Abstract
Background
The cognitive profile among patients with schizophrenia (SZ) and bipolar disorder (BD) has varied widely across different studies. The aim of the current study was to compare different cognitive domains using psychometric and neurophysiological tests in patients with SZ to those with BD. A case–control study was conducted on 30 BD, 30 SZ and 30 age and sex matched control group. Each subject was submitted to the following: Wechsler Adult Intelligence Scale-3rd edition (WAIS-III), Montreal cognitive assessment scale (MoCA), Brief Visuospatial Memory Test-Revised (BVMT-R), Memory Assessment Scales (MAS), and the P300 event related potential (ERP).
Results
SZ and BD patients had significantly lower total and subscales of WAIS-III scores than the control group. SZ patients had significantly higher deterioration index (DI) than controls, while absence of such significant between BD and controls. SZ patients reported significantly lower MoCA scores and subitems, especially in visuospatial, naming, attention, delayed recall, and orientation subtests than controls. Only visuospatial and delayed recall scores were significantly decreased in BD than controls. SZ patients performed poorer on BVMT-R subscales than the control group. Both SZ and BD groups had lower mean values of all subscales except verbal assessment in the four memory tests. P300 latencies and amplitude had no significant difference among the three groups, although the BD group had a shorter P300 latency.
Conclusion
Patients with SZ and BD had significantly lower scores on various cognitive function domains in comparison to controls with more affection in SZ. The frequency of mood episodes, disease duration, and education level must be considered.
Similar content being viewed by others
Background
Cognitive dysfunction is frequently observed in both schizophrenia (SZ) and bipolar disorder (BD) [1] and is significantly associated with impaired psychosocial functioning and diminished quality of life [2]. The extent of cognitive impairment varies widely across different studies and patients, ranging from mild to severe impairment resembling dementia [3]. As a result, cognitive dysfunction has become an essential measure of treatment outcomes for SZ and BD [4].
Recent research over the last two decades has provided clear evidence that BD is also associated with cognitive deficits and functional impairment, even in euthymic patients [5,6,7], while the severity of cognitive deficits in BD is less severe than in SZ, data suggests that the patterns of cognitive deficits in the two disorders are similar. These findings contradict other observations, such as the existence of a connection between BD, creativity, and premorbid academic achievement [8, 9].
However, methodological limitations such as the use of cross-sectional design, the inclusion of patients with chronic illnesses, and the lack of premorbid cognitive development information can limit our capacity to show possible cognitive variations between SZ and BD. One theory proposes that early cognitive abnormalities are specific to SZ [10, 11] since they have been seen during the prodromal stage and initial episode of SZ but not in BD condition. Another theory suggests SZ is characterized by cognitive abnormalities crucial for communication and social functioning (social cognition). Theory of mind (ToM) and other social cognitive deficits are more distinct in SZ than non-social cognitive abnormalities [12]. Some authors have also suggested that premorbid intellectual development issues can differentiate SZ from BD [13, 14]. There is significant neurobiological and clinical data overlap between SZ and BD [15].
Moreover, both illnesses share susceptibility genes. Nonetheless, familial, and genetic studies have provided evidence for schizophrenia- and BD-specific genetic risk factors [16, 17].
Furthermore, research suggests that BD with psychotic symptoms has cognitive deficits similar to SZ, notably in some respects such as episodic and working memory, executive, and attentional skills [18]. Some investigations, for example, showed no disparities in the severity of deficits between BP psychosis and SZ, especially in affective individuals with poor outcomes [19]. Others have discovered that people with SZ and BD with psychotic characteristics have a comparable neurocognitive profile, however the severity is greater in SZ patients [20].
Our objective was to evaluate cognitive function in patients with BD and SZ and compare it to a control group of healthy individuals. Additionally, we aimed to determine if each disorder exhibits a distinct pattern of cognitive dysfunction.
