Abstract
Background
The quality of life of B-thalassemia major (β-TM) patients has improved with the use of frequent blood transfusions. However, this leads to chronic iron overload with its sequelae, as prediabetes and diabetes mellitus. This study aimed to assess insulin resistance and glucose abnormalities in a sample of B-thalassemia major patients in Benha, Egypt.
Results
This case-control study included 40 B-thalassemia major patients on regular blood transfusion and iron chelation. Their ages ranged from 8 to 16 years, and 30 normal age and sex-matched controls. Thorough clinical examination was performed including weight (kg), height (m), body mass index (BMI) (kg/m2), and liver and spleen size. Laboratory investigations were done in the form of complete blood count, liver enzymes, serum ferritin, fasting plasma insulin, and fasting, and 2 h postprandial plasma glucose. Insulin resistance (IR) was calculated using the Homeostasis Model Assessment of insulin resistance (HOMA-IR) index. Insulin resistance was found in 27.5% of thalassemic patients; 18.2% of them had diabetes, 72.7% were prediabetics (with impaired fasting glycemia), and 9.1% had normal fasting and 2 h postprandial plasma glucose level. Insulin resistance increased significantly with increased blood transfusion duration, serum ferritin, liver enzymes, fasting plasma insulin, fasting plasma glucose, and 2 h postprandial plasma glucose (ROC). The curve analysis showed that the duration of blood transfusion, serum ferritin, fasting plasma insulin, fasting, and 2 h postprandial plasma glucose could significantly predict insulin resistance at a certain cut-off point.
Conclusion
Our data show that HOMA-IR can be used to detect insulin resistance in β-TM patients on long-term blood transfusions, especially patients with high serum ferritin and impaired liver enzymes.
Similar content being viewed by others
Background
B-Thalassemia major (β-TM) patients’ life span and quality are highly dependent on regular blood transfusion, which comes with the cost of iron overload [1]. Despite advances in iron-chelating agents, iron overload remains a significant challenge in managing β-TM patients [2]. Diabetes mellitus (DM) is one of the important endocrinal disorders that happen due to iron overload. The precise mechanism of iron-induced diabetes is still unknown, but these three mechanisms are most likely to occur: insulin deficiency, insulin resistance (IR), and liver dysfunction. Evidence suggests that the most crucial factor in the pathogenesis of the disease’s clinical complications is oxidative stress (caused by iron accumulation) [3]. Both IR and impaired insulin secretion lead to impaired glucose tolerance and type 2 diabetes mellitus (T2DM) [4].
It is said that iron excess and its related oxidative stress can mediate pancreatic islet cell apoptosis resulting in reduced insulin secretory capacity [5]. Islet cells are also very susceptible to oxidative damage due to an almost exclusive dependence on mitochondrial glucose metabolism to secrete glucose-induced insulin and have a flawed system of antioxidant defense [6]. Recently, McClain and colleagues have demonstrated a high prevalence of irregular homeostasis of glucose in patients with hemochromatosis as well as impaired insulin secretion and insulin resistance [7].
In several pathophysiological states, IR broadly occurs. Several researchers have indicated that IR is already present in diabetic patients before blood glucose irregularities. Hyperinsulinemia and IGT, in other words, are both T2DM reserve forces. Even in subjects with normal glucose tolerance [8], hyperinsulinemia and IR are dangerous. Homeostasis Model Assessment (HOMA), which involves the measurement of only fasting plasma insulin and fasting plasma glucose, is the easiest and most widely used marker in clinical practice to measure IR [9].
Aim of the study
We aimed in this study to evaluate IR and glucose abnormalities in a sample of β-TM patients in Benha, Egypt.
Methods
Subjects
This case-control study was conducted on 40 children and adolescents with β-TM (27 males and 13 females), aged between 8 and 16 years and 30 normal, age and sex-matched controls. The diagnosis of the patients was confirmed by Hb electrophoresis. They were regularly transfused at the hematology units of the Pediatric Departments, at Benha University Hospitals and Benha Children’s Hospital. Informed consent was taken before conducting the study from parents of both cases and controls.
This research was approved by the ethical committee, Faculty of Medicine, Benha University.
