Abstract
Background
Ameloblastic fibro-dentinoma is considered a rare, benign, mixed odontogenic tumor that occurs mainly in the posterior mandible in the 1st–2nd decade of life. Although the clinical behavior of Ameloblastic fibro-dentinoma is similar to that of ameloblastic fibroma, there is a debate about whether Ameloblastic fibro-dentinoma is a developing hamartomatous odontoma or a separate neoplastic odontogenic tumor like ameloblastic fibroma. However, it is important to understand the histopathogenesis of this rare tumor.
Case presentation
A case report presenting an 11-year-old male child with a swelling in the posterior mandible. Radiographic examination revealed a multilocular lesion with mixed radiodensity related to the impacted lower left second premolar tooth. Incisional biopsy was done, and microscopic examination revealed cords and nests of odontogenic follicles lined by ameloblast-like cells and central stellate reticulum-like cells in the primitive ecto-mesenchymal stroma with areas of dentinoid material and osteodentin. The diagnosis was ameloblastic fibro-dentinoma. Surgical excision of the lesion was done, and the patient was followed up for 1 year without evidence of recurrence.
Conclusion
Reporting such a rare entity clarifies the debate about its nature and the importance of early diagnosis of lesions that are associated with unerupted teeth showing how it is effective in early management and prognosis.
Similar content being viewed by others
Background
Ameloblastic fibro-dentinoma (AFD) accounts for 2% of all odontogenic tumors. It occurs most frequently at a young age, with the mean patient age being 15 years. This tumor predominantly affects the mandible especially the posterior region more than the maxilla with slight male predilection [1, 2] (WHO, 2022). AFD is very similar to ameloblastic fibroma (AF) and ameloblastic fibro-odontoma (AFO). Clinically, it is a slow-growing asymptomatic tumor, it may be associated with impacted or unerupted teeth additionally it could cause cortical perforation. Radiologically, it appears as a well-defined radiolucency with varying degrees of radiopacity [3].
Histologically AFD is composed of odontogenic epithelium, immature connective tissue, and is characterized by the formation of dysplastic dentin [3]. The odontogenic epithelium is in the form of strands and small islands like dental lamina and enamel organs in a highly cellular primitive ectomesenchyme like dental papillae. Tall cuboidal to columnar cells with central stellate reticulum-like cells similar to ameloblastic follicles may be seen lined with a few odontogenic strands or follicles. Osteodentin, dentinoid, and tubular dentin deposition may be evident in relation to the odontogenic epithelium [4]. Enamel or enamel matrix must not seen in cases of AFD [3].
There is a debate about AFO and AFD as they appear to be intermediate between AF and odontoma. The WHO classification in 2005 classified the AFDs and AFOs under AFs however, further studies disapprove of this and concluded that AFs should be regarded as a true neoplasm whereas AFO is more likely a developing odontoma[3, 5,6,7]. The continuum concept with AF, AFO, and odontomas was rejected on the basis of age of occurrence, site of occurrence, histopathology, gender, and evidence studied in recurrent cases [8].
In the WHO 2017 edition and current WHO tumors classification 2022, AFD and AFO are classified as developing odontomas (hamartomatous process). Although the presence of BRAF and V600E mutations in AFD and AFO is similar to AF but absent in odontoma, supported the arguments that at least some of these lesions are in fact neoplastic, particularly those with locally aggressive biological behavior, large size, and recurrence [8,9,10,11]
Case presentation
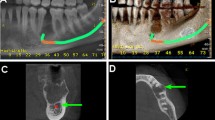
An 11-year-old male child presented to the oral and maxillofacial surgery department, faculty of dentistry, Cairo University with a swelling in the posterior mandible. Radiographic examination revealed a multilocular lesion with mixed radiodensity related to the impacted lower left second premolar tooth. The lesion was large in size extending from the canine to the molars area causing teeth displacement and hindering the eruption of the lower 2nd premolar and one of the lower molars (Fig. 1).
Based on the clinical and radiographic findings, differential diagnoses of odontogenic tumors: ameloblastoma, ameloblastic fibroma, ameloblastic fibro-odontoma, or ameloblastic fibro-dentinoma were made.
