Abstract
Background
Calcified coronary arteries encountered during percutaneous intervention increase the probability of unsuccessful procedures. Heavy calcification of coronary arteries may lead to suboptimal stent expansion. Intravascular lithotripsy (IVL) is a novel method of transmitting sonic waves in pulses, which fractures the calcific plaque in the vessel with minimal soft tissue injury. This study systematically reviews and summarizes the reported clinical scenarios in which IVL was successfully used in coronary lesions.
Main text
Articles were obtained by searching PubMed and Embase databases for IVL use in coronary arteries. We restricted the search to case reports. Our study included 84 patients from 70 case reports/case series. The mean age was 70.3 years (SD 10) and ranged from 27 to 96 years, and 67% were males. The indications for the angiogram that led to the use of IVL include chest pain (37.7%), non-ST elevated myocardial infarction (27.9%), ST elevated myocardial infarction (13.1%), and previous under-expanded stent (8.2%). The IVL was used in the left anterior descending artery (60.7%), right coronary artery (35.7%), left main disease (23.8%), and left circumflex (9.5%). Coronary IVL was safely and successfully used in different clinical scenarios for heavily calcified coronary lesions, including in-stent restenosis of native coronary arteries, saphenous vein grafts, and under-expanded stents. In addition, IVL was successfully used synergistically with orbital and rotational atherectomy and drug-coated balloon angioplasty in select patients.
Conclusion
IVL has successfully been used in an expanding array of clinical scenarios.
Similar content being viewed by others
Background
Calcification of coronary arteries encountered during percutaneous coronary intervention (PCI) increases the probability of an unsuccessful procedure. Heavy calcification of coronary arteries may lead to suboptimal stent expansion, interference in catheter crossing, and problems with balloon dilatation. It may also increase the risk of stent thrombosis and stent stenosis [1, 2]. There is also an increased risk of major adverse cardiovascular events (MACEs).
Recent advancements in medicine have introduced techniques to help with successful intervention in coronary arteries by providing adequate lesion preparation. Rotational atherectomy is commonly used as an intervention for severely calcified plaques. The atherectomy devices modify superficial calcium but do not modify the deep-seated calcium in a vessel, which causes a restriction in the expansion during PCI [1]. Recently, intravascular lithotripsy has been used to help defragment calcium deposition in the coronary arteries.
Intravascular lithotripsy (IVL) is a novel vessel preparation method to facilitate PCI. The technique is based on transmitting sonic waves in pulses, which fractures the calcific plaque in the vessel with minimal soft tissue injury [3]. The fracture in the calcified plaque provides improved vessel compliance and helps facilitate stent expansion. The procedure is being performed in many countries, and it has been reported to have high success rates and a low risk of complications.
There have been a growing number of reported cases of intravascular lithotripsy use in coronary and noncoronary artery vessels. Though clinicians are successfully trying out IVL in new clinical scenarios, there has not been any recent systematic review of these cases. Therefore, this study aims to systematically review and summarize the reported cases of IVL in patients with heavily calcified coronary arteries.
Main text
Articles were obtained by searching PubMed and Embase databases with the keywords coronary artery intravascular lithotripsy. We restricted the search to case reports. Two authors independently reviewed the titles and abstracts to determine the articles that met our inclusion and exclusion criteria. To be included in the study, the article must be a case report or case series on the use of IVL in coronary arteries. We excluded articles not written in English, articles that did not include the demographic data of the cases, and conference abstracts not published in a journal. After selecting the articles that met our inclusion criteria, we reviewed the full texts and extracted the data into a spreadsheet. The data that were extracted included the age and gender of the patients, comorbidities, the coronary vessels where IVL was used, and the complications reported.
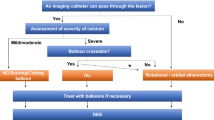
We did a quantitative analysis using means and percentages to describe the patients' age, gender, comorbidities, and the vessels where IVL was done. We also did a qualitative analysis describing the unique situations and off-label use of IVL in calcified coronary arteries reported in the articles. We excluded articles that did not provide patient information, such as age and gender, from the quantitative analysis. However, we included such articles in the qualitative analysis (Fig. 1).
Quantitative result
Our study included 84 patients from 70 case reports/case series. The mean age was 70.3 years (SD 10) and ranged from 27 to 96 years, and 67% were males. The major comorbidities include hypertension (35.7%), diabetes mellitus (33.3%), hyperlipidemia (22.6%), chronic kidney disease (7.1%), and chronic obstructive pulmonary disease (7.1%). The indications for the angiogram that led to the use of IVL include chest pain (37.7%), non-ST elevated myocardial infarction (27.9%), ST elevated myocardial infarction (13.1%), and previous under-expanded stent (8.2%). The IVL was used in the left anterior descending artery (60.7%), right coronary artery (35.7%), left main disease (23.8%), and circumflex artery (9.5%) (Tables 1, 2).
