Abstract
Background
Breast cancer (BC) and its treatment affect women's tissue architecture and physiology, which leads to impaired muscle strength and joint dysfunction, affecting quality of life (QOL). Most evidence has focused on exercises; however, due to the complexity and heterogeneity of patients’ rehabilitation needs, further research is required to investigate more adjunctive methods to help optimal rehabilitation according to patients’ needs, preferences, and effective interventions.
Methods
This study aimed to determine the effect of Kinesiotaping (KT) combined with resistive exercise on muscle strength and QOL in breast cancer survivors (BCS). Forty premenopausal BCS treated with chemotherapy postmastectomy participated in this study. Their age ranged from 40 to 55 years, and their body mass index (BMI) was 25–29.9 kg/m2. They were randomly distributed into two equal groups. The control group received resistive exercise two times/week for 12 weeks, while the study group received resistive exercise and KT applied to the lower limbs. Hip, knee, and ankle muscle strength were measured using a hand-held dynamometer, and QOL was evaluated using 36-Item Short Form (SF-36) before and after treatment.
Results
Both groups showed a significant increase (p = 0.0001) in the strength of hip flexors, knee extensors, flexors, ankle plantar flexors, and dorsiflexors, as well as SF-36 score after treatment. However, the study group showed a more significant increase in strength of hip flexors (p = 0.005), knee extensors (p = 0.01) and flexors (p = 0.02), ankle plantar flexors (p = 0.01), and dorsiflexors (p = 0.01), as well as SF-36 score (p = 0.006) than the control group.
Conclusions
KT plus resistive exercise is more effective than exercise alone for improving muscle strength and QOL in BCS. So, the KT can be recommended as a non-invasive, adjunctive method added to the protocol therapy for BCS to help better outcomes during the rehabilitation period.
Similar content being viewed by others
Background
Breast cancer (BC) is the most prevalent cancer in women all over the world. Advancements in diagnostics and treatment have led to a notable rise in the survival rate, which presents novel challenges for healthcare providers to help patients attain optimal rehabilitation [1]. Several factors increase the risk of developing cancers, which include a sedentary lifestyle, smoking, alcoholism [2], or obesity [3].
Most patients suffer from a muscle-wasting syndrome called cachexia [4] before and after cancer treatment [5]. Cachexia is the continued loss of muscle mass with or without weight loss [6]; it results from systemic inflammation and catabolic stimuli, inhibiting protein synthesis and enhancing muscle catabolism [7].
Additionally, the use of chemotherapy may result in decreased muscle strength and joint dysfunction, which impact the quality of life (QOL). Patients showed 25% lower strength in the maximal voluntary isometric contraction of lower extremities compared with healthy women [8] and reported a reduction of QOL in the aspects of physical function, and body image [9]. Decreased muscle strength and mass negatively affect patients’ performance status [8], tolerance to chemotherapy, and prognosis in both medical and surgical cancer patients [10], and lead to morbidity and mortality in advanced cancer [4, 6, 8].
Most of the research has focused on how exercise can help cancer patients manage chemo-related adverse effects [11] such as fatigue, anxiety, poor QOL [12], and impaired muscle strength. Despite the benefits of resistive exercises, and due to the complexity of the patient’s rehabilitation needs, further clinical studies are required to individualize treatment [13] and to determine the best rehabilitation for patients based on their needs, preferences, and effective interventions [14, 15]. Furthermore, a recent systematic review found that maximum benefits in muscle strength and hypertrophy require light loads with high repetitions or high-load training, which might not be suitable for all subjects. It suggested that other approaches for maximizing motor unit recruitment may be just as productive without performing high repetitions with light loads [16].
Kinesiotaping (KT) is a therapeutic modality used alone or combined with exercise, which improves isokinetic muscle strength due to its facilitative action on the muscles [17, 18]. KT showed superiority over other non-invasive interventions for muscle fatigue recovery [19], increased isometric muscle strength, and decreased muscle soreness after intensive exercise compared with the placebo and stretch groups [20]. However, there is a need for more high-quality and long intervention period research describing the application direction and the tension amount used [17, 18].
