Abstract
Background
Underutilization of implantable cardioverter defibrillators (ICD) to prevent sudden cardiac death (SCD) in post-myocardial infarction (MI) patients remains an issue across several geographies. A better understanding of risk factors for SCD in post-MI patients from regions with low ICD adoption rates will help identify those who will benefit from an ICD. This analysis assessed risk factors for all-cause and cardiovascular-related mortality in post-MI patients from the Improve Sudden Cardiac Arrest (SCA) Bridge Trial.
Results
For the entire cohort, the overall 1-year mortality rate was 5.9% (88/1491) and 3.4% (51/1491) for all-cause and cardiovascular mortality, respectively, with 76.5% of all cardiac deaths being from SCD. A multivariate model determined increased age, reduced left ventricular ejection fraction (LVEF), increased time from myocardial infarction to hospital admission, being female, being from Southeast Asia (SEA), and having coronary artery disease to be significant risk factors for all-cause mortality. The risk factors for cardiovascular-related mortality revealed increased age, reduced LVEF, and being from SEA as significant risk factors.
Conclusions
We show several characteristics as being predictors of cardiovascular-related mortality in post-MI patients from the Improve SCA Bridge study. Patients who experience an MI and present with these characteristics would benefit from a referral to an electrophysiologist for further SCD risk stratification and management and possible subsequent ICD implantation to reduce unnecessary death.
Similar content being viewed by others
Background
Implantable cardioverter defibrillators remain the standard of care to prevent sudden cardiac death (SCD) in indicated patients [1]. However, the rate of ICD implants for those who need them remains low, especially in regions underrepresented in major ICD clinical trials [2, 3]. This is concerning given that SCD remains one of the most common causes of death worldwide.
Patients who experience a myocardial infarction (MI) have been shown to be at heightened risk for SCD, despite recent advancements in the management of these patients [3, 4]. Current guidelines recommend the use of ICDs in post-MI patients who have a reduced left ventricular ejection fraction (LVEF) (≤ 35%) for 40 days after MI [1, 5]. Waiting 40 days to implant post-MI is based on two major studies that showed no benefit of early ICD intervention in post-MI patients [6, 7]. However, more research is needed to better identify post-MI patients at risk of SCD to help determine who would best benefit from an ICD, especially in regions where ICD use remains low.
The Improve Sudden Cardiac Arrest (SCA) Bridge Trial aimed to identify barriers to patient referral for SCD risk stratification and management in regions with low ICD utilization [8]. Understanding risk factors for death following MI in these patients may help inform decisions on SCD risk management in regions where ICD adoption is lagging. Using data from the Improve SCA Bridge cohort, the current study aims to identify risk factors for all-cause and cardiovascular-related mortality to help identify those who may benefit from further SCD risk stratification and management.
Methods
Improve SCA bridge study design and eligibility
The Improve SCA Bridge Trial (ClinicalTrials.gov; Identifier: NCT03715790) was a prospectively enrolled, non-randomized, multicenter, global, post-market study aimed at identifying reasons why post-MI patients were not referred for further SCD risk stratification and management [8]. The six regions that participated in the study were: 1. Mainland China, 2. India Subcontinent (ISC, including India and Bangladesh), 3. South Korea, 4. Middle East, Africa, Central Asia and Turkey (MEACAT, including Egypt, Pakistan, Saudi Arabia, South Africa and Tunisia), 5. Southeast Asia (SEA, including Brunei, Indonesia, Malaysia, The Philippines, Singapore and Thailand), and 6. Taiwan. These regions were chosen due to their low rate of ICD therapy adoption. The full study design details have been published previously [8].
The inclusion criteria for enrollment in the study were as follows: (1) Age 18 and above (and met age requirements per local law); (2) Experienced an acute ST-segment elevation myocardial infarction (STEMI) or non‐ST-segment elevation myocardial infarction (NSTEMI) ≤ 30 days before enrollment, and [3] An LVEF < 50% measured within 14 days of the MI. Exclusion criteria are outlined in Supplemental Table 1. Follow-up visits occurred at 3, 6, and 12 months and were performed either in-person or by phone due to the ongoing COVID-19 pandemic.
Cause of death classification
Classification for the cause of death was determined by each individual site and adjudicated by an outside clinical events committee. For the purposes of this study, deaths were classified as either SCD, non-SCD, non-cardiac death, or unknown. Regulatory reporting of deaths was completed according to local regulatory requirements.
