients’ enrollment and collection of data. All authors revised and approved the manuscript and agree to publish it in the Egyptian Pediatric Association Gazette (EPAG).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Research Ethics Committee of the Faculty of Medicine, Ain Shams University, approved the protocol. Written consent was obtained from the patients’ guardians. The reference number is not available at the time being.
Consent for publication
Nothing to declare
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The research was done in chest clinic and chest department, Ain Shams University Hospital, Cairo, Egypt.
Supplementary Information
Additional file 1.
Supplementary data. Tables S1–S3.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, H.A., Deraz, T.E., Mohamed, D.A. et al. Impact of vitamin D status on CF and non-CF bronchiectasis outcomes. Egypt Pediatric Association Gaz 70, 3 (2022). https://doi.org/10.1186/s43054-021-00095-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-021-00095-7