Qualitative result and discussion
In-stent restenosis of native arteries
IVL was effectively and safely used in many in-stent restenosis cases, including in-stent restenosis secondary to under-expanded stents [4,5,6,7]. IVL has been used in calcified in-stent restenosis lesions, especially when atherectomy is technically contraindicated [4]. IVL was used in in-stent restenosis after multiple attempts with a balloon failed to expand the lesion. A drug-coated balloon angioplasty was deployed after IVL [4, 5]. Kaniappan et al. successfully deployed the same IVL balloon catheter on the left anterior descending (LAD) and left circumflex artery (LCX), showing that deploying the same IVL balloon catheter in two different vessels was feasible [5]. However, deploying the same balloon catheter on multiple vessels is now more common.
Rotational and halfway rotational atherectomy and IVL
Rotational atherectomy is the most commonly used atherectomy approach for heavily calcified coronary artery lesions [8]. It works in a drill-like fashion with a maximum burr-to-vessel ratio of 0.7 [9]. It is recommended to treat severely calcified or fibrotic lesions that may be difficult to cross or dilate before stent placement [8]. There were reported cases of rotational atherectomy effectively and safely combined with IVL [10, 11]. In the case reported, rotational atherectomy was initially used to treat the heavily calcified lesions. Then, IVL provided further and more profound calcium modification, resulting in successful stent placement [10]. Safe and successful synergistic use of halfway rotational atherectomy with IVL was also reported [11]. In the halfway rotational atherectomy, the Burr was not advanced beyond any acute angle within the calcified lesion because of wire kinking, and IVL was then used for the lesion that the Burr did not get to [11].
Post-dilation of under-expanded coronary stents
There were reports of IVL used in previously implanted but under-expanded coronary stents [12, 13]. Though an off-label use, IVL has been reported as safe and effective in cases where the stent was not fully expanded in the first PCI, and a second PCI was done with IVL, resulting in full stent expansion [13]. IVL was also safe and effective in treating under-expanded stents at the de novo PCI, where IVL was used for post-dilatation, resulting in full stent expansion [12]. There was also a case of a poorly expanded stent that failed treatment with several inflations with a noncompliant balloon, with persistent residual 70% stenosis. IVL was safely and successfully deployed, resulting in 0% residual stenosis [14]. Though IVL effectively treats under-expanded coronary stents, adequately modifying the plaque before stent deployment is a priority [12].
IVL and drug-coated balloon angioplasty
Drug-coated balloon (DCB) angioplasty has emerged as an attractive strategy for leaving nothing behind during PCI [15]. DCB results in a homogenous and fast release of antiproliferative drugs into the vessel wall and inhibits neointimal hyperplasia without leaving a permanent metallic frame behind, as seen with drug-eluting stents. This eliminates the risk of in-stent thrombosis and decreases the length of dual antiplatelet therapy [16]. DCB is used for in-stent restenosis of both bare-metal and drug-eluting stents, as well as in de novo small-vessel disease and patients with high bleeding risk [17, 18].
IVL was used successfully with DCB in multiple cases. Jun Sim et al. reported seven patients safely and successfully treated with IVL and drug-coated balloon angioplasty for de novo-calcified coronary lesions [19]. Angiographic success, defined as < 30% residual stenosis, was achieved in six patients (86%), while one patient had post-procedure 50% residual stenosis. Furthermore, Ashari et al. reported that IVL was successfully used synergistically in a patient with recurrent in-stent restenosis [4]. After attempted pre-dilatation failed to achieve good lesion preparation, IVL was used. Following the use of IVL, the intravascular ultrasound showed multiple cracks within the calcified lesion. Drug-coated balloon angioplasty was then deployed, resulting in good angiographic results with good flow [4].
IVL and orbital atherectomy
Orbital atherectomy is approved to treat severely calcified coronary artery lesions to facilitate stent delivery. Orbital atherectomy utilizes centrifugal force to create cracks in heavily calcified lesions and change the lesions' compliance and morphology [8, 20]. Chiang et al. reported a case of an unsuccessful use of IVL in a heavily calcified mid-RCA lesion. Orbital atherectomy was performed, and IVL was done again with success [21]. This case shows that IVL can be safely and effectively used synergistically with orbital atherectomy. Orbital atherectomy debulked the calcium and allowed further lesion cracking with IVL [21].