Study objectives
The main objective of this study was to determine risk factors for all-cause and cardiovascular-related mortality using data from the Improve SCA Bridge cohort. These risk factors could help inform decisions on SCD risk stratification and management to better determine those who would benefit from ICD therapy. Secondary objectives included all-cause and cardiovascular-related mortality in STEMI and NSTEMI patients, separately. We also reported the causes of death and Kaplan–Meier estimated 1-year all-cause and cardiovascular-related mortality rates.
Statistical analysis
Quantitative data were reported as the mean and standard deviation while categorical data was reported as the number and percent ratio. Kaplan–Meier estimates of the survival function were used to determine the 1-year rate of all-cause mortality and SCD in our patient population. For the risk factor analysis, a total of 21 patient characteristics were used as candidates (same as baseline characteristics in Table 1). For the sake of comparison, potential risk factors were first assessed using a univariate Cox proportional-hazards model. All 21 predictors were then entered into a multivariate Cox proportional-hazards model applying a backward selection process. Predictors were removed from the multivariate model if their p-value was greater than 0.15. At the conclusion of the selection process, any predictors with a p-value < 0.05 were considered significant. The univariate and multivariate Cox analyses were performed for the entire patient cohort using all 21 characteristics, and for STEMI and NSTEMI populations separately, using 20 predictors (STEMI status was naturally excluded as a predictor). A multivariate Cox proportional-hazards model assumes that the effect of different variables on survival is constant over time. All statistical analysis was performed using SAS software, version 9.4 (SAS Institute Inc., Cary NC).
Ethics statement
The primary Improve SCA Bridge Study and this sub-analysis of Improve SCA Bridge were conducted in compliance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee at each participating site before enrollment.
Results
Baseline characteristics
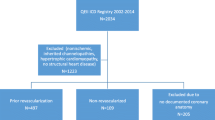
A total of 1491 post-MI patients were enrolled in the Improve SCA Bridge study (Fig. 1). Baseline characteristics for all patients can be found in Table 1. The average age of enrolled patients was 60.2 ± 12 years, with 82.4% being male, 35.6% having type 2 diabetes, and 49.8% presenting with hypertension (Table 1). Nearly two-thirds (66.1%) of all patients had an MI that was ST-elevated (STEMI) with SEA having the highest percentage of STEMI patients among all regions at 74% (Table 1). In most geographies the time from MI to hospital admission was under 24 h, with the exception being Mainland China having a mean time of 2.1 ± 3.4 days (Table 1).
Risk factors for all-cause mortality
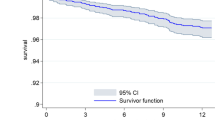
Mortality of any etiology occurred in 88 patients (5.9% of all enrolled patients) during the study period (Table 2). The Kaplan–Meier estimated all-cause mortality at 1 year was 7% (Fig. 2A). Twenty-one characteristics were screened as possible risk factors for all-cause mortality, of which 12 were significant via a univariate model (Table 3). A separate multivariate model revealed six independent risk factors for all-cause mortality which included: increased age (p = 0.0001), low LVEF (p = 0.004), increased time from MI to hospital admission (p < 0.013), female gender (p = 0.017), being from SEA (p = 0.006), and presence of CAD (p = 0.011) (Table 3).
Risk factors for cardiovascular-related mortality
Cardiovascular mortality represented 57.9% (51/88) of all deaths during the study, 76.5% (39/51) of which were a result of SCD (Table 2). The Kaplan–Meier estimate of 1-year SCD was 3.9% (Fig. 2B). Of the 21 potential risk factors analyzed for cardiovascular mortality, three were significant at alpha = 0.05 after applying the multivariate model including increased age (p = 0.023), low LVEF (p = 0.0002), and being from SEA (p = 0.009) (Table 4).
Mortality risk factors in STEMI vs. NSTEMI patients
Baseline characteristics separated by STEMI and non-STEMI patients can be found in Supplemental Table 2. In STEMI patients, six factors were identified as significant for all-cause mortality with the univariate model, while only two factors, low LVEF (p < 0.0001) and presence of CAD (p = 0.002), were significant at alpha = 0.05 after the multivariate analysis (Supplemental Table 3). As with all-cause mortality, low LVEF (p < 0.0001) and presence of CAD (p = 0.008) were risk factors for cardiovascular-related mortality in STEMI patients, in addition to the presence of renal disease (p = 0.037) (Supplemental Table 4).
Univariate analysis revealed seven risk factors for all-cause mortality in NSTEMI subjects. Of these, four were significant after multivariate analysis: increased age (p < 0.0001), increased time from MI to hospital admission (p = 0.003), being from SEA (p = 0.0002), and having diabetes (p = 0.0009). In addition, having hypertension was not significant in the univariate model but was significant in the multivariate analysis (p = 0.017) (Supplemental Table 5). The significant risk factors for cardiovascular-related mortality in NSTEMI subjects, identified with the multivariate model, were age (p = 0.0036), being female (p = 0.038), being from SEA (p = 0.001), and being from the MEACAT region (p = 0.034) (Supplemental Table 6).