To the best of our knowledge, there were no studies investigating the effect of adding KT to strength exercises in BCS to determine the best clinical intervention, that would counteract and improve chemotherapy-related side effects and improve functional performance during the rehabilitation period. Therefore, the present study aimed to investigate the effect of combined KT and resistive exercise on muscle strength and QOL in BCS compared to resistive exercise alone. The hypothesis was that adding KT to resistive exercise would improve muscle strength and QOL than exercise alone in BCS.
Methods
Participants
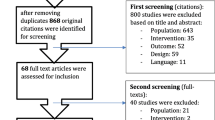
The study is a prospective, controlled, randomized clinical trial. A physician recruited a sample of 46 sedentary overweight middle-aged women diagnosed with BC stage I–III from the Outpatient Clinic, Oncology Hospital. Their ages ranged from 40 to 55 years, and their body mass index (BMI) was 25 to 29.9 kg/m2. They had regular menstrual cycles. All BCS received chemotherapy for 1–12 weeks after mastectomy. The exclusion criteria included respiratory or heart problems affecting mobility, lymphedema, marked skeletal deformity, neuropathy, visual system affection, cognition problems, previous surgeries at their back or lower limbs, or skin over-sensitivity to tape.
Out of 46 participants, two did not meet the inclusion criteria, two refused to sign a consent form, and two did not accept to join the study (Fig. 1). A blinded investigator created a computer-generated simple randomization sequence to allocate participants using a sealed envelope procedure to the control or study group. The control group performed a resistive exercise program two times/week for 12 weeks, while the study group received the same program and application of KT for 12 weeks. However, due to the nature of the intervention, the participants were aware of the group allocation. The data analysis and the primary outcome assessors were blinded to the participant allocation. No subjects dropped out of the study.
Based on a prior study [21], the sample size was calculated using the statistical program G*POWER (version 3.1.9.2; Franz Faul, University Kiel, Germany). It revealed that the sample size required was 20 subjects in each group with α = 0.05, power = 80%, and effect size = 0.91.
Outcome measures
1-Assessment of muscle strength
A hand-held dynamometer (HHD) (Lafayette Instrument Company, IN 47904 USA) was the method used to record the peak isometric force of the hip flexors, knee flexors, and extensors, as well as ankle plantar flexors and dorsiflexor muscles. It consisted of a lightweight (10.6 oz) microprocessor-control unit that measured peak force in kilograms while storing up to 52 tests. It allowed test times ranging from 1–10 s and had an audible tone indicating the end of the preset time. The unit provided a built-in calibration routine that verified a valid calibration. It was a valid and reliable tool for measuring lower limb muscle isometric strength among adults [22, 23].
Testing positions and strength assessments were performed as described by Mentiplay et al. [24]. Before starting, the manufacturer validated the dynamometer. Each patient maintained contraction for each muscle group for 5 s with a one-minute rest between measurements to decrease fatigue impact. An examiner blinded to the patient’s assignment performed the measurements three times and recorded the averages before and after 12 weeks of the treatment.
2-Assessment of QOL
The most common tool used to measure health-related QOL, especially among cancer patients, is the 36-item short-form survey (SF-36). This survey evaluated perceived health status across broad physical and emotional health domains. It comprised 36 questions that covered eight domains of health in BCS. It assessed physical functioning, role limitations due to physical health, bodily pain, general health, energy/fatigue, social functioning, role limitations due to emotional problems, and emotional well-being. Each patient received thorough instructions about the survey and enough time to fill it out. She gave each question a score between 0 and 100, with 100 indicating the maximum degree of functioning. The investigator calculated the final score by averaging the results of the questions that addressed each domain [25] before and after the 12-week course of treatment.
Interventions
A-Resistive exercises
The resistive exercise program included warm-up, active, and cool-down phases. Warm-up and cool-down phases consisted of 5 to 10 min of stretching exercise for the pectorals major, hamstring, hip flexors, and calf muscles, with each static stretch lasting at least 20 s in the first week and then 30 s for the remaining 11 weeks. In the active phase, strengthening exercises using sandbags were applied for hip flexors, knee flexors and extensors, and ankle plantar flexors and dorsiflexors. The therapist determined one repetition maximum (1RM) for each muscle group, which was the maximum weight the patient lifted for one time. Then, the load applied was 50–80% of 1RM along the treatment course. Each exercise repetition was 8–12 times, and the number of sets (1–3) continuously increased over the first 2–3 weeks. The program lasted approximately 60 min, twice a week, for 12 weeks [13].
