Abstract
Aims
Both S100A4 and Glypican-3 have been known to be engaged in HCC development and progression. This study aimed to evaluate both S100A4 and GPC3 expression in HCC tissues as a prognostic markers.
Methods
Tissues from 70 patients of HCC in cirrhotic HCV patients were evaluated by immunohistochemistry using antibodies against SA100A4 and GPC3 and compared with tumor-adjacent tissue (controls). All cases were followed for 40 months.
Results
GPC3 was more expressed in HCC (79%) than S100A4 (21%). S100A4 was more significantly expressed in cases showing metastasis, microscopic vascular emboli, necrosis, and grade III tumors. There was no relationship between overall survival and both S100A4 and GPC3. The only significant independent predictor for recurrence was decompensation (OR 3.037), while metastasis was significantly predicted by S100A4 expression (OR 9.63) and necrosis (OR 8.33).
Conclusion
S100A4 might be used as a prognostic marker for HCC, while GPC3 is a reliable marker of HCC diagnosis.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is one of the most unfavorable malignancies worldwide. HCC ranked globally from third to second cancer. It is currently the second leading cause of cancer-related deaths in men and the fifth in women [1]. The overall 5-year survival rate for HCC is only 5%. The relapse rate within 5 years of undergoing surgery is 70%, and the recurrence rate within the remaining liver tissue is more than 80%. Although there are similar clinicopathological parameters in HCC patients, the outcome is entirely different, confirming the unsolved biological behavior of this tumor [2]. In Egypt, HCV is considered to be a significant risk factor for HCC [3, 4].
Expression of S100A4 is related to epithelial mesenchymal transition (EMT). EMT is a process of tumor-cell invasion leading to loss of epithelial characteristics and acquiring mesenchymal features. In HCC, S100A4 expression, together with other EMT-related proteins, is equivalent to metastasis and overall survival [5]. A more vital metastatic ability has been reported in S100A4-expressing tumor cells than other S100A4-negative cells [6]. The expression of S100P gradually progressed from dysplasia, precancerous lesions to HCC. S100P may be a contributing factor for HCC formation or progression. Although S100P expression is well-known to be linked to many neoplastic disorders, its role in HCC has not been extensively studied. Malignant hepatocyte cell lines express higher S100P levels than normal nonmalignant cells, which lack S100P [7]. Glypican-3 (GPC3) is one of the members of the heparin sulfate (HS) proteoglycan family. GPC3 is strikingly expressed in human embryos regulating morphogenesis probably depending on insulin-like growth factor, bone morphogenic protein, fibroblast growth factor (FGF), or hedgehog signaling. GPC3 is profusely expressed during pregnancy in fetal organs such as the liver, lung, kidney, and placenta. On the other hand, GPC3 expression is reduced in adults [8]. GPC3 is highly expressed in early HCC, so it is a sensitive and specific biomarker for diagnosis. Moreover, GPC3expression was proved as a prognostic factor and could predict the poor outcome for HCCs [9].
The aim of this study is to evaluate the prognostic value of tissue expression of both S100A4 protein and GPC3 in HCC in Egyptian patients with HCV cirrhosis.
Materials and methods
Patients
Only 70 out of 400 patients with primary HCC (56 males and 14 females) with a mean age 63.03 ± 8.17 years were examined and investigated at both Tropical Medicine Department and Gastroenterology Surgery Center Mansoura University between January 2016 and November 2019. The 70 patients met the inclusion criteria for hepatectomy and were recruited in the current study. Laboratory investigations were conducted in the Molecular Genetic Unit of Endemic Hepatogastroenterology and Infectious Diseases (MGUHID) of Mansoura Faculty of Medicine. This study was approved by the Institutional Review Board of Mansoura Faculty of Medicine, and written informed consent was obtained from all patients. Diagnosis of HCC depended on one dynamic imaging technique for lesions beyond 2 cm in diameter with the typical hypervascular pattern in the arterial phase with washout in the portal venous or delayed phases. For the diagnosis of lesions less than 2 cm, two dynamic techniques were obtained [10]. Patients who met all of the following criteria were included in the analysis: (i) patients with Child-Pugh class A, (ii) no main portal vein branch thrombosis or extrahepatic metastasis, (iii) prothrombin concentration greater than 50%, (iv) platelet count greater than 50.000/mm3, and (v) accepted performance status for hepatectomy.