Patients suffering from any acute illness, liver disease, or those previously diagnosed with DM were excluded from this study.
Methods
All cases were subjected to detailed history taking regarding blood transfusion duration and the chelation therapy details. Then, clinical examination including measurement of weight (kg), height (m), body mass index (BMI) (kg/m2), and liver and spleen size was performed for all the subjects. Laboratory investigations were done for all the subjects, including complete blood picture (CBC) (performed by Sysmex XS-8001 cell counter), liver enzymes; alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (performed by Biosystem A 15 autoanalyzer), and serum ferritin (performed by AIA 15 fluorescence, chemiluminescent immunoassay system, TOSOH Corporation, Tokyo, JAPAN). Moreover, fasting plasma glucose was obtained after 8 h of fasting (3 ml of venous blood (done by Glucose TR, SPINREACT), and at the same time, fasting plasma insulin level was collected (measured by DRG® Insulin ELISA, USA (EIA-2935)), then after taking a meal, 2 h postprandial plasma glucose (2 h pp plasma glucose) was obtained (2 ml of venous blood (done by Glucose TR, SPINREACT)).
DM and prediabetes diagnosis is based on the American Diabetes Association criteria [10]:
-Prediabetes (impaired fasting glycemia (IFG)) was diagnosed if fasting plasma glucose was 100-125 mg/dL (5.6-6.9 mmol/L).
-Diabetes was diagnosed if fasting plasma glucose was > 126 mg/dL (11.1 mmol/L).
Evaluation of the IR index was done using the Homeostasis Model Assessment (HOMA-IR) = (fasting plasma glucose (mmol/L) X fasting plasma insulin (μu/mL))/22.5). HOMA-IR value of ≥ 2.7 was considered to be an indicator of IR [11].
Statistical methods
The collected data were tabulated and analyzed using SPSS version 16 software (SpssInc, Chicago, ILL Company). Categorical data were presented as numbers and percentages, chi-square (χ2) and Fisher’s exact tests were used to analyze them. Quantitative data were tested for normality using the Shapiro-Wilks test, assuming normality at P > 0.05. Normally distributed variables were expressed as mean ± standard deviation and analyzed by St. “t” for two independent groups. Simultaneously, non-parametric data were presented as a median and inter-quartile range (IQR) and analyzed by Mann Whitney U test (ZMWU) test. Spearman’s correlation coefficient (rho) was used to assess the correlation between non-parametric variables. Receiver operator characteristic curve (ROC) curves were constructed to detect the studied parameters’ cut-off values to early diagnose IR among β-TM patients. P value ≤ 0.05 was considered significant(s), P > 0.05 was non-significant (NS), P ≤ 0.001 is highly significant (HS) [12].
Results
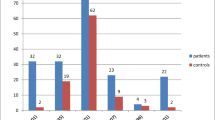
The patient group included forty thalassemic patients with a mean age of 11 ± 2.86 ys (range, 8-16 years); 67.5% were males (27 patients), and 32.5% were females (13 patients). The control group consisted of thirty healthy subjects with a mean age of 10.8 ± 2.49 years (range, 8-16 years); 60.0% were males (18 patients), and 40% were females (12 patients), with no statistically significant difference between the two groups (P = 0.51) (Table 1).
All patients were on iron chelating therapy (34 patients were on Desferasirox, four patients were on Deferiprone, and two patients were on combined therapy (deferasirox and deferoxamine)). Two of our patients were splenectomized.
Thalassemic patients had significantly higher serum ferritin, ALT, fasting, 2 h pp plasma glucose levels, and HOMA-IR than controls (Table 1).
A total of 27.5% of the thalassemic patients (11 patients) had IR, while none of the controls group had IR. Patients with IR included two patients with DM (18.2%), eight patients with high fasting plasma glucose (72.7%), and one patient with regular fasting and 2 h pp plasma glucose levels (no glycemic abnormality) (9.1%).
Patients with IR had a significantly longer duration of blood transfusions, higher serum ferritin, ALT, fasting plasma insulin, fasting, and 2 h pp plasma glucose than those with no IR (Table 2).