Methods
Participants and procedures
A case–control study was done at the outpatient clinic of the Psychiatry Department at Assiut University Hospital between August 2018 and July 2019. Patients diagnosed with schizophrenia and bipolar I disorder according to Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [21] diagnostic criteria, aged between 18 and 60 years, were included in the study. For schizophrenia patients, the inclusion criteria required recurrent episodes, current full remission, while bipolar disorder patients should be in full remission. All patients must complain on their medication at least 6 months. Moreover, bipolar individuals were required to be lacking in acute mood symptoms for at least one month prior to enrolment, with euthymia defined as a 1-month score of 7 or less [22] on the Hamilton Depression Rating Scale 17-item (HAMD) [23] and Young Mania Rating Scale (YMRS) [24]. Exclusion criteria included comorbid substance use or personality disorders, medical illness or neurological disease, and illiterate subjects.
The recruited patients consisted of 2 groups (30 per group): group1 with BP and group 2 consisted of SZ. Additionally, a control group (group 3) was included, consisting of healthy volunteers.
Assessment tools
Psychiatric interview
Participants underwent a standardized psychiatric interview using the semi-structured Interview based on DSM-5 diagnostic criteria prepared by researchers to collect psychiatric and medical history upon enrolled to the study. Controls also underwent the interview to ensure the absence of psychiatric or personality disorders.
Neuropsychological assessment
Wechsler Adult Intelligence Scale-3rd edition (WAIS-III)
This test measures intelligence in adults and older adolescents, evaluating verbal, performance, total intelligence quotient (IQ), and deterioration index (DI). Various classifications are used based on IQ scores [25].
Montreal Cognitive Assessment Scale (MoCA)
This test is a brief 30-point assessment that measures various aspects of cognitive function. It includes tasks for short-term memory recall, visuospatial abilities, executive functions (alternation, phonemic fluency, and verbal abstraction), attention, concentration, working memory, language skills, and orientation to time and place [26].
Brief Visuospatial Memory Test-Revised (BVMT-R)
This test assesses visual memory through tasks involving recall, learning, and recognition of figures presented on a plate. Participants reproduce the figures from memory and recognize target and non-target figures [27].
Memory Assessment Scales (MAS)
This set of tests assesses short-term, verbal, and visual memory through tasks that test recall and recognition directly and after a period following stimulus presentation. The battery includes 12 subscales, covering verbal span, list learning, prose memory, visual span, visual recognition, visual reproduction, and names-face recognition [28].
Neurophysiological assessment: event-related potential (ERP) test, P300 wave
The test was conducted using electromagnetic stimulation (EMS) biomedical equipment. Before running the test, the subjects were placed in a semi-dark acoustically quiet room, and they were allowed to adapt to the surrounding environment for a suitable duration. An oddball paradigm was applied, where the subjects had to recognize an infrequent target signal in a chain that included “frequent” signals. The stimuli were applied at a specific intensity and with varying interstimulus intervals. captured using scalp electrodes implanted at three different locations: central (Cz), frontal (Fz), and parietal (Pz), as well as their average value. The measured variables included P300 wave latency and amplitude. The test was repeated twice for each subject, and the mean result was obtained for analysis [29].
Statistical analysis
Continuous variables were reported as mean and standard deviation, whereas categorical variables were expressed with numbers and percentages. The normal distribution of continuous variables was tested using the Kolmogorov–Smirnov test and Q-Q Plots. The chi-square test was used to compare categorical variables, and the Mann–Whitney test was used for non-parametric variables, while ANOVA was used for normally distributed data. The spearman correlation coefficient was used to evaluate association factors of cognitive impairment among participants. A significance level of P < 0.05 was set for all statistical analyses, and the analysis was performed using SPSS 27 software (IBM Armonk, NY, USA).
Results
Sociodemographic data
There were no significant differences regarding demographic data between the three studied groups. Patients with BD had a mean 4.2 ± 3.3 episodes. Details illustrated in Table 1.
Neuropsychological assessment
The Wechsler Adult Intelligence Scale-3rd edition (WAIS-III)
There were significant differences between the three groups in total, verbal, performance, and DI. These differences were due to: The total score, verbal, and performance assessment of the WAIS-III in SZ patients were significantly poor compared to control group. Furthermore, SZ patients exhibited a higher DI than control as depicted in Table 2.