Clinical data were collected, including age, gender, complete blood count, prothrombin time, albumin, bilirubin, α-fetoprotein (AFP), and alkaline phosphatase. Tissue samples were taken from resection specimens from both tumorous tissue and adjacent para-tumorous cirrhotic tissue (control). Diagnosis of HCC in all cases was confirmed histopathologically (Fig. 1). Moreover, pathological data were collected, including tumor size, location, number, grading, growth pattern, mitotic count, necrosis, and microscopic vascular emboli. Follow-up of the patients was done for 40 months to detect local recurrence, metastasis, and/or decompensation.
Tissue microarray
After selecting areas for construction of TMAs on the donor blocks, sampling was done using a manual arraying instrument (Beecher Instruments, Sun Prairie, WI, USA). Four TMA blocks were constructed using 1-mm tissue cores (Alphelys, Plaisir, France). The blocks were cut into 5-μm sections and coated with paraffin for future use (Fig. 2).
Immunohistochemistry
Mouse monoclonal antibodies against SA100A4 (Novus Biologicals USA) and GPC3 (1G12; Cell Marque, Rocklin, CA, USA) were used for IHC on the slides of TMAs. The staining procedures were performed according to the manufacturers’ instructions. Two independent investigators examined all samples. The slides were examined using Olympus CX41 microscopy, and photos were taken using Amscope MU1000 camera. Positive staining for S100A4 protein and GPC3 was observed as yellow or brown staining in the cytoplasm of HCC tumor cells. Samples were categorized into one of two groups, based on the level of immune-staining: positive:\(\ge\) 5%of cells stained and negative: < 5% of cells stained.
Statistical analysis
Data were analyzed with SPSS version 21. The normality of data was first tested with a one-sample Kolmogorov-Smirnov test. Qualitative data were described using numbers and percentages. Association between categorical variables was tested using the chi-square test and Fisher exact test. Continuous variables were presented as mean ± SD (standard deviation) for parametric data and median for nonparametric data. The two groups were compared with the Student t-test (parametric data) and Mann-Whitney test (nonparametric data). p-value is considered significant when ≤ 0.05. The markers with p < 0.05 were analyzed by stepwise logistic regressions to evaluate independent variables. Survival was expressed in months, and survival analysis was performed by the Kaplan-Meier method with log-rank analysis.
Results
Clinicopathological characteristics of the study group
The clinicopathological features of patients in the study group are summarized in Table 1. Microscopic vascular emboli were present in 26 patients (37.1%). Necrosis within tumor tissue was present in 29 cases (41.4%). The main growth pattern was trabecular and was found in 37 cases (52.9%). Local recurrence was reported in 17 cases (24.3%), metastasis in 6 cases (8.6%), and decompensation in 20 cases (28.6%). The median survival time was 7 months (range: 1–40 months).
Immunohistochemistry of S100A4 in liver tissues
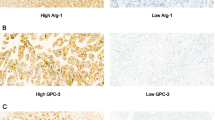
HCC tissues showed more staining intensity for S100A4 protein than their tumor-adjacent tissue (control) (Figs. 3 & 4) as 21% (15/70) of HCC cases were positive for S100A4 protein, whereas only one weak positive control was observed (4.2%, p = 0.001).
Significance of S100A4 protein detection in HCC tissues
The detection of S100A4 protein in HCC tissue was found to be significantly associated with metastasis (26.7% versus 3.6% (p < 0.005), microscopic vascular emboli (60% versus 30.9% (p = 0.039)), necrosis (66.7% versus 34.5% (p = 0.025), high-grade tumors (p = 0.014), and higher AFP levels (p = 0.004). No significant association was observed between S100A4 expression and HCC tumor size, local recurrence, rate of decompensation, or survival time (p > 0.05) (Table 2).