HOMA-IR showed a significant positive correlation with blood transfusion duration, ALT, serum ferritin (Fig. 1), fasting plasma glucose, and 2 h pp plasma glucose, and a highly significant positive correlation between HOMA-IR and fasting plasma insulin. There was a significant positive correlation between serum ferritin and fasting plasma insulin, fasting, 2 h pp plasma glucose levels, HOMA-IR, and ALT level. There was a significant positive correlation between fasting plasma insulin and serum ferritin and fasting and 2 h pp plasma glucose. There was a significant positive correlation between fasting plasma glucose and duration of blood transfusions and serum ferritin and a highly significant positive correlation with the 2 h pp plasma glucose. There was a significant positive correlation between the 2 h pp plasma glucose and serum ferritin (Table 3).
ROC curve analysis showed that the duration of blood transfusions (mean, 10.2 years), serum ferritin (mean, 3173 ng/ml), fasting insulin, fasting, and 2 h pp plasma glucose can significantly predict IR at the shown cut-off values (Table 4) (Fig. 2).
Discussion
Regular and frequent blood transfusions usage in patients with β-TM has improved patients’ lifespan and quality of life. However, it contributes to chronic iron overload, sometimes causing endocrine issues, mainly DMD [13].
In our study, 27.5% of the thalassemic patients (11 patients) had IR. Patients with IR included two patients with DM (18.2%), eight patients with high fasting plasma glucose (72.7%), and one patient with regular fasting and 2 h pp plasma glucose levels (no glycemic abnormality) (9.1%).
Patients with IR had a significantly longer duration of blood transfusions and higher serum ferritin, ALT, fasting plasma insulin, fasting, and 2 h pp plasma glucose compared with those with no IR.
This study showed that fasting plasma glucose and 2 h pp plasma glucose were significantly higher in thalassemic patients than the control group (P = 0,002 and P < 0.001, respectively), similar to the study of Metwally and El-Said [14]. Moreover, IR was significantly higher in patients than in controls (P = 0.015). This is consistent with that reported in a study on Chinese children with β-TM, where IR was significantly higher in patients than controls [15]. In this study, 5% of thalassemic patients had fasting plasma glucose in the range of the provisional diagnosis of diabetes. This is similar to the Egyptian study conducted by Metwalley and El-Saied, who reported that the incidence of diabetes was 5% among studied β-TM cases [14]. This is also nearly similar to a recent study that found that the prevalence of DM in β-TM patients was 6.54% [16].
The incidence of DM in our study was higher than the overall prevalence of diabetes in Chinese thalassemic children under 18 years, which was 2% [15]. Our results were also higher than the reported incidence in the study conducted by Bhat and Periasamy, where diabetes was not diagnosed in any of the β-TM patients included in their study [17].
Our study showed that there was a significant positive correlation between HOMA-IR in β-TM patients and duration of blood transfusion and serum ferritin, in agreement with the study of Hafez et al., who reported that there was a positive correlation between serum ferritin and HOMA IR [18], and in agreement with Bhat and Periasamy, who found that there was a progressive increase in IR with the increase in the number of units transfused and age [17]. Also, in agreement with Ansari et al., who found that the association between serum ferritin values and HOMA-IR index value was highly statistically significant (P < 0.001) [19].
In this study, HOMA-IR significantly increased with the increase in ALT level. This is similar to a study done by Liang et al., which reported a significant positive correlation between HOMA-IR, the gold standard marker of IR, and age, serum ferritin, and ALT levels, suggesting that the degree of iron overload and hepatic dysfunction were responsible for the IR [15]. This study demonstrated a significant positive correlation between serum ferritin and fasting plasma insulin, fasting plasma glucose and 2 h pp plasma glucose, and HOMA-IR. This was similar to the study, which reported that the ferritin level was positively correlated with the fasting plasma glucose and 2 h pp plasma glucose [20]. This study showed a highly significant positive correlation between serum ferritin in thalassemic patients and ALT level (P = 0.001). This was in agreement with Ezzat et al., who found a significant positive correlation between serum ferritin and liver enzymes; AST (r = 0.978, P ˂0.001), and ALT (r = 0.98, P ˂ 0.001) [21]. In our study, 11 patients had IR, two of them had DM, eight patients had IFG, and one patient did not have any glycemic abnormality, suggesting that IR precedes the glycemic abnormalities (prediabetes and DM). This was similar to the study of Soliman et al., who reported that three of their adolescents with B-TM showed IR state, one of them had DM, one had prediabetes, and the third one did not have any glycemic abnormality [20]. This state of IR may overwork the beta-cell function and, in addition to iron toxicity, leads to prediabetes and DM later.