Discussion
Previous studies have revealed age, diabetes, hypertension, smoking, peripheral artery disease, chronic liver disease, chronic renal disease, history of stroke, history of cancer, and chronic obstructive pulmonary disease as being potential risk factors for all-cause mortality in post-MI patients [9,10,11,12,13,14,15,16]. In the current study, we also found older age, low LVEF, female gender, increased time from MI to hospital admission, being from SEA, and the presence of CAD as potential risk factors for all-cause mortality. Ye et al. showed LVEF dysfunction and presence of pump failure to be potential risk factors for all-cause mortality after MI [16]. In another study, Drybus et al. found the risk of all-cause mortality post-MI to be higher in patients with stage 3/4 chronic kidney disease, diabetes, or hypercholesterolemia [17]. Vega et al. also showed that patients with elevated blood pressure and diabetes were at higher risk of all-cause death following MI [18]. Hence, valid prediction models for patients with post-MI mortality is essential and must consider different variables and comorbidities that influence heart disease [19].
Of the deaths in our study, the most common cause of death was SCD, which is not surprising given that SCD remains the most common type of cardiac mortality post-MI [20]. Also, SCD made up more than 75% of all cardiovascular deaths in our cohort and so the risk factors for cardiovascular mortality revealed in our analysis can be used as a surrogate for SCD risk in this population. In regards to risk factors for cardiac mortality, we found increased age, reduced LVEF, and being from SEA to be the strongest predictors of cardiac-related death for 1-year post-MI. Age is a key factor in predicting post-MI mortality, specifically because older individuals are more likely to experience vascular complications after an MI [16, 21, 22]. Reduced LVEF was also an independent risk factor for 1-year cardiac-related death in our analysis. This agrees with previous studies where an LVEF < 40% is a strong predictor of cardiac death post-MI [16, 23]. This finding further supports timely treatment of heart failure patients with low LVEF using β-blockers, angiotensin-converting enzyme inhibitors, and ICD therapy to decrease the mortality rate of patients with MI [24].
Interestingly, we also show that individuals from SEA are at a higher risk for cardiac-related death post-MI. Previous studies have documented a higher rate of cardiac-related deaths in SEA than in other regions [25, 26]. Additionally, cardiac-related deaths occur 5 to 10 years earlier in affected individuals from SEA than those from Western countries [25, 27]. A previous study also found a higher prevalence of CAD, diabetes, hypertension, and ischemic heart failure in SEA countries [28]. This has raised the hypothesis that SEA has a unique propensity for MI that is not accounted for by traditional risk variables [25]. Higher rates of smoking and air pollution have been proposed as contributors to the higher incidences of cardiac disease in SEA, with smoking being identified as a major risk factor for MI [25]. These previous results are in line with our finding that those individuals from SEA who experience an MI are at higher risk for death than post-MI patients from other regions.
Also, we found that female gender is a risk factor for all-cause mortality but not cardiovascular mortality in post-MI patients. This is an interesting finding that implies that females in our cohort experienced higher rates of non-cardiac deaths or “unknown” deaths and is worthy of further investigation. It has been shown that in Asian countries the rate of cardiovascular death is significantly higher in men than women [29]. This explains why female gender was not associated with cardiovascular death, and also helps explain the high rate of males in the trial who experienced an MI compared to females.
Lastly, we found no overlap in risk factors between STEMI and NSTEMI patients for either all-cause or cardiovascular-related death within 1-year post-MI. Contrary to our results, a previous analysis of 2,151 patients from France found similar risk factors between STEMI and NSTEMI patients, notably increased age and diabetes, indicating possible geographical differences [30]. Takeji et al. showed that within 6 months of MI, NSTEMI patient deaths were more often caused by weakened post-resuscitation status or HF, while STEMI patient deaths were more often a result of mechanical cardiac complications or cardiogenic shock [31]. Thus, differences in the death etiology may help explain the contrast in risk factors between STEMI and NSTEMI groups given that most of the deaths in our study occurred within 6 months of MI.