Immunohistochemistry of GPC3 in liver tissues
GPC3 was more expressed in HCC samples than in tumor-adjacent tissue (control) samples (Fig. 5) as 79% (55/70) of HCC tissues showed positive staining for GPC3, while only 4.2% (3/70) of tumor-adjacent tissues (control) had weak and focal staining of duct epithelium (but not hepatocytes) (p < 0.001).
Significance of GPC3 expression in HCC tissues
Detection of GPC3 in HCC tissues was found to be associated with short tumor-free survival and rapid recurrence (p = 0.02). Moreover, the levels of AFP were higher in positive cases than in negative cases (p = 0.04). No significant association was present between GPC3 expression and patient survival, tumor metastasis, or recurrence rate (p > 0.05). Also, no association was observed with tumor grading, necrosis, microscopic vascular emboli, or tumor size (p > 0.05) (Table 2).
Logistic regression analysis
Stepwise logistic regression analysis was applied to evaluate variables independently to predict tumor recurrence. Only decompensation was found to predict recurrence (p = 0.05) (OR 3.037) (Table 3).
Many variables were found to be associated with tumor metastasis: S100A4, necrosis, size of the tumor, AST level, and ALT level (p = 0.017, 0.029, 0.03, 0.03, and 0.006, respectively). After univariate regression analysis, S100A4 expression (OR 9.63) and necrosis (OR 8.33) were independent predictors. After multivariate regression analysis, S100a4 expression was found to be the most significant predictor of tumor metastasis (OR 8.4) (Table 4).
Kaplan-Meier log-rank analysis of survival
The cumulative percentage for survival at 6, 12, 18, 24, 30, 36, and 40 months was 55.7, 15.9, 14.3, 4.3, 2.9, 4.3, and 2.9, respectively (Fig. 6). The median overall survival was shorter in decompensated patients and higher-grade tumors (p = 0.012 and p = 0.046, respectively) (Table 5).
Discussion
Hepatocellular carcinoma (HCC) is the most common form of liver malignancies worldwide [4, 11]. There are many risk factors for HCC recurrence and progression, including tumor size, associated cirrhosis, HBV infection, HCV infection, histological grade of tumor differentiation, vascular permeation, and the absence of capsule [12,13,14].
The molecular pathogenesis of HCC disease remains mysterious even after both causative factors and cellular changes have been recognized [15,16,17,18]. Furthermore, therapeutic modalities for HCC remain limited. More interpretation of the underlying biology of HCC is needed for further development in subsequent targeted therapies against essential molecular mechanisms of HCC [19].
S100A4 and other S100P (proteins) have been studied in many in vitro studies on HCC cell lines, revealing higher S100P levels than normal nonmalignant hepatocytes, which frequently lack S100P. Also, silencing of endogenous S100P was observed to decrease HCC cell growth by Kim et al. [7].
The present study reported that expression of S100A4 in HCC patients is associated with metastasis, higher grades of HCC, microscopic vascular embolization, and tumor necrosis. These findings may implicate that more biologically aggressive tumors are related to the positivity of S100A4 in tumor tissues.
Cui et al. (2006) reported that S100A4 was overexpressed only in the metastatic cells among other metastasis candidate proteins in HCC cell lines and was selected for further series of assays. The authors suggested that S100A4 might contribute to HCC invasion and metastasis through two matrix metalloproteinase (MMP9) secretion regulation paths and strengthened motility and invasion properties [6].
Yan et al. (2013) reported that S100A4 expression was significantly higher in liver cancer-associated mesenchymal stem cells (LC-MSCs) compared with liver normal MSCs (LN-MSCs) from adjacent cancer-free tissues. They also revealed that S100A4 secreted from LC-MSCs has a role in HCC progression and may be a potential therapeutic target [20].