In our study, there was a significant increase in DM, and prediabetes with the increase of the duration of blood transfusion, ALT, 2 h pp plasma glucose, and HOMA-IR than those with no glycemic abnormality, and there was a highly significant increase in serum ferritin of DM and IFG (prediabetic) than those with no glycemic abnormality.
The only patient in our study who had IR with no glycemic abnormality had the youngest age, the least duration of blood transfusion, and the least serum ferritin. This might show that IR precedes prediabetes and DM, which may develop after that with age progression and increased blood transfusion duration. This highlights the importance of good observation and close monitoring of glycemic parameters of these patients. However, to confirm our results, this requires more extensive longitudinal studies rather than cross-sectional studies. This was in agreement with Metwally and El-Saied. They found that fasting and 2 h pp glucose, fasting insulin, HOMA-IR, ALT, and serum ferritin levels showed a significant increase in thalassemic patients with DM and prediabetes than patients with standard glucose tolerance [14].
In this study, there was no significant difference between patients with IR and those with no IR regarding liver span and spleen size; this was in agreement with Bhat and Periasamy, who found that the size of the liver and spleen did not correlate with any of the parameters like IR, age, ferritin, number of transfusions or glycemic indices, like fasting glucose and fasting insulin [17].
ROC curve analysis in our study showed that the duration of blood transfusion at a cut-off value ≥ 10.2 years and serum ferritin at a cut-off value ≥ 3173 ng/dl could significantly predict IR, with a sensitivity of 63.6% and 81.8% and a specificity of 69% and 82.8%, respectively. Also, ROC curve analysis showed that fasting plasma glucose at a cut-off value ≥ 115.5 mg/dl, 2 h pp glucose at a cut-off value ≥ 162.5 mg/dl, and fasting plasma insulin at a cut off value ≥ 9.13 could significantly predict IR, with a sensitivity of 81.8% and a specificity of 79.3%, 62.15%, and 72.4%, respectively. This was in disagreement with Ghergherehchi and Habibzadeh, where they found that only ALT could predict DM occurrence, unlike other variables such as serum ferritin or blood transfusion duration [22].
Conclusion
Our results show that HOMA-IR can be used to detect IR in (β-TM) patients on long-term transfusions, especially patients with high serum ferritin and liver enzymes (ALT).
Recommendation
Adolescents and children with β-TM on long-term transfusions should be periodically monitored with glycemic indices and serum ferritin levels for early detection of DM. More efficient therapeutic strategies that could ameliorate insulin resistance should be considered in treating transfusion-dependent thalassemic patients.
Availability of data and materials
Collected from outpatient clinics pediatric department
Abbreviations
- β-TM:
-
B-Thalassemia major
- HOMA-IR:
-
Homeostasis Model Assessment of insulin resistance index
- IR:
-
Insulin resistance
- IFG:
-
Impaired fasting glycemia
- DM:
-
Diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- PP:
-
Postprandial
- BMI:
-
Body mass index
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ROC curve:
-
Receiver operator characteristic curve
- HS:
-
Highly significant
- NS:
-
Non-significant
- CBC:
-
Complete blood count
References
Musallam KM, Cappellini MD (2012) AT Wood Taher. Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood reviews. 26:16–S9
Koohi F, Kazemi T, Miri-Moghaddam E (2019) Cardiac complications and iron overload in beta thalassemia major patients—a systematic review and meta-analysis. Annals of hematology. 98(6):1323–1331
Swaminathan S, Fonseca VA, Alam MG et al (2007) The role of iron in diabetes and its complications. Diabetes Care. 30:1926–1933
Praveen EP, Sahoo J, Khurana ML et al (2012) Insulin sensitivity and 훽-cell function in normoglycemic offspring of individuals with type 2 diabetes mellitus: impact of line of inheritance, Indian. Journal of Endocrinology and Metabolism 16(1):105–111
American Diabetes Association (2004) Diagnosis and classification of diabetes mellitus. Diabetes Care. 27:S5–S10
Tiedge M, Lortz S, Drinkgern J et al (1997) Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 46:1733–1742
McClain DA, Abraham D, Rogers J et al (2006) High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia. 49:1661–1669
Rubins HB, Robins SJ, Collins D et al (2002) Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs High-density Lipoprotein Intervention Trial (VA-HIT). Arch Intern Med 162(22):2597–2604
Govindarajan G, Gill H, Rovetto M et al (2006) what is insulin resistance? Heart Metab 30:30–34
American Diabetes Association, Diabetes Care 2020; 43 (Supplement 1): 14–S31 https://doi.org/10.2337/dc20-S002.