B-Kinesiotaping application
Elastic Ares KT (made in Korea) was applied to the muscles of the lower extremities. For sartorius, the I-strip extended from the anterior superior iliac spine to the medial edge of the patella; for rectus femoris, the I-strip extended from the anterior inferior iliac spine to the patella upper edge [26]. Also, two I-strip were applied, one for the semimembranosus/semitendinosus from the ischial tuberosity to the posterior surface of the medial tibial condyle and the other for the biceps femoris from the ischial tuberosity to the fibular head posterior surface [27]. The patellar KT application used two Y strips cut about 2/3 of the length of the strip down the middle to create two tails; the uncut portion of one Y-strip was above the superior portion of the patella with the tails winged medially and laterally to the medial and lateral femoral condyles, respectively; the other uncut portion was above on the tibial tuberosity with the tails winged medially and laterally around the patella [28]. The tibialis anterior KT application used one I-strip extending from the tibia proximal lateral portion to the first metatarsal and medial cuneiform [29]. Each strip was placed with a 20–25% stretch and downward pressure, with no tension applied to the first and last inch of the strip. The study group received KT applications two times/week for 12 weeks.
Statistical analysis
The Statistical Package for Social Studies (SPSS) version 25 for Windows (IBM SPSS, Chicago, IL, USA) was the software used for all statistical analysis. The Shapiro–Wilk test evaluated the normal distribution of data. Levene’s test for homogeneity of variances revealed the homogeneity between groups. Mixed MANOVA was the method used to investigate the effect of treatment. The Bonferroni correction was the post-hoc test used for subsequent multiple comparisons. The level of significance for all statistical tests was at p < 0.05.
Results
Table 1 represents the patient characteristics of the control and study groups; unpaired t-tests showed no significant difference between groups in age (p = 0.16), weight (p = 0.16), height (p = 0.24), BMI (p = 0.29), and duration of chemotherapy (p = 0.46).
As shown in Table 2, the control and study groups showed a significant increase (p = 0.001) in the strength of hip flexors, knee extensors and flexors, ankle plantar flexors, and dorsiflexors, as well as the SF-36 score after treatment. Compared to the control group, the study group showed a significant increase in the strength of hip flexors (p = 0.005), knee extensors (p = 0.01), flexors (p = 0.02), ankle plantar flexors, and dorsiflexors (p = 0.01), as well as the SF36 score (p = 0.006). None of the patients reported discomfort, limitations of motion, or other side effects while using KT.
Discussion
BC women suffer from many adverse effects resulting from chemotherapy such as fatigue, and impaired muscle strength, which affect QOL [30]. Due to complicated patient rehabilitation needs, further studies are warranted to investigate adjunctive modalities to achieve maximum benefits for patients’ outcomes during the treatment course. Therefore, the current study aimed to determine the effect of adding KT to resistive exercise on the lower limb muscle strength and QOL compared to exercise alone in BCS. Results revealed that adding KT to resistive exercise showed more improvement in the hip flexors, knee flexors, extensors, ankle plantar flexors, and dorsi flexors strengths, as well as QOL than resistive exercise alone.
A recent systematic review [11] confirmed the results about the efficacy of resistive activities. It suggested resistive exercise as part of supportive treatment for BCS following anticancer treatment because it improved QOL, muscle strength, endurance, physical performance, mental function, and leisure time in BCS. Exercise-induced benefits could be related to lowered cytokines, which play a significant role in cancer development; however, this was not addressed in this study [31]. Resistive exercise may counteract some mechanisms underlying muscle wasting in cancer patients by reducing the production of pro-inflammatory cytokines and enhancing the phosphorylation of intramuscular amino acid-signaling molecules [32, 33]. Also, exercise enhances metabolism and metabolic phenotype in a variety of tissues, which are mediated in part by transcriptional responses [34]; the increase in PGC1α, a transcriptional coactivator, in response to exercise, activates branched-chain amino acid (BCAA) metabolism, which increases protein synthesis [35]. Increased muscle content of proteins is associated with enhanced protein stability and may be attributed to a chaperone-dependent mechanism and/or reduced regulation by proteolysis [36]. These adaptive responses increase oxidative capacity [34]. Moreover, resistive exercise increases the activity of cellular antioxidant enzymes such as superoxide dismutase 1 (SOD1), SOD2, catalase (CAT), and peroxidase (GPX), which improve antioxidant capacity and protect against oxidative stress induced by contraction, delaying muscle fatigue [37]. Moreover, increased muscle strength may result from neuromuscular adaptations resulting from increased motoneuron recruitment and excitability which enhance muscle cell activation during exercises [16].