On the other hand, BD patients showed lower scores compared to the control group on the total and verbal scores of the WAIS-III (P < 0.001 for each), and the performance score (P = 0.02). However, there was no significant difference between BD patients and the control group in terms of the DI. Comparison between SZ versus BD showed that: SZ had significantly lower scores than BP in total score, verbal and performance scores and higher deterioration index than BP group as illustrated in Table 2.
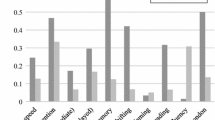
Montreal Cognitive Assessment Scale score (MoCA)
There were significant differences between the three groups in total and all subitems scores except in Language subitems score. These significant were due to:
-
Patients with SZ had significantly lower total score of the MoCA scale compared to control group particularly in visuospatial, naming, attention, delayed recall, and orientation subtests. However, BP had significantly lower scores than controls in visuospatial and delayed recall only.
-
Comparison between SZ versus BD showed that SZ was significantly more affection than BP in total score, visuospatial, naming, attention, abstraction, and orientation as illustrated in Table 3.
Brief Visuospatial Memory Test-Revised (BVMT-R)
Table 4 demonstrated that there were significant differences between the three groups in all items of BVMT-R measures except in learning and % retained and Rec. Response Bias. These differences come from SZ patients performed worse than healthy controls in the same subscales of BVMT-R. Moreover, the BD group had poor scores in compared to the control group on trial 2 and total recall only. In comparison between the two patients’ groups: SZ group had more affection in trial 1, 2, 3, total recall, and delayed recall with higher RDI than BP.
Memory Assessment Scale (MAS)
Table 5 show that BD and SZ groups performed significantly worse than the control group on all four memory assessment tests (P < 0.001) with significantly more affection in SZ than BP in all subscales except in verbal assessment were recorded.
Neurophysiological assessment: event-related potential (ERP) test, P300
The P300 latencies and amplitudes were within normal ranges of patient’s groups, with no significant differences between the three groups at different points of assessment recorded. However, BD patients displayed a significantly shorter P300 latency compared to SZ at the Pz point of assessment (P = 0.024) (Table 6).
Correlation studies
Table 7 presents the correlations observed between various clinical variables and psychometric scales, as well as event-related potentials (ERPs), among individuals diagnosed with mood disorders. An increase in the number of years of education was found to be associated with higher scores on MOCA (r = 0.521, p = 0.003), total IQ (r = 0.456, p = 0.022), and all subscales of the BVMT-R, while no such correlation with other psychometric tests. Furthermore, an increase in the number of episodes experienced by the patients inversely correlated with total scores on the MOCA (r = − 0.709, p = 0.0001), as well as performance on trial 1 (r = − 0.412, p = 0.024), trial 3 (r = − 0.377, p = 0.040), total recall (r = − 0.408, p = 0.025), and delayed recall (r = − 0.395, p = 0.031). On the other hand, an increase in the duration of illness was associated with a decrease in the total score on the MOCA (r = − 0.588, p = 0.001), whereas an early onset of illness was associated with an more deterioration of the total IQ score (r = − 0.456, p = 0.011).
In the case of individuals diagnosed with schizophrenia, the correlations observed between clinical variables, psychometric scales, and ERPs had no significant correlation between different parameters with each other.
Discussion
The current study's main findings include patients with SZ and BD had significantly lower scores on the total, verbal, and performance assessment of the WAIS-III compared with controls. However, SZ patients exhibited a higher Deterioration Index (DI) compared with controls; however, there was no difference in DI between BD patients and controls.
Previous research utilizing the WAIS-III to assess cognitive function has consistently demonstrated that SZ patients perform worse than BD and control individuals in verbal and performance domains. Compared to controls, BD patients also exhibit lower scores on overall IQ, verbal, and performance assessment subtests [30]. Another meta-analysis comparing the intellectual capacity of BD and SZ patients before and after illness onset with a control group found that SZ patients experienced a significant decline in premorbid intellectual function, whereas BD individuals did not [31]. Additionally, SZ were found to have slightly lower general intelligence than BD, with both groups displaying significantly lower general intelligence than healthy individuals [32]
In this study, individuals with SZ exhibited significantly lower total scores on the MoCA assessment, particularly in the visuospatial, naming, attention, delayed recall, and orientation subtests, as well as all subscales of the BVMT-R assessment, compared with controls. On the other hand, individuals with BD had significantly lower scores than controls in visuospatial and delayed recall subtests, as well as on trial 2 and total recall only. Moreover, the SZ group demonstrated more impairment in trials 1, 2, 3, total recall, and delayed recall, as evidenced by higher RDI scores than the BD group.