Incisional biopsy was done (Fig. 2) and referred to the oral and maxillofacial pathology department, faculty of dentistry, Cairo University, and the microscopic examination revealed cords and nests of odontogenic follicles like enamel organs lined by tall cuboidal to columnar cells with central stellate reticulum-like cells similar to ameloblastic follicles surrounded by juxta epithelial hyalinization (Figs. 3 and 4). The surrounding connective tissue stroma showed primitive immature ectomesenchymal cells resembling dental lamina with areas of dentinoid material (Fig. 5 black arrow) and osteodentin (Figs. 6 and 7 black arrows). The microscopic features were consistent with ameloblastic fibro-dentinoma. Surgical excision of the lesion was done, and the patient was followed up for 1 year without evidence of recurrence.
A photomicrograph of Hematoxylin and eosin (H&E) stained sections showing the cords and nests of odontogenic follicles lined by ameloblast-like cells (red arrows) and central stellate reticulum-like cells (yellow arrows) and surrounded by juxta epithelial hyalinization (black arrow). It also shows highly proliferative ectomesenchymal primitive cells in the surrounding stroma
Discussion
AFs, AFDs, AFOs, and odontomas are lesions that almost share the same histopathological, clinical, and radiographical features this in turn results in an argument about whether they can be categorized as a separate pathological entity or as developmental stages of the same lesion. That is why some researchers consider AFs and odontomas to be the extremes while, AFDs and AFOs are intermediate stages in between them [7, 8]. Philipsen et al. supposed that AF and AFD are expressed in two forms. The neoplastic one if left more time without treatment will not mature into odontoma. The other variant is a hamartomatous lesion that has the potential to mature into an AFO and complex odontoma. Gardner has an opinion that the AFD should be referred to simply as AF [12].
In 2005, WHO classified the AFDs under AFs. The recent WHO classification (2017) and (2022) considered AFDs to be a hamartomatous process supposing that once the hard tissue is formed, they mature to form odontomas [10, 11]. However, cases have been reported with significant large growth patterns causing cortical plate perforation. Moreover, many reports of cases with malignant transformation and recurrence have been reported. Additionally, AF, AFO, and AFD harbor the same BRAF p.V600E mutation in 46%, 34%, and 69% of cases respectively [8, 13, 14].
AFD is considered is a rare mixed odontogenic tumor that arises mainly in the posterior region mandible and is usually with an unerupted molar which in accordance with the present case too. AFD is more commonly reported in males as in this case. AFD often grows with no symptoms and may reach large sizes [13].
Histologically, the tumor is characterized by epithelial and ectomesenchymal neoplastic components. The epithelial component consists of odontogenic nest or cords lined by tall ameloblastic-like cells and central stellate reticulum-like cells. The ectomesenchymal component resembles the dental lamina with dysplastic dentin deposition.
AFD could be distinguished from other similar lesion like AF and AFO by that AFD exhibit dysplastic or tubular dentin, whereas enamel matrix deposits are found in the AFO but, regarding AF there is no any type of dental hard tissue deposits [8]. In this case, microscopically, we observed long narrow cords and islands of odontogenic follicles with juxtaepithelial hyalinization. The epithelial strands resided in a primitive ectomesenchymal stroma with stellate-shaped fibroblasts exhibiting long slender cytoplasmic extensions that resembled dental papilla. The lesions exhibited non-tubular dentin entrapping cells in multiple lacunae in close relation to odontogenic epithelium resembling dentinoid. The presence of dentinoid excludes AF tumor. Additionally, there is no enamel matrix or enamel spaces, so AFO was excluded. All of these features are consistence with the AFD.
As reported by Gardner and Farquhar, in AFDs different forms of dentin could be seen such as osteodentin, dentinoid, and dentin undergoing globular mineralization [15]. All of this tissue is considered dentinal induction; however, the juxtaepithelial hyalinization is categorized as non-dentinal induction in the ecto-mesenchyme [16]. In the present case, these features are present in the form of dentenoid material, osteodentin, and juxtaepithelial hyalinization surrounding the odontogenic follicles and cords.
Since AFDs have with low recurrence rate and usually with good biological behavior a conservative approach is usually recommended for this tumor [17]. In 2012, Giraddi, G.B., and V. Garg reported an aggressive atypical AFD presented with resorption and perforation of the cortical plate which was treated radically treated [4]
Occasionally, ameloblastic fibro-dentinosarcoma arises from the malignant transformation of AFD [18]. The 2022 WHO classification of odontogenic sarcomas presented three tumors: ameloblastic fibro-sarcoma, ameloblastic fibro-dentinosarcoma, and ameloblastic fibro-odontosarcoma. They arise from the malignant transformation of the mesenchymal component while the epithelial component does not show any malignant changes. In the present case the lesion was large in size with no evidence of malignant transformation so, conservative surgical treatment was done with a follow-up of 1 year.