In a study on mainly HBV patients, Liu et al. found that S100A4 correlated with tumor differentiation, invasion, recurrence, and overall survival and concluded that it could be a valuable marker of tumor aggressiveness and prognosis [21].
In the current study, there was a significant positive correlation between AFP level and S100A4 expression. At the same time, Liu et al. found no significant association between S100A4 expression and AFP level [21].
On the other hand, Cho et al. found that S100A4 expression in HCC was not statistically correlated with clinicopathologic parameters, including histologic grade, stage, capsular invasion, intrahepatic metastasis, and portal vein invasion. Also, they found that gene amplification of S100A4 was not associated with clinical parameters [22].
Sample sizes, population admixture, differences in ethnic backgrounds, differences in etiology, and different criteria for selection of target population may all contribute to these conflicting results and discrepancies.
The primary etiology of liver disease in HCC patients significantly impacts patient outcome and cannot be neglected as a cofactor with other variables [23, 24]. Many studies concluded that survival is significantly better in HCV-related HCC than in HBV-related HCC. Cantarini et al. reported that hepatitis C virus-related HCC has a lesser aggressiveness than hepatitis B virus-related HCC, as HCV-related HCC becomes clinically manifest once they have reached an advanced stage [17, 25]. Sinn et al. concluded that HCV-related HCC has a better prognosis than HBV [26]. The discrepancy in survival has also been previously described in breast cancer studies with S100A4. The discrepancy has been attributed to fewer case numbers, the difference in stages of disease included in different studies, a relatively short period of follow-up, and differences in sample storage conditions or fixation methods [27, 28].
GPC3 has been established as a diagnostic marker for HCC. Further studies have evaluated its value in prognosis suggesting its role as a promising prognostic biomarker. Many meta-analytic studies confirmed that GPC3 expression is of prognostic value as it was correlated with shorter overall survival (OS) and disease-free survival (DFS) of HCC patients [29].
In the current study, the diagnostic value of GPC3 in HCC has been confirmed, supporting its established value. However, regarding its prognostic value, its expression was only correlated with the time of recurrence of HCC, and there was no correlation with patient overall survival. Similar results were obtained with Chen et al., who revealed that GPC3 expression was a significant independent prognostic factor for disease-free survival. However, overall survival was not affected, but this study included only cases of early HCC [29].
Unlike most previous studies, the present study found no association between GPC3 and tumor differentiation and other clinicopathological parameters [29].
This conflict between this study and previous studies might be explained by some limitations which were addressed in some of those studies. First, positive results were more published than negative ones, suggesting a potential risk of bias. In addition, the total number of included studies and the total sample size were relatively small, which might influence the validity of the analysis to some extent [29]. Moreover, many of these studies were based on the Asian population. Also, there was a lack of data about other races. In addition, the IHC analysis technique has many related factors such as the type of antibody used, detection method, evaluation method of results, and interobserver variation that lead to the heterogeneity of IHC studies [29].
There are a few limitations to this study. All the patients were HCV-induced HCC, and no comparison was made with other causes of liver disease. The small sample size (70 patients), a short period of follow-up, and operative complications may have an impact on survival, and it was not clarified here. Moreover, in patients who had decompensated liver disease, there was a defect in follow-up for recurrence of HCC, suggesting a possibility of missed recurrence or metastasis in this group of patients.
Conclusion
This study suggests that S100A4 protein levels may be a valuable tool in predicting poor outcomes in HCC, and this may play a role as a therapeutic target in the future. Additionally, it is recommended that patients with positive S100A4 expression may need more close follow-up and more intensive treatment modalities. We also confirmed the diagnostic value of GPC3 in HCC.