Several systematic reviews and meta-analyses have been performed to compare cognitive domains in SZ and BD, yielding contradictory results. One of this meta-analysis reported that patients with SZ performed worse than BP. BD patients performed worse in 9 of 11 cognitive domains, including verbal fluency, verbal working memory, delayed visual memory, executive control, and mental speed. SZ patients also performed worse in verbal memory, intelligence, and concept information, albeit with minor effect sizes [33].
Previous research has identified lower IQ, executive function, visual memory, language, and linguistic expertise in SZ patients, with varying patterns of decline at different ages. However, impairments in verbal memory, working memory, processing speed, and visuospatial ability were present from the early stages and remained stable.
In contrast, BD patients displayed worse performance in IQ, verbal knowledge, and language, but only in verbal functions and at different ages. Deficits in verbal memory, processing speed, and executive function also remained stable. SZ and BD patients experienced cognitive impairment in general and specialized functions following the initial episode, but the specific nature of the impairments varied with age. For effective cognitive rehabilitation, focusing on specific adult functions may be the most successful approach [3].
Additionally, some studies have reported that patients with SZ performed worse than BD in visual learning, working memory, and attention persistence, while other studies failed to confirm these findings [34, 35]. Conversely, meta-analysis on executive dysfunction tests indicated that the kind of cognitive deficits did not differentiate between BD and SZ [36] However, Jenkins et al. found that the BD group with psychosis outperformed the SZ group in processing speed, attention, visual, and working memory [34].
Genetic factors have been linked to brain function and cognitive deficiencies, although the precise mechanisms remain unknown [37, 38], while the structural and functional differences in various brain regions between SZ and BD patients are still unclear, studies suggested that neurodevelopmental variables may play a role in cognitive deficits in SZ and may be in a subgroups of BD patients [39].
A meta-analysis encompassing all available research found that individuals with BD generally performed better than SZ patients. The differences in cognitive impairment profiles observed in previous studies may be attributed to using different cognitive function assessment instruments. Other factors that could potentially explain the contradictory findings in cognitive function assessment in euthymic BD patients include the number of episodes, chronicity of the illness, length of hospitalization, and types of antipsychotic medication [40], comorbidities such as substance misuse, definitions of euthymia, and the specific cognitive tests employed. Additionally, factors that may introduce bias in assessing cognitive function, the representativeness of the samples, the specific cognitive domains assessed, and the recruitment methods employed [41].
Regarding memory assessment, both BD and SZ groups performed significantly worse than the controls across all four subscales (P < 0.001). Patients with SZ had significant impairment more than patients with BD in all subscales except verbal assessment.
Some studies have suggested that SZ and BD differ in working memory [42, 43]. Working memory is recognized as a core cognitive domain that is impacted by major mental illness [44], so it serves as a suitable endophenotypic marker for distinguishing between SZ and BD [45]. Another investigation assessing the cognitive profiles of people with early-onset SZ and pediatric-onset BD showed the prevalence of abnormalities in numerous domains in SZ and a comparable but less severe of cognitive impairment in BD [46]. Conversely, another study found no significant reduction in the Working Memory index, indicating that illness duration may impact cognitive decline.
The study also employed event-related potentials (ERPs), specifically the P300 component, to assess cognitive function. The P300 ERP is a late cognitive-related signal linked to attention and memory functions [47].
P300, an indicator of the process of discriminating between significant and insignificant brain stimuli, is dissected into two fundamental components. These two basic components are known as amplitude and delay. While the amplitude of the P300 changes due to the inability of the targets, the latency changes due to the difficulty of distinguishing the target stimulant from the standard stimulants. In most individuals with a psychiatric disease and reduced cognitive skills, latency is often shortened while P300 amplitude decreases.