IVL use in saphenous vein graft stenosis
Saphenous vein grafts (SVGs) are commonly used in coronary artery bypass surgery, although their long-term patency is worse than arterial bypass grafts [22]. Percutaneous coronary intervention (PCI) to SVG is sometimes done despite the high incidence of stent failure [23]. PCI to calcified saphenous vein grafts can be challenging, and the use of laser and rotational atherectomy has been reported with limited data [23]. Øksnes et al. presented a case series of five patients with calcified de novo SVG disease or SVG stent failure where IVL was successfully utilized. In four cases, IVL was used before drug-eluting stents (DES) were placed. In the fifth case, the patient had SVG in-stent restenosis, and IVL was followed by drug-eluting balloons, with good outcomes [23]. Although the use of IVL in SVG is currently off-label, these cases suggest that IVL can be safely and effectively used to treat de novo-calcified SVG lesions and SVG stent failure in selected patients.
Success of IVL procedures
Our study showed nearly 100% clinical and angiographic success in the use of IVL in patients with heavily calcified coronary artery lesions. While the near 100% success might be due to a reporting bias because authors are more likely to publish a successful case of IVL, previous meta-analyses of IVL observational studies have reported high success rates. A systematic review and meta-analysis involving eight observational studies with 980 patients showed clinical success with IVL in 95.4% of patients and angiographic success in 97% of patients [24]. Clinical success was defined as successful stent delivery with IVL that results in less than 50% residual diameter stenosis without in-hospital major adverse cardiac events (MACEs), such as myocardial infarction, or revascularization of the same lesion after the completion of the initial procedure. Angiographic success was defined as successful stent delivery with IVL that results in less than 50% residual diameter stenosis without significant angiographic complications such as coronary perforation, persistent slow flow, no-reflow, or abrupt closure [24]. Similarly, multiple systematic reviews of IVL have reported significant improvement in the post-IVL lumen diameter and a significant reduction in the luminal calcium angle and maximum calcium thickness [2, 24, 25].
Limitations of the study
One limitation of our review of case reports is that the study has a small sample size. Small sample sizes can lead to variability in outcomes and may not fully capture the diversity of patient responses to IVL. Furthermore, authors will likely publish cases that had a successful outcome, leading to publication bias. This potential publication bias could skew the overall impression of IVL's efficacy and safety. The high success rates reported may not accurately reflect the real-world performance of IVL, where less favorable outcomes might be underreported. Therefore, more studies are required to assess the efficacy and safety of IVL in some of the clinical scenarios described in the articles. Additionally, this review focuses on short-term procedural success and immediate angiographic results, as the case reports do not address long-term outcomes, including the results' durability and potential late complications. Finally, given the small number of cases and the potential for selective reporting, the generalizability of the findings to broader patient populations is limited.
Conclusions
Our study showed that IVL has been successfully used in different clinical scenarios for heavily calcified coronary lesions, including in-stent restenosis of native coronary arteries, SVG, and under-expanded stents. In addition, IVL was successfully used synergistically with orbital and rotational atherectomy and drug-coated balloon angioplasty in select patients.
Availability of data and materials
All papers analyzed during this study are included in this published article in Table 2.
Abbreviations
- DCB:
-
Drug-coated balloon
- DES:
-
Drug-eluting stents
- IVL:
-
Intravascular lithotripsy
- LAD:
-
Left anterior descending artery
- LCX:
-
Left circumflex artery
- MACE:
-
Major adverse cardiovascular events
- PCI:
-
Percutaneous coronary intervention
- RCA:
-
Right coronary artery
- SVG:
-