Limitation of the study
Small number size of the HCC patients and the absence of the Western blot technique are considered some of the limiting factors in this study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- GPC3:
-
Glypican-3
- EMT:
-
Epithelial mesenchymal transition
- S100A4:
-
S100 calcium-binding protein A4
- HS:
-
Heparin sulfate
- FGF:
-
Fibroblast growth factor
- MGUHID:
-
Molecular genetic unit of endemic hepatogastroenterology and infectious diseases
- AFP:
-
α-Fetoprotein
- MMP9:
-
Matrix metalloproteinase
- LC-MSCs:
-
Liver cancer-associated mesenchymal stem cells
- IHC:
-
Immunohistochemistry
References
Global Burden of Disease Cancer C. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505–27. https://doi.org/10.1001/jamaoncol.2015.0735.
Xiao W, Zhao W, Li L, Wu Q, Zhu L, Zhang Q, Dai W, Wang Y, Zhang B. Preliminary investigation of the role of BTB domain-containing 3 gene in the proliferation and metastasis of hepatocellular carcinoma. Oncology Lett. 2017;14(2):2505–10. https://doi.org/10.3892/ol.2017.6369. (PMID: 28789460).
Shaker MK, Abdella HM, Khalifa MO, Dorry AKE. Epidemiological characteristics of hepatocellular carcinoma in Egypt: a retrospective analysis of 1313 cases. Liver Int. 2013;33(10):1601–6. https://doi.org/10.1111/liv.12209. (PMID: 23714212).
El-Bendary M, Nour D, Arafa M, Neamatallah M. Methylation of tumour suppressor genes RUNX3, RASSF1A and E-cadherin in HCV-related liver cirrhosis and hepatocellular carcinoma. Br J Biomed Sci. 2020;77(1):35–40. https://doi.org/10.1080/09674845.2019.1694123. (PMID: 31790342).
Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, Cho JY, Yoo JE, Choi JS, Park YN. Human hepatocellular carcinomas with “stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54(5):1707–17. https://doi.org/10.1002/hep.24559. (PMID: 22045674).
Cui JF, Liu YK, Zhang LJ, Shen HL, Song HY, Dai Z, Yu YL, Zhang Y, Sun RX, Chen J, Tang ZY, Yang PY. Identification of metastasis candidate proteins among HCC cell lines by comparative proteome and biological function analysis of S100A4 in metastasis in vitro. Proteomics. 2006;6(22):5953–61. https://doi.org/10.1002/pmic.200500460. (PMID: 17051636).
Kim JK, Jung KH, Noh JH, Eun JW, Bae HJ, Xie HJ, Ahn YM, Ryu JC, Park WS, Lee JY, Nam SW. Targeted disruption of S100P suppresses tumor cell growth by down-regulation of cyclin D1 and CDK2 in human hepatocellular carcinoma. Int J Oncol. 2009;35(6):1257–64 (PMID: 19885547).
Haruyama Y, Kataoka H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma. World J Gastroenterol. 2016;22(1):275–83. https://doi.org/10.3748/wjg.v22.i1.275. (PMID: 26755876).
Wu Y, Liu H, Ding H. GPC-3 in hepatocellular carcinoma: current perspectives. J Hepatocell Carcinoma. 2016;3:63–7. https://doi.org/10.2147/JHC.S116513jhc-3-063. (PMID: 27878117).
EASL-EORTC clinical practice guidelines. management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–94 S0168-8278(11)00873-7. PMID: 22424438.
Neamatallah MA, El-Missiry MA, Said MMA, Elbendary M, Othman AI, Ammar OA. TP53 polymorphism as a risk factor for hepatocellular carcinoma in hepatitis C virus-infected Egyptian patients. Egypt J Basic Appl Sci. 2014;1(1):9–15. https://doi.org/10.1016/j.ejbas.2014.01.001.
Farid K, Elalfy H, El-Bendary M, Besheer T, El-Eraky A, Abdelbadie M, Elmokadem A, Moawad A, El-khair SA, Sakr S, El-Deek B, El-Morsy A, Elmorsy RA, Amer T, Abed S. Cardiopulmonary assessment of patients with unresectable hepatocellular carcinoma treated by transarterial chemoembolization. J Liver Transplant. 2022;5:100063. https://doi.org/10.1016/j.liver.2021.100063.