This study observed no significant differences in P300 latencies and amplitudes between the three groups at different assessment points. However, BD had significantly shorter P300 latency compared with controls and compared with SZ group separately. This is due to changes in the information processing in the brain [48] as BD had increased physiological arousal and rapid information process. Previous research has indicated that BD and SZ patients have lower P300 amplitudes than healthy individuals [49, 50] suggesting that P300 amplitude alone may not distinguish between these disorders. These findings support the notion that lower amplitude and delayed latency of P300 may be implicated in the pathogenesis of both BD and SZ, irrespective of psychotic history [51, 52]. In the current study, absence of significant correlation between P300 parameters and different clinical variables in both ZS and BD could be due to the normal P300 latency values ranging between 250 and 350 ms were established in adults by Kraus et al. [53]. However, wider latency ranges, were also reported [54] and this wide range of normal values as well as the small sample size of each group may explain the absence of such correlation and may explain why P300 is not routinely used in clinical practice.
While early results suggested that SZ and BD could potentially be spectrum of psychoses [36] investigations have shown that SZ has higher grey matter atrophy [55] and worse global connectivity than BD. Poor global connectivity could lead to worse verbal learning, processing speed, and attention performance in SZ [36, 56].
In the present study, an increased number of episodes in individuals with mood disorders and a longer duration of illness were found to be associated with a decline in cognitive function. Previous research has consistently reported that heightened cognitive dysfunction, particularly in areas such as attention and memory, is frequently observed alongside greater symptom severity [57]. Additionally, it has been suggested that frontal dysfunction may be linked to a prolonged duration of illness [58].
In the context of mood disorders, higher scores on cognitive function assessments were observed in individuals with more years of formal education. Previous studies have consistently demonstrated a positive correlation between the level of formal education and cognitive abilities [59]. Furthermore, it has been noted that education pertaining to mental health plays a significant role in fostering educational resources, cognitive skills, and social integration [60].
Our study has several limitations, such as a small sample size, we did not control for the types, dosage, or duration of psychotropics, duration of untreated [61], possible biological etiology [62], and the study design being an observational case–control study with no follow-up data.
In conclusion, patients with SZ and BP had significantly lower scores on various cognitive function domains compared with the control group with more affection in SZ. The importance of considering factors such as number of mood episodes, duration of illness, education, and specific cognitive domains in understanding the cognitive profiles of these psychiatric conditions. Further studies are warranted to investigate the underlying mechanisms of cognitive dysfunction in both SZ and BD patients, which may help in developing better treatment strategies for these patients.
Availability of data and materials
All data generated or analyzed during this study are available from corresponded on request.
Abbreviations
- SZ:
-
Schizophrenia
- BD:
-
Bipolar disorder
- Cz:
-
Central
- Fz:
-
Frontal
- Pz:
-
Parietal
- ERP:
-
Event-related potential
- MoCA:
-
Montreal Cognitive Assessment Scale
- WAIS-III:
-
Wechsler Adult Intelligence Scale-3rd edition
- BVMT-R:
-
Brief Visuospatial Memory Test-Revised
- MAS:
-
Memory Assessment Scales
- DI:
-
Deterioration Index
- RDI:
-
Recognition Discrimination Index
References
Barch DM, Ceaser A (2012) Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci 16(1):27–34. https://doi.org/10.1016/j.tics.2011.11.015