Saphenous vein grafts
References
Neleman T, Ziedses des Plantes AC, Daemen J (2022) Coronary lithotripsy—a state of the art review. Trends Cardiovasc Med. https://doi.org/10.1016/j.tcm.2022.01.003
Sattar Y, Ullah W, Virk HUH, Doshi R, Rauf H, Desai H, Panchal A, Nasir M, Almas T, Ullah I, Pacha HM, Zaher N, Alraies MC (2021) Coronary intravascular lithotripsy for coronary artery calcifications—systematic review of cases. J Commun Hosp Intern Med Perspect 11(2):200–205. https://doi.org/10.1080/20009666.2021.1883219
Cubero-Gallego H, Calvo-Fernandez A, Tizon-Marcos H, Aparisi A, Gomez-Lara J, Amat-Santos I, Fuertes M, Santos-Martinez S, Salvatella N, Garcia-Guimaraes M, Negrete A, Mohandes M, Gomez-Hospital JA, Moris C, Vaquerizo B (2022) Real-world multicenter coronary lithotripsy registry: long-term clinical follow-up. J Invasive Cardiol 34(10):E701–E708
Ashari A, Aris FA, Lim YH, Rosman A (2022) Third in-stent restenosis in 5 years—are we doing enough? Role of intravascular ultrasound and lithotripsy in severely calcified ISR. J Am Coll Cardiol 79(15_Supplement):S107–S108. https://doi.org/10.1016/j.jacc.2022.03.150
Kaniappan K, Kandasamy B, Rusani BI, Haroon AY, Yong D (2022) The road less travelled. J Am Coll Cardiol 79(15_Supplement):S104–S106. https://doi.org/10.1016/j.jacc.2022.03.149
Raza A, Yousafzai OK, Lee J, Safi LM, Faraz HA, Vaidya PJ, Patel AK (2022) Revisiting an old adversary—coronary calcium. J Am Coll Cardiol 79(9_Supplement):2875. https://doi.org/10.1016/S0735-1097(22)03866-9
Xu J, Cross MS Jr, Patel S, Alaref SJ (2022) Utilizing shockwave therapy for the treatment of recurrent instent restenosis and significant coronary calcification. J Am Coll Cardiol 79(9_Supplement):2284. https://doi.org/10.1016/S0735-1097(22)03275-2
Goel S, Pasam RT, Chava S, Gotesman J, Sharma A, Malik BA, Frankel R, Shani J, Gidwani U, Latib A (2020) Orbital atherectomy versus rotational atherectomy: a systematic review and meta-analysis. Int J Cardiol 15(303):16–21
Abdel-Wahab M, Richardt G, Joachim Büttner H, Toelg R, Geist V, Meinertz T, Schofer J, King L, Neumann FJ, Khattab AA (2013) High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv 6(1):10–19
Dargan J, Miles C, Wilson S et al (2022) 74 Rotashock left main bifurcation primary angioplasty with balloon pump support and intravascular image guidance. Heart 108:A55–A56
Chu HYZ (2022) A case using halfway rotational atherectomy. J Am Coll Cardiol 79(15_Supplement):S94–S96. https://doi.org/10.1016/j.jacc.2022.03.144
Sawhney R, Arman P, Christensen J, Alom M, Dib C, Al-Azizi K, Thomas SP, Potluri SP, Sayfo S (2022) Intravascular lithotripsy for de-novo post-dilation of coronary drug-eluting stents. J Am Coll Cardiol 79(9_Supplement):2282. https://doi.org/10.1016/S0735-1097(22)03273-9
Priolo L, Ferrarello S, Buccheri D, Lombardo R, Vinci D (2022) C27 shockwave deferred use in a case of inferior stemi. Eur Heart J Suppl 24((Supplement_C)):suac011-026. https://doi.org/10.1093/eurheartj/suac011.026
Raxwal T, Balhara C, Parekh D (2022) intravascular lithotripsy for underexpanded stent in heavily calcified coronary artery disease. Case Rep Cardiol 2022:7075933. https://doi.org/10.1155/2022/7075933
Jeger RV, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, Alfonso F, Latib A, Ong PJ, Rissanen TT, Saucedo J (2020) Drug-coated balloons for coronary artery disease: third report of the International DCB Consensus Group. Cardiovasc Interv 13(12):1391–1402
Ho HH, Lee JH, Khoo DZ, Hpone KK, Li KF (2021) Shockwave intravascular lithotripsy and drug-coated balloon angioplasty in calcified coronary arteries: preliminary experience in two cases. J Geriatr Cardiol JGC 18(8):689
Rissanen TT, Uskela S, Eränen J, Mäntylä P, Olli A, Romppanen H, Siljander A, Pietilä M, Minkkinen MJ, Tervo J, Kärkkäinen JM (2019) Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): a single-blind, randomised, non-inferiority trial. Lancet 394(10194):230–239
Jeger RV, Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Leibundgut G, Weilenmann D, Wöhrle J, Richter S, Schreiber M, Mahfoud F (2018) Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet 392(10150):849–856
Sim EWJ, Li KFC, Ho HH (2022) Novel use of shockwave intravascular lithotripsy with drug coated balloon angioplasty in de novo calcified coronary lesions. J Am Coll Cardiol 79(15_Supplement):S29. https://doi.org/10.1016/j.jacc.2022.03.064