Elalfy H, Besheer T, El-Maksoud M, Farid K, Elegezy M, El Nakib A, El-Aziz M, El-Khalek A, El-Morsy A, Elmokadem A. Monocyte/granulocyte to lymphocyte ratio and the MELD score as predictors for early recurrence of hepatocellular carcinoma after trans-arterial chemoembolization. Br J Biomed Sci. 2018;75(4):187–91. https://doi.org/10.1080/09674845.2018.1494769. (PMID: 29991324).
Farid K, Elalfy H, Abo El-khair SM, Elgamal H, Besheer T, Elmokadem A, Shabana W, Abed S, Elegezy M, El-khalek AA, El-Morsy A, Negm A, Elsamanoudy AZ, El deek B, Amer T, El-Bendary M. Prognostic value of vascular endothelial growth factor in both conventional and drug eluting beads transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma in HCV patients. Exp Rev Gastroenterol Hepatol. 2020; 14(12): 1203-1214. https://doi.org/10.1080/17474124.2020.1823215.
Besheer T, El-Bendary M, Elalfy H, Abd El-Maksoud M, Salah M, Zalata K, Elkashef W, Elshahawy H, Raafat D, Elemshaty W. Prediction of fibrosis progression rate in patients with chronic hepatitis C genotype 4: role of cirrhosis risk score and host factors. J Interferon Cytokine Res. 2017;37(3):97–102. https://doi.org/10.1089/jir.2016.0111. (PMID: 28068153).
Besheer T, Arafa M, El-Maksoud MA, Elalfy H, Hasson A, Zalata K, Elkashef W, Elshahawy H, Raafat D, Elemshaty W. Diagnosis of cirrhosis in patients with chronic hepatitis C genotype 4: role of ABCB11 genotype polymorphism and plasma bile acid levels. Turk J Gastroenterol. 2018;29(3):299. https://doi.org/10.5152/tjg.2018.17570. (PMID: 29755014).
Neamatallah M, El-Bendary M, Elalfy H, Besheer T, El-Maksoud MA, Elhammady D, Abed S, Elegezy M, Kandeel L, Eldeib D. Impact of toll-like receptors 2 (TLR2) and TLR 4 gene variations on HCV susceptibility, response to treatment and development of hepatocellular carcinoma in cirrhotic HCV patients. Immunol Investig. 2019: 1-15. https://doi.org/10.1080/08820139.2019.1673772 PMID: 29755014.
Abdel-Azziz MY, Zalata KR, El-Bendary MM. Insulin resistance and liver fibrosis progression in patients with chronic hepatitis C virus infection. Arab J Gastroenterol. 2010;11(1):30–4. https://doi.org/10.1016/j.ajg.2010.01.004.
Staib F, Krupp M, Maass T, Itzel T, Weinmann A, Lee JS, Schmidt B, Muller M, Thorgeirsson SS, Galle PR, Teufel A. Cell MinerHCC: a microarray-based expression database for hepatocellular carcinoma cell lines. Liver Int. 2013;34(4):621–31. https://doi.org/10.1111/liv.12292. (PMID: 24016071).
Yan X-L, Jia Y-L, Chen L, Zeng Q, Zhou J-N, Fu C-J, Chen H-X, Yuan H-F, Li Z-W, Shi L, Xu Y-C, Wang J-X, Zhang X-M, He L-J, Zhai C, Yue W, Pei X-T. Hepatocellular carcinoma-associated mesenchymal stem cells promote hepatocarcinoma progression: role of the S100A4-miR155-SOCS1-MMP9 axis. Hepatology. 2013;57(6):2274–86. https://doi.org/10.1002/hep.26257. (PMID: 23316018).