KT plus resistive exercises improved QOL, which was a significant predictor of better treatment outcomes [38]. Previous research showed the same finding; one study has reported that KT applied for 5 weeks achieved significant change in QOL variables, which included global health, fatigue, pain, and well-being in BCS treated with an Aromatase inhibitor [39]. Another study has shown that KT and lifestyle changes improved physical function, pain, and QOL in women with dysmenorrhea [40].
Regarding the effect of KT on muscle strength, the results confirmed earlier findings of previous studies. They reported that KT increased isometric muscle strength and reduced muscle soreness after intensive exercise [20], and increased muscle torque of the vastus medialis, laterals, and rectus femoris [26].
However, these findings contraindicated previous studies; they found no substantial difference in QOL [41] in women with chronic venous insufficiency and the quadriceps muscle strength after short application of KT in healthy subjects [19] and patients with osteoarthritis [17, 18]. These contradictions may result from the difference in the participants’ muscle strength and duration of the KT treatment among studies.
The more significant increase in the muscle strength in the group receiving KT and exercise than exercise alone may result from the additive facilitative action of KT on the muscles, which was supported by the electromyography (EMG) findings of increased activity of the vastus medialis muscle following 24 h of using KT [26]; KT could induce tactile stimulation, which results in the firing of large-diameter afferent fibers and the inhibition of small-diameter afferent fibers, hence limiting pain transmission, decreasing muscular soreness, and improving muscle strength. It may also help with muscle strengthening by transmitting a pulling force to the muscle and fascia and activating mechanoreceptors; application of KT in the direction of the muscle contraction could enhance the muscle spindle reflex and increase the excitability of the motor units, improving the intensity of the muscle stimulation. Also, KT could raise the skin away from the underlying fascia, promoting blood and lymphatic flow, which enhances muscle activity and increases oxygen distribution to the muscle. Also, KT could improve muscle fatigue recovery after exercise, enhancing exercise performance [19]. However, the physiological underlying mechanisms, such as protein synthesis and antioxidant enzyme activity in response to KT application, are still unknown and require more research.
Limitations
There are some limitations to the current study. Firstly, this study does not investigate the mechanisms underlying the effect of combined resistive exercise and KT. Therefore, further research is warranted to investigate the effect of combined interventions on cytokines in BCS. Secondly, the study is restricted to a narrow range of women’s ages and BMI, so more research is needed to assess the effect of the interventions using large sample sizes with different ages, a wide range of BMI, and more objective instruments like EMG to investigate muscle activity and isokinetic to assess muscle strength and torque. Thirdly, the long-term effect of the interventions and follow-up in BCS needs to be investigated. Fourthly, there is a need to determine the effect of the application of KT to the back muscles on back muscle activities, strength, and torque in BCS.
Conclusions
KT plus resistive exercise is more effective than exercise alone to counteract and improve related side effects of chemotherapy such as impaired muscle strength and poor QOL. KT can be added to the protocol therapy of cancer as it enhances the functional performance of the BCS.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- BC:
-
Breast cancer
- BCS:
-
Breast cancer survivors
- BMI:
-
Body mass index
- HHD:
-
Hand-held dynamometer
- KT:
-
Kinesiotaping
- 1RM:
-
One repetition maximum
- QOL:
-
Quality of life
References
Rodríguez-Cañamero S, Cobo-Cuenca AI, Carmona-Torres JM, Pozuelo-Carrascosa DP, Santacruz-Salas E, Rabanales-Sotos JA, Cuesta-Mateos T, Laredo-Aguilera JA. Impact of physical exercise in advanced stage cancer patients: Systematic review and meta-analysis. Cancer Med. 2022;11:3714–27.
Ng R, Sutradhar R, Yao Z, Wodchis WP, Rosella LC. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int J Epidemiol. 2020;49:113–30.
Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep. 2019;21:41.
Biswas AK, Acharyya S. Understanding cachexia in the context of metastatic progression. Nat Rev Cancer. 2020;20:274–84.