As previously mentioned, adoption of ICD therapy in qualified patients from regions included in this analysis is low. Several factors contribute to this low rate including cost, patient education, and lack of resources to name a few. While the current analysis cannot eliminate these barriers to access, they help inform a clinician’s decision to refer post-MI patients with reduced LVEF for further assessment of risks. The guidelines recommend an ICD for post-MI patients with a reduced LVEF more than 40 days post-MI and, at the very least, these individuals should be referred for SCD risk stratification and management [32]. If these patients also hold any of the risk factors identified in this study, they may be at even increased risk and thus referral is even more imperative.
Limitations
The biggest limitation of this study was the small sample size. Although the primary Improve SCA Bridge study had a large sample size, the number of patients who died represented a small subset, which limited the strength of our risk factors model given the number of characteristics considered as potential risk factors. This likewise limited our ability to appropriately test the proportionality of hazards to ensure that variables met assumptions for the multivariate Cox proportional-hazards model. The follow-up duration of this study was also short, which limited our ability to capture more deaths and develop a more robust analysis. Also, there were many patients that exited the study if not referred for further risk stratification and management or were lost to follow-up, which also reduced the number of deaths to be analyzed. Other limitations included the retrospective nature of the analysis, high percentage of males, which could limit the generalizability of the results, and number of deaths that were classified as “unknown”. Given that this study was performed with patients from specific regions on the Asian continent, the generalizability of the results onto other populations outside of these regions is limited and not recommended. Future studies utilizing large patient databases or a longer follow-up period to capture more deaths would allow for a more robust analysis of mortality risk factors in post-MI patients from these regions. Also a future analysis looking at the risks specific to each geography or the impact of differing healthcare structures across countries would be meaningful.
Conclusion
Low LVEF, increased age, and being from SEA were predictors of both all-cause and cardiovascular-related mortality in patients from the Improve SCA Bridge study. Additionally, being female, presence of CAD, and increased MI to hospital time were predictors of cardiovascular-related death post-MI. Post-MI patients in these regions who possess these characteristics are potentially at heightened risk of SCD and, therefore, SCD risk stratification and management strategies should reflect this increased risk to improve outcomes in regions where ICD use is low. While all patients in the original study should have been referred to an electrophysiologist, per guidelines, the results of this sub-analysis identify several subgroups who would have especially benefited from referral.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- CAD:
-
Coronary artery disease
- ICD:
-
Implantable cardioverter defibrillators
- LVEF:
-
Left ventricular ejection fraction
- MEACAT:
-
Middle East, Africa, Central Asia and Turkey
- MI:
-
Myocardial infarction
- NSTEMI:
-
Non‐ST-segment elevation myocardial infarction
- SCA:
-
Sudden Cardiac Arrest
- SCD:
-
Sudden cardiac death
- SEA:
-
Southeast Asia
- STEMI:
-
ST-elevated myocardial infarction
References
Al-Khatib SM, Stevenson WG, Ackerman MJ et al (2018) 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol 72:1677–1749
Chia YMF, Teng TK, Tan ESJ et al (2017) Disparity between indications for and utilization of implantable cardioverter defibrillators in Asian patients with heart failure. Circ Cardiovasc Qual Outcomes 10:e003651
Zhang S, Ching CK, Huang D et al (2020) Utilization of implantable cardioverter-defibrillators for the prevention of sudden cardiac death in emerging countries: Improve SCA clinical trial. Heart Rhythm 17:468–475
Solomon SD, Zelenkofske S, McMurray JJ et al (2005) Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med 352:2581–2588
Priori SG, Blomstrom-Lundqvist C, Mazzanti A et al (2015) Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). Endorsed by: association for European paediatric and congenital cardiology (AEPC). Eur Heart J 36:2793–2867
Hohnloser SH, Kuck KH, Dorian P et al (2004) Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med 351:2481–2488
Steinbeck G, Andresen D, Seidl K et al (2009) Defibrillator implantation early after myocardial infarction. N Engl J Med 361:1427–1436
Zhang S, Chen WJ, Sankardas MA et al (2022) Improve the prevention of sudden cardiac arrest in patients with post-acute myocardial infarction. JACC Asia 2:559–571
Deckers JW, van Domburg RT, Akkerhuis M, Nauta ST (2013) Relation of admission glucose levels, short- and long-term (20-year) mortality after acute myocardial infarction. Am J Cardiol 112:1306–1310