Liu Z, Liu H, Pan H, Du Q, Liang J. Clinicopathological significance of S100A4 expression in human hepatocellular carcinoma. J Int Med Res. 2013;41(2):457–62. https://doi.org/10.1177/0300060513478086. (PMID: 23781010. DOI: 41/2/457 [pii]).
Cho YG, Kim CJ, Kim SY, Nam SW, Lee SH, Yoo NJ, Lee JY, Park WS. Overexpression of S100A4 is closely associated with the progression of gastric cancers and hepatocellular carcinomas. Cancer Res. 2004;64(7):1166. https://doi.org/10.1034/j.1600-0463.2003.1110502. (PMID:12887505).
Abdel Allah HMM, Zahran WE, El-Masry SA, El-Bendary M, Soliman AF. Association of MTHFR and TYMS gene polymorphisms with the susceptibility to HCC in Egyptian HCV cirrhotic patients. Clin Exp Med. 2021. https://doi.org/10.1007/s10238-021-00747-3. (PMID: 34297238).
Elbaz T, Waked I, El-Akel W, Shaker MK, Abdelaziz AO, Yousif M, El-Bendary M, Zaky S, AbdAllah M, Hassany M, Esmat G, Doss W. Impact of successful HCV treatment using direct acting antivirals on recurrence of well ablated hepatocellular carcinoma. Exp Rev Anti-infective Ther. 2021: https://doi.org/10.1080/14787210.2021.1951230. PMID: 34253123.
Cantarini MC, Trevisani F, Morselli-Labate AM, Rapaccini G, Farinati F, Del Poggio P, Di Nolfo MA, Benvegnu L, Zoli M, Borzio F, Bernardi M. Effect of the etiology of viral cirrhosis on the survival of patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101(1):91–8. https://doi.org/10.1111/j.1572-0241.2006.00364.x. (PMID: 16405539 DOI: AJG364 [pii]).
Sinn DH, Gwak GY, Cho J, Paik SW, Yoo BC. Comparison of clinical manifestations and outcomes between hepatitis B virus- and hepatitis C virus-related hepatocellular carcinoma: analysis of a nationwide cohort. PLoS One. 2015;9(11):e112184. https://doi.org/10.1371/journal.pone.0112184. (PMID: 25372403. PONE-D-14-28985 [pii]).
Helfman DM, Kim EJ, Lukanidin E, Grigorian M. The metastasis associated protein S100A4: role in tumour progression and metastasis. Br J Cancer. 2005;92(11):1955–8. https://doi.org/10.1038/sj.bjc.6602613. (PMID: 15900299).
Pedersen KB, Nesland JM, Fodstad Ø, Mælandsmo GM. Expression of S100A4, E-cadherin, α- and β-catenin in breast cancer biopsies. Br J Cancer. 2002;87(11):1281–6. https://doi.org/10.1038/sj.bjc.6600624. (PMID: 12439718).
Xiao WK, Qi CY, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of glypican-3 in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:104. https://doi.org/10.1186/1471-2407-14-104. (PMID: 24548704: 1471-2407-14-104).
Acknowledgements
We thank prof. Abdel-Hady Elgilany for his great effort in performing statistical analysis of this study. Our great thanks extend to Professor Dina Elhammady (native English speeker) for her help to perform language editing of the manuscript.
STROBE statement
The guidelines of the STROBE statement have been adopted.
Funding
No funding agencies.
Author information
Authors and Affiliations
Contributions
MB, KF and AM were involved in study conception design and write the manuscript. KF and AM acquired the data, analysis and interpretation of data. MA and WK performed pathological diagnosis of the liver biopsies. TA obtain surgical biopsy specimens. All authors were involved in the drafting and critical revision of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Mansoura Faculty of Medicine, and written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Bendary, M., Farid, K., Arafa, M. et al. Prognostic value of S100A4 and Glypican-3 in hepatocellular carcinoma in cirrhotic HCV patients. J Egypt Natl Canc Inst 35, 26 (2023). https://doi.org/10.1186/s43046-023-00184-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43046-023-00184-1