Pin F, Barreto R, Couch ME, Bonetto A, O’Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle. 2019;10:140–54.
Sadeghi M, Keshavarz-Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: diagnosis, assessment, and treatment. Crit Rev Oncol Hematol. 2018;127:91–104.
Setiawan T, Sari IN, Wijaya YT, Julianto NM, Muhammad JA, Lee H, et al. Cancer cachexia: molecular mechanisms and treatment strategies. J Hematol Oncol. 2023;22(16):54.
Klassen O, Schmidt ME, Ulrich CM, Schneeweiss A, Potthoff K, Steindorf K, et al. Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle. 2017;8:305–16.
Ettridge K, Scharling-Gamba K, Miller C, Roder D, Prichard I. Body image and quality of life in women with breast cancer: Appreciating the body and its functionality. Body Image. 2022;40:92–102.
Bozzetti F. Chemotherapy-induced sarcopenia. Curr Treat Options Oncol. 2020;21:7.
Schutz S, Aidar FJ, Souza RLM, Dos Santos JL, Voltarelli FA, Vieira Junior RC, et al. Different methods of physical training applied to women breast cancer survivors: A Systematic Review. Front Physiol. 2021;12:639406.
Moraes RF, Ferreira-Júnior JB, Marques VA, Vieira A, Lira CAB, Campos MH, et al. Resistance training, fatigue, quality of life, anxiety in breast cancer survivors. J Strength Cond Res. 2021;35:1350–6.
Montaño-Rojas LS, Romero-Pérez EM, Medina-Pérez C, Reguera-García MM, de Paz JA. Resistance training in breast cancer survivor: a systematic review of exercise programs. Int J Environ Res Public Health. 2020;17:6511.
Gerland L, Baumann FT, Niels T. Resistance exercise for breast cancer patients? evidence from the last decade. Breast care (Basel). 2021;16:657–66.
Olsson Möller U, Beck I, Rydén L, Malmström M. A comprehensive approach to rehabilitation interventions following breast cancer treatment - a systematic review of systematic reviews. BMC Cancer. 2019;19:472.
Lopez P, Radaelli R, Taaffe DR, Newton RU, Galvão DA, Trajano GS, et al. Resistance training load effects on muscle hypertrophy and strength gain: systematic review and network meta-analysis. Med Sci Sports Exerc. 2021;53:1206–16.
Mao HY, Hu MT, Yen YY, Lan SJ, Lee SD. Kinesio taping relieves pain and improves isokinetic not isometric muscle strength in patients with knee osteoarthritis- a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:10440.
Wu H, Yao R, Wu J, Wen G, Wang Y. Does kinesio taping plus exercise improve pain and function in patients with knee osteoarthritis?: a systematic review and meta-analysis of randomized controlled trials. Front Physiol. 2022;13:961264.
Yam ML, Yang Z, Zee BC, Chong KC. Effects of Kinesio tape on lower limb muscle strength, hop test, and vertical jump performances: a meta-analysis. BMC Musculoskelet Disord. 2019;20:212.
Boobphachart D, Manimmanakorn N, Manimmanakorn A, Thuwakum W, Hamin MJ. Effects of elastic taping, non-elastic taping and static stretching on recovery after intensive eccentric exercise. Res Sports Med. 2017;25:181–90.
Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and QOL in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer. 2015;137:471–80.
Chamorro C, Armijo-Olivo S, De la Fuene C, Fuentes J, Javier CL. Absolute reliability and concurrent validity of hand held dynamometry and isokinetic dynamometry in the hip, knee and ankle joint: Systematic review and meta-analysis. Open Med (Wars). 2017;12:359–75.
Pinto-Ramos J, Moreira T, Costa F, Tavares H, Cabral J, Costa-Santos C, et al. Hand held dynamometer reliability to measure knee extension strength in rehabilitation patients-a cross-sectional study. PLoS one. 2022;17:e0268254.
Mentiplay BF, Perraton LG, Bower KJ, Adair B, P YH, Williams GP, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. Plos One, 2015;10:e0140822.
Bunevicius A. Reliability and validity of the SF-36 Health Survey Questionnaire in patients with brain tumors: a cross-sectional study. Health Qual Life Outcome. 2017;15:92.