Shlofmitz E, Martinsen BJ, Lee M, Rao SV, Genereux P, Higgins J, Chambers JW, Kirtane AJ, Brilakis ES, Kandzari DE, Sharma SK (2017) Orbital atherectomy for the treatment of severely calcified coronary lesions: evidence, technique, and best practices. Expert Rev Med Devices 14(11):867–879
Chiang CSM, Alan Chan KC, Lee M, Chan KT (2020) Orbital-tripsy: novel combination of orbital-atherectomy and intravascular-lithotripsy, in calcified coronaries after failed intravascular-lithotripsy. JACC Case Rep 2(15):2437–2444. https://doi.org/10.1016/j.jaccas.2020.10.027
Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR (1996) Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 28:616–626
Øksnes A, McEntegart M (2021) Intravascular lithotripsy in saphenous vein grafts and graft stent failure: a case series. Catheter Cardiovasc Interv 97(7):E945–E950
Mhanna M, Beran A, Nazir S, Sajdeya O, Srour O, Elzanaty A, Eltahawy EA (2022) Efficacy and safety of intravascular lithotripsy in calcified coronary lesions: a systematic review and meta-analysis. Cardiovasc Revasc Med 1(36):73–82
Sheikh AS, Connolly DL, Abdul F, Varma C, Sharma V (2021) Intravascular lithotripsy for severe coronary calcification: a systematic review. Minerva Cardiol Angiol 71(6):643–652. https://doi.org/10.23736/s2724-5683.21.05776-8
Simsek C, Vos J, IJsselmuiden A, Meuwissen M, van den Branden B, den Heijer P, Schölzel BE (2020) Coronary artery perforation after shockwave intravascular lithotripsy. Case Rep 2(2):247–249
Lee TJ, Wan Rahimi WFB, Low MY, Nurruddin AA (2021) Type E coronary artery dissection caused by intravascular lithotripsy balloon rupture; vessel anatomy and characteristics in a lithoplasty complication case as detailed by optical coherence tomography: a case report. Eur Heart J Case Rep. 5(12):ytab432. https://doi.org/10.1093/ehjcr/ytab432
Donisan T, Mertens A, Luay S (2021) Kawasaki related coronary artery disease refractory to angioplasty: the role of intravascular shockwave lithotripsy. Cureus 13(10):e19020. https://doi.org/10.7759/cureus.19020
Agrawal Y, Zoltowska D, Nazroo JR, Halabi AR (2021) Impella-assisted intracoronary lithotripsy of severely calcified left main coronary artery bifurcation for NSTEMI with cardiogenic shock. Cureus 13(4):e14772. https://doi.org/10.7759/cureus.14772
Tehrani S, Rathore S, Achan V (2020) Changing paradigm for treatment of heavily calcified coronary artery disease. A complementary role of rotational atherectomy and intravascular lithotripsy with shockwave balloon: a case report. Eur Heart J Case Rep 5(1):ytaa456. https://doi.org/10.1093/ehjcr/ytaa456
Górny B, Balak W, Bielawski G, Ziołkowski M, Walukiewicz M, Grześk G (2020) Underexpanded stent in left anterior descending coronary artery treated with intravascular lithotripsy. Postepy Kardiol Interwencyjnej 16(2):216–218. https://doi.org/10.5114/aic.2020.96068
Pawłowski T, Legutko J, Modzelewski P, Gil RJ (2021) Synergistic application of high-speed rotational atherectomy and intravascular lithotripsy for a severely calcified undilatable proximal left anterior descending coronary artery bifurcation lesion: Case of rotalithoplasty-facilitated DK-CRUSH. Cardiol J 28(1):181–182. https://doi.org/10.5603/CJ.2021.0014
Opolski MP, Wolny R, Grodecki K, Debski A, Witkowski A (2019) Intravascular lithotripsy for heavily calcified subtotal occlusion of right coronary artery. Cardiol J 26(5):608. https://doi.org/10.5603/CJ.2019.0101
Yap LB, Choy CN, Balachandran K (2022) Intravascular ultrasound guided treatment of severe coronary artery calcification with shockwave intravascular lithotripsy. Med J Malays 77(1):116–118
Pineda A, Puri A, Jahangiri B (2019) Successful intravascular lithotripsy for severely calcified left anterior descending coronary artery stenosis. N Z Med J 132(1491):93–95
Çimci M, Iglesias JF, Huber C, Mach F, Roffi M (2020) Intravascular lithotripsy to treat an ostial left main coronary artery stenosis due to porcelain aorta in a patient with congenital high-density lipoprotein deficiency. Anatol J Cardiol 24(5):345–346. https://doi.org/10.14744/AnatolJCardiol.2020.62254