Isiklar A, Karsidag K (2018) Association between thalassemia trait and insulin resistance. Acta Medica Mediterranea. 34:323–327
Khothari CR (2004) Research methodology: methods and techniques. New Age International, New Delhi
Delvecchio M, Cavallo L (2010) Growth and endocrine function in thalassemia major in childhood and adolescence. J Endocrinol Invest 33:61–68
Metwalley KA, El-Saied AA (2014) Glucose homeostasis in Egyptian children and adolescents with β-Thalassemia major. Indian J Endocr Metab 18:333–339
Liang Y, Bajoria R, Jiang Y et al (2017) Prevalence of diabetes mellitus in Chinese children with thalassaemia major. Trop Med Int Health 22(6):716–724
He L, Chen W, Yang Y, Xie Y et al (2019) Elevated prevalence of abnormal glucose metabolism and other endocrine disorders in patients with B-thalassemia major: a meta-analysis. Biomed Res Int 2019:1–13
Bhat KG, Periasamy PK (2016) Effect of long-term transfusion therapy on the glycometabolic status and pancreatic beta cell function in patients with beta thalassemia major. J Fam Med Primary Care. 3:119–123
Hafez M, Yousry I, Hamed FA et al (2009) abnormal glucose tolerance in B-thalassemia major. Hemoglobin 33(2):101–108
Ansari AM, Bhat K G, Dsa SS et al (2018) Study of insulin resistance in patients with B-thalassemia major patients and validity of TYG index. J Pediatr Hematol Oncol 40(2):128–131
Soliman AT, Yasin M, El-Awwa A, De Sanctis V (2013) Detection of glycemic abnormalities in adolescents with beta thalassemia using continuous glucose monitoring and oral glucose tolerance in adolescents and young adults with β-thalassemia major: pilot study. Indian J Endocrinol Metab 17(3):490–495
Ezzat AM, Abdelmotaleb GS, Shaheen AM, Ismail YM, Diab AM (2016) Peroxidative stress and antioxidant enzymes in children with b-thalassemia major. Medical Research Journal 15:57–62
Ghergherehchi R, Habibzadeh A (2015) Insulin resistance and beta cell function in B-thalassemia major patients. Hemoglobin 39(1):69–73
Acknowledgements
The authors thank all included patients.
Funding
This study had no funding from any resource.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. AD: Writing this manuscript and collecting data. GA: Share in writing this manuscript and revising data. KE: Collecting data and revising data. EM: Laboratory step. EA: Writing this manuscript and collecting data
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research accepted by the Research Ethics Committee (REC) of the Faculty of Medicine, Benha University (chairman: Prof/ Nermeen Adly Mahmoud), Ethics committee reference number RC 1.11.2020.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. A written informed consent was obtained from each patient after explaining all steps of this study.
Consent for publication
All patients or their parents have been consented for taking their laboratory results for scientific researches
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
M. Diab, A., S. Abdelmotaleb, G., Abdel-Azim Eid, K. et al. Evaluation of glycemic abnormalities in children and adolescents with β-thalassemia major. Egypt Pediatric Association Gaz 69, 9 (2021). https://doi.org/10.1186/s43054-021-00052-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-021-00052-4