Conclusion
This paper is reporting on one of the rare entities with illustrating the historical and current debate about it. Additionally, the presented case showed the importance of early diagnosis of lesions related to the delayed eruption or missing teeth and how it’s effective in early management and hence prognosis of the patient.
Availability of data and materials
The data used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AFD:
-
Ameloblastic fibro-dentinoma
- AF:
-
Ameloblastic fibroma
- AFO:
-
Ameloblastic fibro-odontoma
- WHO:
-
World Health Organization
References
Galvão CF, et al. Loss of heterozygosity (LOH) in tumour suppressor genes in benign and malignant mixed odontogenic tumours. J Oral Pathol Med. 2012;41(5):389–93.
Buchner A, Vered M. Ameloblastic fibroma: a stage in the development of a hamartomatous odontoma or a true neoplasm? Critical analysis of 162 previously reported cases plus 10 new cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(5):598–606.
Barnes L, et al. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. 2005.
Giraddi GB, Garg V. Aggressive atypical ameloblastic fibrodentinoma: Report of a case. Contemp Clin Dent. 2012;3:97–102.
de Sousa Lopes MLD, et al. Ameloblastic fibro-odontoma: case report and immunohistochemical profile. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology. 2017;29(1):77–82.
Wright JM, et al. Odontogenic Tumors, WHO 2005: Where Do We Go from Here? Head Neck Pathol. 2014;8(4):373–82.
Chen Y, et al. Ameloblastic fibroma and related lesions: a clinicopathologic study with reference to their nature and interrelationship. J Oral Pathol Med. 2005;34(10):588–95.
Chrcanovic BR, Gomez RS. Ameloblastic fibrodentinoma and ameloblastic fibro-odontoma: an updated systematic review of cases reported in the literature. J Oral Maxillofac Surg. 2017;75(7):1425–37.
Brown NA, et al. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 2014;20(21):5517–26.
Wright JM, Vered M. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Odontogenic and Maxillofacial Bone Tumors. Head Neck Pathol. 2017;11(1):68–77.
Soluk-Tekkesin M, Wright JM. The World Health Organization classification of odontogenic lesions: a summary of the changes of the 2022 (5th) Edition. Turk Patoloji Derg. 2022;38(2):168–84.
Gardner DG. The mixed odontogenic tumors. Oral Surgery, Oral Medicine, Oral Pathology. 1984;57(4):395–7.
Shwetha V, et al. Ameloblastic fibrodentinoma: A rare occurrence. International Journal of Medical and Dental Case Reports. 2016;3(1):1–4.
Jacob OA, et al. Ameloblastic Fibrodentinoma: A 12 Years follow-up of a Rare Entity. 2015.
Gardner DG, Farquhar DA. A classification of dysplastic forms of dentin. J Oral Pathol. 1979;8(1):28–46.
Bavle RM, et al. Ameloblastic fibrodentinoma: a case with varied patterns of dysplastic dentin. Cureus. 2017;9(6):e1349.
Bhargava D, et al. Ameloblastic fibrodentinoma. Indian J Dent Res. 2011;22(2):345.
Umashankara KV, Nagaveni NB, Manjunath S. Ameloblastic fibro-dentinoma: Report of a rare tumor with literature review. J Cranio Max Dis. 2012;1:141–4.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors analyzed and interpreted the patient data. HA performed the histological examination, and NM was a major contributor to writing the manuscript. ND substantively revised the work. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work was approved by the Research Ethical Committee at the Faculty of Dentistry, Cairo University, done in compliance with the Helsinki Declaration. Written informed consent to participate in the study was provided by the patient’s father.
Consent for publication
Written informed consent was obtained from the patient’s father for publication of this case report and accompanying images before the submission of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Darwish, N.M.A., Amer, H.W.AF. & Mahrous, N.N.M. Ameloblastic fibro-dentinoma: a rare mixed odontogenic tumor case report with review of literature. J Egypt Natl Canc Inst 35, 34 (2023). https://doi.org/10.1186/s43046-023-00193-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43046-023-00193-0