Choi I, Lee J. Effect of kinesiology tape application direction on quadriceps strength. Medicine. 2018;97:e11038.
Christofel HK, da Silva RA, Masser El Afch FH, da Escobar Silva L, Pires OI, Iida LM, et al. Evaluation of the effects of different applications of kinesio taping on postural control in healthy women. J Bodyw Mov Ther. 2021;28:1–5.
Donec V, Kubilius R. The effectiveness of Kinesio Taping® for mobility and functioning improvement in knee osteoarthritis: a randomized, double-blind, controlled trial. Clin Rehabil. 2020;34:877–89.
Saki F, Romiani H, Ziya M, Gheidi N. The effects of gluteus medius and tibialis anterior kinesio taping on postural control, knee kinematics, and knee proprioception in female athletes with dynamic knee valgus. Phys Ther Sport. 2022;53:84–90.
Marques VA, Ferreira-Junior JB, Lemos TV, Moraes RF, Junior JRS, Alves RR, et al. Effects of chemotherapy treatment on muscle strength, quality of life, fatigue, and anxiety in women with breast cancer. Int J Environ Res Public Health. 2020;17:7289.
Lan T, Chen L, Wei X. Inflammatory cytokines in cancer: Comprehensive understanding and clinical progress in gene therapy. Cells. 2021;10:100.
Liu YC, Hung TT, Konara Mudiyanselage SP, Wang CJ, Lin MF. Beneficial exercises for cancer-related fatigue among women with breast cancer: a systematic review and network meta-analysis. Cancers (Basel). 2022;15:151.
Khosravi N, Stoner L, Farajivafa V, Hanson ED. Exercise training, circulating cytokine levels and immune function in cancer survivors: a meta-analysis. Brain Behav Immun. 2019;81:92–104.
McGee SL, Hargreaves M. Exercise adaptions: molecular mechanisms and potential targets for therapeutics benefits. Nat Rev Endocrinol. 2020;16:495–505.
Kamei Y, Hatazawa Y, Uchitomi R, Yoshimura R, Miura S. Regulation of skeletal muscle function by amino acids. Nutrients. 2020;12:261.
Makhnovskii PA, Zgoda VG, Bokov RO, Shagimardanova EI, Gazizova GR, Gusev OA, et al. regulation of proteins in human skeletal muscle: The role of transcription. Sci Rep. 2020;26(10):3514.
Powers SK, Goldstein E, Schrager M, Ji LL. Exercise training and skeletal muscle antioxidant enzymes: An update. Antioxidants (Basel). 2022;12:39.
Marta GN, Moraes FY, Leite ET, Chow E, Cella D, Bottomley A. A critical evaluation of quality of life in clinical trials of breast cancer patients treated with radiation therapy. Ann Palliat Med. 2017;6:S223–32.
Conejo I, Pajares B, Alba E, Cuesta-Vargas AI. Effect of neuromuscular taping on musculoskeletal disorders secondary to the use of aromatase inhibitors in breast cancer survivors: a pragmatic randomized clinical trial. BMC Complement Altern Med. 2018;18:180.
Doğan H, Eroğlu S, Akbayrak T. The effect of kinesio taping and lifestyle changes on pain, body awareness and quality of life in primary dysmenorrhea. Complement Ther Clin Pract. 2020;39:101120.
Ferreira R, Resende R, Roriz P. The effects of Kinesio Taping in lower limb musculoskeletal disorders: a systematic review. Int J Ther Rehabil Res. 2017;6:1.
Acknowledgements
We would like to thank everyone who contributed to completing this work.
Funding
This research received no specific grant from the public, commercial, or not-for-profit funding agencies.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design, data collection, statistical analyses, interpretation of data, and manuscript preparations. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethical Committee of the Faculty of Physical Therapy, Cairo University (No: P.T.REC/0.12/004048). Also, it was approved by the Secretariat of Specialized Medical Centers, Ministry of Health, Egypt. Each patient signed an informed consent and was given a detailed explanation of the protocol at the start of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramadan, A.M., ElDeeb, A.M., Ramadan, A.A. et al. Effect of combined Kinesiotaping and resistive exercise on muscle strength and quality of life in breast cancer survivors: a randomized clinical trial. J Egypt Natl Canc Inst 36, 1 (2024). https://doi.org/10.1186/s43046-023-00205-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43046-023-00205-z