Depp CA, Mausbach BT, Harmell AL, Savla GN, Bowie CR, Harvey PD et al (2012) Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord 14(3):217–226. https://doi.org/10.1111/j.1399-5618.2012.01011.x
Zanelli J, Reichenberg A, Sandin S, Morgan C, Dazzan P, Pilecka I et al (2022) Dynamic and static cognitive deficits in schizophrenia and bipolar disorder after the first episode. Schizophr Bull 48(3):590–598. https://doi.org/10.1093/schbul/sbab150
Solé B, Jiménez E, Torrent C, Reinares M, Bonnin CDM, Torres I et al (2017) Cognitive impairment in bipolar disorder: treatment and prevention strategies. Int J Neuropsychopharmacol 20(8):670–680. https://doi.org/10.1093/ijnp/pyx032
Robinson LJ, Ferrier IN (2006) Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 8(2):103–16. https://doi.org/10.1111/j.1399-5618.2006.00277.x
Bora E, Yucel M, Pantelis C (2009) Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 113(1–2):1–20. https://doi.org/10.1016/j.jad.2008.06.009
Samalin L, de Chazeron I, Vieta E, Bellivier F, Llorca PM (2016) Residual symptoms and specific functional impairments in euthymic patients with bipolar disorder. Bipolar Disord. 18(2):164–73. https://doi.org/10.1111/bdi.12376
Kumar CT, Frangou S. Clinical implications of cognitive function in bipolar disorder. Ther Adv Chronic Dis. 2010;1(3):85–93.https://doi.org/10.1177/2040622310374678
Kyaga S, Lichtenstein P, Boman M, Hultman C, Långström N, Landén M (2011) Creativity and mental disorder: family study of 300,000 people with severe mental disorder. Br J Psychiatry. 199(5):373–9. https://doi.org/10.1192/bjp.bp.110.085316
Demjaha A, MacCabe JH, Murray RM (2012) How genes and environmental factors determine the different neurodevelopmental trajectories of schizophrenia and bipolar disorder. Schizophr Bull. 38(2):209–14. https://doi.org/10.1093/schbul/sbr100
Kahn RS, Keefe RS (2013) Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 70(10):1107–12. https://doi.org/10.1001/jamapsychiatry.2013.155
Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry. 2013;170(3):334–41.https://doi.org/10.1176/appi.ajp.2012.12040490
Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C (2004) A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 71(2–3):405–16. https://doi.org/10.1016/j.schres.2004.03.002
Goodwin GM, Martinez-Aran A, Glahn DC, Vieta E (2008) Cognitive impairment in bipolar disorder: neurodevelopment or neurodegeneration? An ECNP expert meeting report. Eur Neuropsychopharmacol. 18(11):787–93. https://doi.org/10.1016/j.euroneuro.2008.07.005
Pearlson GD (2015) Etiologic, phenomenologic, and endophenotypic overlap of schizophrenia and bipolar disorder. Annu Rev Clin Psychol. 11:251–81. https://doi.org/10.1146/annurev-clinpsy-032814-112915
Craddock N, Owen MJ. The Kraepelinian dichotomy - going, going... but still not gone. Br J Psychiatry. 2010;196(2):92–5.https://doi.org/10.1192/bjp.bp.109.073429
Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF et al (2009) Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 373(9659):234–9. https://doi.org/10.1016/S0140-6736(09)60072-6
Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL et al (2007) The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry 62(8):910–916. https://doi.org/10.1016/j.biopsych.2007.02.001
Quraishi S, Frangou S (2002) Neuropsychology of bipolar disorder: a review. J Affect Disord 72(3):209–226. https://doi.org/10.1016/s0165-0327(02)00091-5
Schretlen DJ, Cascella NG, Meyer SM, Kingery LR, Testa SM, Munro CA et al (2007) Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry 62(2):179–186. https://doi.org/10.1016/j.biopsych.2006.09.025
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). APA, Washington, DC, p 2013
Tsapekos D, Strawbridge R, Cella M, Wykes T, Young AH (2021) Cognitive impairment in euthymic patients with bipolar disorder: Prevalence estimation and model selection for predictors of cognitive performance. J Affect Disord. 294:497–504. https://doi.org/10.1016/j.jad.2021.07.036
Max H (1960) A RATING SCALE FOR DEPRESSION. J Neurol Neurosurg Psychiatry 23(1):56. https://doi.org/10.1136/jnnp.23.1.56
Young RC, Biggs JT, Ziegler VE, Meyer DA (1978) A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133(11):429–435. https://doi.org/10.1192/bjp.133.5.429