Sharma SK (2022) Shock wave intravascular lithotripsy (IVL)-assisted staged percutaneous coronary intervention (PCI) for a calcified right coronary artery in a patient with unstable angina: shock the rock. Cureus 14(4):e24489. https://doi.org/10.7759/cureus.24489
Curtis E, Khan A, El-Jack S, Glenie T (2019) Precipitation of de novo atrial fibrillation during Shockwave Intravascular Lithotripsy® after pacing capture during the treatment of proximal right coronary artery disease: a case report. Eur Heart J Case Rep 3(4):1–4. https://doi.org/10.1093/ehjcr/ytz147
Kozinski L, Orzałkiewicz Z (2020) Lithotripsy and ultrasound: Useful armamentarium in the case of ostial calcified stenosis of the right coronary artery. Cardiol J 27(1):89–90. https://doi.org/10.5603/CJ.2020.0019
Tomasiewicz B, Kosowski M, Zimoch W, Telichowski A, Kübler P, Reczuch K (2019) Heavily calcified coronary lesion treated by shockwave intravascular lithotripsy. Kardiol Pol 77(9):890–891. https://doi.org/10.33963/KP.14917
Wong B, El-Jack S, Newcombe R, Glenie T, Armstrong G, Cicovic A, Khan A (2019) Shockwave Intravascular lithotripsy of calcified coronary lesions in ST-elevation myocardial infarction: first-in-man experience. J Invasive Cardiol 31(5):E73–E75
Kaur N, Pruthvi CR, Sharma Y, Gupta H (2021) Rotatripsy: synergistic effects of complementary technologies: a case report. Eur Heart J Case Rep. 5(4):083. https://doi.org/10.1093/ehjcr/ytab083
McQuillan C, Alkhalil M, Johnston PW (2019) A paced heart without a pacemaker. Eur Heart J 40(10):819a. https://doi.org/10.1093/eurheartj/ehy749
Seif S, Kumar A, Arya S, Karthikeyan VJ (2021) Intravascular lithotripsy to treat an underexpanded coronary stent during index procedure: a case report study. Avicenna J Med 11(1):54–57. https://doi.org/10.4103/ajm.ajm_200_20
Legutko J, Niewiara Ł, Tomala M, Zajdel W, Durak M, Tomaszewski P, Szolc P, Żmudka K, Guzik B (2019) Successful shockwave intravascular lithotripsy for a severely calcified and undilatable left anterior descending coronary artery lesion in a patient with recurrent myocardial infarction. Kardiol Pol 77(7–8):723–725. https://doi.org/10.33963/KP.14859
Salazar CH, Travieso A, Gonzalo N, Escaned J (2019) Intracoronary lithotripsy in percutaneous treatment of calcific left main coronary stenoses. JACC Case Rep 1(1):46–49. https://doi.org/10.1016/j.jaccas.2019.05.008
Chan KCA, Luk NHV, Lee KYM, Chan KT (2019) A case of rota-shock-pella. JACC Case Rep 1(5):765–770. https://doi.org/10.1016/j.jaccas.2019.10.028
Pradhan A, Vishwakarma P, Bhandari M, Sethi R (2022) Intravascular lithotripsy for coronary calcium: a case report and review of the literature. World J Cardiol 14(9):496–507. https://doi.org/10.4330/wjc.v14.i9.496
Ali ZA, McEntegart M, Hill JM, Spratt JC (2020) Intravascular lithotripsy for treatment of stent underexpansion secondary to severe coronary calcification. Eur Heart J 41(3):485–486. https://doi.org/10.1093/eurheartj/ehy747
Marchese A, Tarantini G, Tito A, Margari V, Resta F, Dhojniku I, Paparella D, Speziale G (2021) Mechanical circulatory support and intravascular lithotripsy in high-risk patients undergoing percutaneous coronary intervention and transcatheter aortic valve replacement: a case series. Eur Heart J Case Rep. 5(12):ytab498. https://doi.org/10.1093/ehjcr/ytab498
Dimitriadis K, Aznaouridis K, Tsiamis E, Tsioufis K (2022) Intravascular lithotripsy as bail-out in an acute coronary syndrome patient with severe underexpansion of a previously implanted stent. J Invasive Cardiol 34(9):E692–E693
Yousif N, Bardooli F, Hussain T, Noor HA (2021) Precision percutaneous coronary intervention of a complex lesion. Rev Recent Clin Trials 16(2):220–224. https://doi.org/10.2174/1574887115666201009123721
Del Val D, Rivero F, Cuesta J, Bastante T, Alfonso F (2022) Coronary perforation after intravascular lithotripsy for severe stent underexpansion in a heavily calcified lesion. Coron Artery Dis 31(1):e17–e18. https://doi.org/10.1097/MCA.0000000000001078
Warisawa T, Goto S, Salazar CH, Akashi YJ, Escaned J (2019) Safety and feasibility of coronary lithotripsy supported by guide extension catheter for the treatment of calcified lesion in angulated vessel. Cardiovasc Revasc Med 20(11):6–8
Tizón-Marcos H, Rodríguez-Costoya I, Tevar C, Vaquerizo B (2020) Intracoronary lithotripsy for calcific neoatherosclerotic in-stent restenosis: a case report. Eur Heart J Case Rep 4(4):1–4. https://doi.org/10.1093/ehjcr/ytaa117