Wechsler D. WAIS-R : manual : Wechsler adult intelligence scale--revised. New York, NY: Harcourt Brace Jovanovich [for] Psychological Corp.; 1981.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I et al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B (1996) Revision of the brief visuospatial memory test: studies of normal performance, reliability, and validity. Psychol Assess 8:145–153. https://doi.org/10.1037/1040-3590.8.2.145
Williams JM (1991) Memory assessment scales. Odessa, Psychological Assessment Resources, 199(1):1–127
Picton TW (1992) The P300 wave of the human event-related potential. J Clin Neurophysiol 9(4):456–479. https://doi.org/10.1097/00004691-199210000-00002
Fujino H, Sumiyoshi C, Sumiyoshi T, Yasuda Y, Yamamori H, Ohi K et al (2014) Performance on the W echsler A dult I ntelligence S cale-III in J apanese patients with schizophrenia. Psychiatry Clin Neurosci 68(7):534–541
Trotta A, Murray R, MacCabe J (2015) Do premorbid and post-onset cognitive functioning differ between schizophrenia and bipolar disorder? A systematic review and meta-analysis. Psychol Med 45(2):381–394
Afshari B, Shiri N, Ghoreishi FS, Valianpour M (2020) Examination and comparison of cognitive and executive functions in clinically stable schizophrenia disorder, bipolar disorder, and major depressive disorder. Depress Res Treat 2020:2543541. https://doi.org/10.1155/2020/2543541
Krabbendam L, Arts B, van Os J, Aleman A (2005) Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res 80(2–3):137–149
Jenkins LM, Bodapati AS, Sharma RP, Rosen C (2018) Working memory predicts presence of auditory verbal hallucinations in schizophrenia and bipolar disorder with psychosis. J Clin Exp Neuropsychol 40(1):84–94
Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF (2013) Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry 170(3):334–341
Argyelan M, Ikuta T, DeRosse P, Braga RJ, Burdick KE, John M et al (2014) Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr Bull 40(1):100–110
Butcher LM, Davis OS, Craig IW, Plomin R (2008) Genome-wide quantitative trait locus association scan of general cognitive ability using pooled DNA and 500K single nucleotide polymorphism microarrays. Genes Brain Behav 7(4):435–446
Le Hellard S, Håvik B, Espeseth T, Breilid H, Løvlie R, Luciano M et al (2009) Variants in doublecortin-and calmodulin kinase like 1, a gene up-regulated by BDNF, are associated with memory and general cognitive abilities. PLoS ONE 4(10):e7534
Schneider MR, DelBello MP, McNamara RK, Strakowski SM, Adler CM (2012) Neuroprogression in bipolar disorder. Bipolar Disord 14(4):356–374
Dixon T, Kravariti E, Frith C, Murray R, McGuire P (2004) Effect of symptoms on executive function in bipolar illness. Psychol Med 34(5):811–821
Cullen B, Ward J, Graham NA, Deary IJ, Pell JP, Smith DJ et al (2016) Prevalence and correlates of cognitive impairment in euthymic adults with bipolar disorder: a systematic review. J Affect Disord 205:165–181. https://doi.org/10.1016/j.jad.2016.06.063
Bora E, Pantelis C (2015) Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophr Bull 41(5):1095–1104
Stefanopoulou E, Manoharan A, Landau S, Geddes JR, Goodwin G, Frangou S (2009) Cognitive functioning in patients with affective disorders and schizophrenia: a meta-analysis. Int Rev Psychiatry 21(4):336–356. https://doi.org/10.1080/09540260902962149
Žakić Milas D, Milas G (2019) Working memory in patients with schizophrenia and bipolar affective disorder: quantitative or qualitative differences? Psychiatr Danub 31(1):54–61
Pirkola T, Tuulio-Henriksson A, Glahn D, Kieseppä T, Haukka J, Kaprio J et al (2005) Spatial working memory function in twins with schizophrenia and bipolar disorder. Biol Psychiat 58(12):930–936
Nieto RG, Castellanos FX (2011) A meta-analysis of neuropsychological functioning in patients with early onset schizophrenia and pediatric bipolar disorder. J Clin Child Adolesc Psychol 40(2):266–280
Nehra R, Grover S, Chetri D, Sood A, Das CP (2012) P300 latency and neurocognitive functioning in recently diagnosed human immunodeficiency virus patients. Indian J Psychol Med 34(4):376–380. https://doi.org/10.4103/0253-7176.108225
Poyraz B, Sakallı Kani A, Aksoy Poyraz C, Öcek Baş T, Arıkan MK (2017) Cognitive psychophysiological substrates of affective temperaments. Clin EEG Neurosci. 48(2):96–102. https://doi.org/10.1177/1550059416650112
Turetsky BI, Dress EM, Braff DL, Calkins ME, Green MF, Greenwood TA et al (2015) The utility of P300 as a schizophrenia endophenotype and predictive biomarker: clinical and socio-demographic modulators in COGS-2. Schizophr Res 163(1–3):53–62. https://doi.org/10.1016/j.schres.2014.09.024
Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS et al (2015) Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biological Psychiatry 77(2):127–36. https://doi.org/10.1016/j.biopsych.2014.03.032
Bestelmeyer PE (2012) The visual P3a in schizophrenia and bipolar disorder: effects of target and distractor stimuli on the P300. Psychiatry Res 197(1–2):140–144. https://doi.org/10.1016/j.psychres.2011.09.030
Johannesen JK, O’Donnell BF, Shekhar A, McGrew JH, Hetrick WP (2013) Diagnostic specificity of neurophysiological endophenotypes in schizophrenia and bipolar disorder. Schizophr Bull 39(6):1219–1229
Kraus N, McGee T (1994) Auditory event-related potentials. In: Hand book of clinical audiology. Baltimore, Williams and Wilkins, pp. 406–426
McPherson DL (1996) Late potentials of the auditory system. Singular Pub Group
Birur B, Kraguljac NV, Shelton RC, Lahti AC (2017) Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder-a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophr 3:15. https://doi.org/10.1038/s41537-017-0013-9
Li W, Zhou F-C, Zhang L, Ng CH, Ungvari GS, Li J et al (2020) Comparison of cognitive dysfunction between schizophrenia and bipolar disorder patients: a meta-analysis of comparative studies. J Affect Disord 274:652–661
Marvel CL, Paradiso S. Cognitive and neurological impairment in mood disorders. Psychiatr Clin North Am. 2004;27(1):19–36, vii-viii.https://doi.org/10.1016/S0193-953X(03)00106-0
Buoli M, Caldiroli A, Caletti E, Zugno E, Altamura AC (2014) The impact of mood episodes and duration of illness on cognition in bipolar disorder. Compr Psychiatry 55(7):1561–6. https://doi.org/10.1016/j.comppsych.2014.06.001
Lövdén M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker-Drob EM (2020) Education and Cognitive Functioning Across the Life Span. Psychol Sci Public Interest 21(1):6–41. https://doi.org/10.1177/1529100620920576
Jiang W, Lu Y, Xie H (2020) Education and mental health: Evidence and mechanisms. J Economic Behav Organization. 180:407–37. https://doi.org/10.1016/j.jebo.2020.09.032
Ahmed GK, Elbeh K, Khalifa H, Samaan MR (2021) Impact of duration of untreated illness in bipolar I disorder (manic episodes) on clinical outcome, socioecnomic burden in Egyptian population. Psychiatry Research 296:113659. https://doi.org/10.1016/j.psychres.2020.113659
Hussein EAM, Khalifa H, Ramadan GK, Hassaan SH, Shaaban I, Farrag HMM. Seroprevalence of Toxoplasma gondii among patients with schizophrenia and bipolar disorder in Upper Egypt: a comparative study with a control group. Ann Parasitol. 2020;66(2):183–92.https://doi.org/10.17420/ap6602.253
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EK, NG, KE, GA, and BF recruited participants, analysis, and interpreted data, and were the contributors in writing the manuscript. All authors revised data interpretation, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Aswan University faculty of medicine’s institutional review board approved the study for ethical reasons. To participate in the study, all subjects provided written informed permission. All participant personal information was kept private, and all patient personal data was anonymized immediately following data collection. We verified that all processes were carried out in accordance with the applicable norms and regulations.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khedr, E.M., Ghanima, N.E., Elbeh, K.A. et al. Cognitive impairment among an Egyptian sample of patients with schizophrenia and bipolar disorders: a comparative study. Middle East Curr Psychiatry 30, 70 (2023). https://doi.org/10.1186/s43045-023-00344-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43045-023-00344-y