López-Lluva MT, Jurado-Román A, Sánchez-Pérez I, Abellán-Huerta J, Lozano R-P (2019) Shockwave: useful but potentially dangerous. JACC Cardiovasc Interv 12(5):500–501. https://doi.org/10.1016/j.jcin.2018.12.035
Ciardetti N, Ristalli F, Nardi G, Di Mario C (2021) Bail-out intravascular lithotripsy for severe stent underexpansion during primary angioplasty: a case report. Eur Heart J Case Rep 5(11):ytab448. https://doi.org/10.1093/ehjcr/ytab448
Karacsonyi J, Nikolakopoulos I, Vemmou E, Rangan BV, Brilakis ES (2021) Intracoronary lithotripsy: a new solution for undilatable in-stent chronic total occlusions. JACC Case Rep 3(5):780–785. https://doi.org/10.1016/j.jaccas.2021.03.014
Hlinomaz O, Tejc M, Sabbah M (2021) Shockwave lithotripsy vs rotational atherectomy: mechanistic differences from optical coherence tomography. J Invasive Cardiol 33(2):E136–E137
Baudinet T, Seguy B, Cetran L, Luttoo MK, Coste P, Gerbaud E (2021) Bail-out therapy in ST-segment elevation myocardial infarction due to calcified lesion causing stent underexpansion: Intravascular lithotripsy is in the lead. J Cardiol Cases 23(6):264–266. https://doi.org/10.1016/j.jccase.2020.12.014
Rodríguez Costoya I, Tizón Marcos H, Vaquerizo Montilla B, Salvatella Giralt N, Martí Almor J, Millán SR (2019) coronary lithoplasty: initial experience in coronary calcified lesions. Rev Esp Cardiol (Engl Ed) 72(9):788–790. https://doi.org/10.1016/j.rec.2018.11.017
Azzalini L, Ancona MB, Bellini B, Chieffo A, Carlino M, Montorfano M (2019) Intravascular lithotripsy and microaxial percutaneous left ventricular assist device for complex and high-risk percutaneous coronary intervention. Can J Cardiol 35(7):940.e5-940.e7. https://doi.org/10.1016/j.cjca.2019.04.017
Cicovic A, Cicovic S, Wong B, Stottrup NB, Ghattas A, Glenie T (2019) A quicker pace: shockwave lithotripsy pacing with electromechanical capture. JACC Cardiovasc Interv 12(17):1739–1740
Marchese A, Tito A, Resta F, Colombo A (2020) Intracoronary lithoplasty in percutaneous treatment of challenging calcified coronary lesions. Case Rep 2(11):1679–1683
Chen G, Zrenner B, Pyxaras SA (2019) Combined rotational atherectomy and intravascular lithotripsy for the treatment of severely calcified in-stent neoatherosclerosis: a mini-review. Cardiovasc Revasc Med 20(9):819–821
Bawamia B, Williams P (2021) combined rotational atherectomy and intravascular lithotripsy to treat a calcified vein graft stenosis. Cardiovasc Revasc Med 1(28):201–202
Nagaraja V, Ubaid S, Khoo C, Ratib K (2020) Intravascular lithotripsy for stent underexpansion despite utilization of rotational atherectomy for plaque modification. Cardiovasc Revasc Med 21(11):147–148
Wong B, El-Jack S, Khan A, Newcombe R, Glenie T, Cicovic A, Armstrong G (2019) Treatment of heavily calcified unprotected left main disease with lithotripsy: the first case series. J Invasive Cardiol 31(6):E143–E147
Warisawa T, Salazar CH, Gonzalo N, Akashi YJ, Escaned J (2019) Successful disruption of massive calcified nodules using novel shockwave intravascular lithotripsy. Circ J 84(1):131
Sgueglia GA, Gioffrè G, Chiastra C, Di Giorgio A, Gaspardone A (2020) First report of the one-point transradial two sheathless catheters insertion (OTRANTO) technique. JACC Cardiovasc Interv 13(1):e9–e10. https://doi.org/10.1016/j.jcin.2019.09.004
Jurado-Román A, Gonzálvez A, Galeote G, Jiménez-Valero S, Moreno R (2019) RotaTripsy: combination of rotational atherectomy and intravascular lithotripsy for the treatment of severely calcified lesions. JACC Cardiovasc Interv 12(15):e127–e129. https://doi.org/10.1016/j.jcin.2019.03.036
Macaya F, Yeoh J, Hill J, Dworakowski R (2020) Adjunctive rotational atherectomy and intravascular lithotripsy for heavily calcified left main disease via radial access. J Invasive Cardiol 32(4):E99
Tovar Forero MN, Van Mieghem NM, Daemen J (2020) Stent underexpansion due to heavy coronary calcification resistant to rotational atherectomy: a case for coronary lithoplasty? Catheter Cardiovasc Interv 96(3):598–600. https://doi.org/10.1002/ccd.28641
Morabito G, Tripolino C, Tassone EJ, Grillo P, Missiroli B (2018) A case of stent under-expansion due to calcified plaque treated with shockwave lithoplasty. Cardiology 141(2):75–77. https://doi.org/10.1159/000493747
Wańczura PM, Rogala M (2021) Percutaneous coronary intervention of recurrent ostial restenosis in the course of incomplete expansion of two layers of stents–intravascular lithotripsy under guidance of optical coherence tomography. Adv Interv Cardiol 17(4):421–422. https://doi.org/10.5114/aic.2021.110925
Bulak L, Zimoch WJ, Rakotoarison O, Protasiewicz M, Reczuch K, Kübler P (2021) Strategy of rotational atherectomy guided by optical coherence tomography. Adv Interv Cardiol 17(4):415–418. https://doi.org/10.5114/aic.2021.111888
Giacchi G, Contarini M, Ruscica G, Brugaletta S (2021) The, “RotaTripsy Plus” approach in a heavily calcified coronary stenosis. Cardiovasc Revasc Med 28S:203–205. https://doi.org/10.1016/j.carrev.2021.04.022
McGarvey M, Kumar S, Violaris A, Elghamaz A, Salukhe TV, Yeh JS (2020) Ventricular fibrillation induced by a lithotripsy-pulse on T during coronary intravascular shockwave lithotripsy. Eur Heart J Case Rep 4(6):1
Taneja A, Viswanathan G, Suresh V (2020) Combined use of rotational atherectomy and intravascular lithotripsy balloon (Rotatripsy) for percutaneous coronary intervention of heavily calcified right coronary artery lesion. IHJ Cardiovasc Case Rep (CVCR) 4(1):1–3
Tumminello G, Cavallino C, Demarchi A, Rametta F (2019) Bail-out unexpanded stent implantation in acute left main dissection treated with intra coronary lithotripsy: a case report. Eur Heart J Case Rep 3(4):1–5. https://doi.org/10.1093/ehjcr/ytz172
Watkins S, Good R, Hill J, Brinton TJ, Oldroyd KG (2019) Intravascular lithotripsy to treat a severely underexpanded coronary stent. EuroIntervention 15(1):124–125. https://doi.org/10.4244/EIJ-D-18-00780
Ocaranza-Sánchez R, Abellás-Sequeiros RA, González-Juanatey C (2019) First left main coronary revascularization with adyuvant intracoronary lithotripsy in Spain. Arch Cardiol Mex 89(4):403–405. https://doi.org/10.24875/ACM.19000164
Bagur MV (2022) Management of densely calcified coronary lesions using OPN–NC balloon and shockwave intravascular lithotripsy procedure: a single-center study. J Indian Coll Cardiol 12(3):123. https://doi.org/10.4103/jicc.jicc_28_21
Sharma S (2022) Efficacy of shockwave C2 coronary intravascular lithotripsy for management of severely calcified left anterior descending stenosis. Heart Vessel Transpl 6(2):96–99
Kiron V, Mustepally PK, Agarwala MK, Rath PC (2022) Case report–post-cardiac injury syndrome (PCIS) following coronary intravascular lithotripsy (IVL) assisted left-main angioplasty. IHJ Cardiovasc Case Rep (CVCR) 6(3):122–125
Gabryel ŁM, Bugla K, Jafra J, Gabryel J (2022) A pacemaker lead indenting the wall of the left anterior descending artery. Adv Interv Cardiol 18(2):178–179. https://doi.org/10.5114/aic.2022.118537
Lee TJ, Wan Rahimi WF, Low MY, Nurruddin AA (2021) Type E coronary artery dissection caused by intravascular lithotripsy balloon rupture; vessel anatomy and characteristics in a lithoplasty complication case as detailed by optical coherence tomography: a case report. Eur Heart J Case Rep 5(12):ytab532. https://doi.org/10.1093/ehjcr/ytab432
Moretti A, Dato I, Gatto MC, Schiavoni M, Bernardo V, Kol A (2021) 272 Combined rotational atherectomy and intravascular lithotripsy for heavy calcified coronary artery. Eur Heart J Suppl 23(9(Supplement_G)):suab134-035. https://doi.org/10.1093/eurheartj/suab134.035
Goel PK, Sahu AK (2021) Intravascular lithotripsy in heavily calcified unprotected left main with involvement of LAD ostium taking-off at extreme angulation—taking upon an “Armageddon.” IHJ Cardiovasc Case Rep (CVCR) 5(3):153–155. https://doi.org/10.1016/j.ihjccr.2021.07.003
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors conceptualized and revised the study design. The data were extracted by CU, HK, SP, GB, IS, and KR. CU analyzed the data and wrote the first draft of the paper. MC, AR, RG, HK, SP, GB, IS, and KR reviewed and revised the paper. HK and SP led and coordinated the research and writing of the manuscript. MC, AR, and RG supervised the project. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umeh, C.A., Kaur, H., Paknoosh, S. et al. Intravascular lithotripsy in coronary arteries: a review of case reports. Egypt Heart J 76, 121 (2024). https://doi.org/10.1186/s43044-024-00555-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-024-00555-6