Nauta ST, Deckers JW, Akkerhuis KM, van Domburg RT (2012) Short- and long-term mortality after myocardial infarction in patients with and without diabetes: changes from 1985 to 2008. Diabetes Care 35:2043–2047
Nauta ST, van Domburg RT, Nuis RJ, Akkerhuis M, Deckers JW (2013) Decline in 20-year mortality after myocardial infarction in patients with chronic kidney disease: evolution from the prethrombolysis to the percutaneous coronary intervention era. Kidney Int 84:353–358
Nielsen S, Bjorck L, Berg J et al (2014) Sex-specific trends in 4-year survival in 37 276 men and women with acute myocardial infarction before the age of 55 years in Sweden, 1987–2006: a register-based cohort study. BMJ Open 4:e004598
Rapsomaniki E, Shah A, Perel P et al (2014) Prognostic models for stable coronary artery disease based on electronic health record cohort of 102 023 patients. Eur Heart J 35:844–852
Singh B, Rao HB, Pandurangi U et al (2022) Identifying high risk patients post myocardial infarction with reduced left ventricular function using loop recorders INSPIRE-ELR clinical study. Indian Heart J 74:194–200
van Loo HM, van den Heuvel ER, Schoevers RA et al (2014) Sex dependent risk factors for mortality after myocardial infarction: individual patient data meta-analysis. BMC Med 12:242
Ye Q, Zhang J, Ma L (2020) Predictors of all-cause 1-year mortality in myocardial infarction patients. Medicine 99:e21288
Dyrbus K, Gasior M, Desperak P et al (2021) Risk-factors associated with extremely high cardiovascular risk of mid- and long-term mortality following myocardial infarction: analysis of the hyperlipidaemia therapy in tertiary cardiological center (TERCET) registry. Atherosclerosis 333:16–23
Vega G, Martinez S, Jimenez PA, Navarro A, Bernad F (2007) Effect of cardiovascular risk factors on long-term morbidity and mortality following acute myocardial infarction. Rev Esp Cardiol 60:703–713
Ketchum ES, Dickstein K, Kjekshus J et al (2014) The seattle post myocardial infarction model (SPIM): prediction of mortality after acute myocardial infarction with left ventricular dysfunction. Eur Heart J Acute Cardiovasc Care 3:46–55
Tsao CW, Aday AW, Almarzooq ZI et al (2022) Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation 145:e153–e639
Booth GL, Kapral MK, Fung K, Tu JV (2006) Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 368:29–36
Nauta ST, Deckers JW, Akkerhuis KM, van Domburg RT (2013) Age-dependent care and long-term (20 year) mortality of 14,434 myocardial infarction patients: changes from 1985 to 2008. Int J Cardiol 167:693–697
Bosch X, Theroux P (2005) Left ventricular ejection fraction to predict early mortality in patients with non-ST-segment elevation acute coronary syndromes. Am Heart J 150:215–220
Ambrosioni E, Borghi C, Magnani B (1995) The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The survival of myocardial infarction long-term evaluation (SMILE) study investigators. N Engl J Med 332:80–85
Joshi P, Islam S, Pais P et al (2007) Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA 297:286–294
Anand SS, Yusuf S, Vuksan V et al (2000) Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the study of health assessment and risk in ethnic groups (SHARE). Lancet 356:279–284
Murray CJ, Lopez AD. The Global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020 : summary. Cambridge, MA: Harvard School of Public Health on behalf of the World Health Organization and the World Bank, 1996.
Lam CS, Teng TK, Tay WT et al (2016) Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J 37:3141–3153
Zhao D (2021) Epidemiological features of cardiovascular disease in Asia. JACC Asia 1:1–13
Montalescot G, Dallongeville J, Van Belle E et al (2007) STEMI and NSTEMI: are they so different? 1 year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry). Eur Heart J 28:1409–1417
Takeji Y, Shiomi H, Morimoto T et al (2021) Differences in mortality and causes of death between STEMI and NSTEMI in the early and late phases after acute myocardial infarction. PLoS ONE 16:e0259268
Kusumoto FM, Schoenfeld MH, Wilkoff BL et al (2017) 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 14:e503–e551
Acknowledgements
The authors thank Amy Molan, PhD, Medtronic Inc, for critical review of the article and Bart Gerritse, PhD, Medtronic Inc, for help with statistical analysis.
Funding
This study was sponsored by Medtronic Inc.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the conception, planning, and design of the manuscript and to data interpretation. DK, FB, AT, and TRH were responsible for writing the first draft of the manuscript. WC, MAS, WHA, HL, HG, and SZ were primary investigators of the Improve SCA Bridge Trial and were involved in data collection. BVD performed the statistical analyses and generated tables and figures. All authors critically reviewed and provided intellectual input on the manuscript. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol for the Improve SCA Bridge Trial was approved by the ethics committee at each participating institution and associated national and local regulatory agencies. All patients who participated in the trial provided written informed consent before undergoing study procedures.
Consent for publication
Not applicable.
Competing interests
Dr Chen has received honorariums from Medtronic, Biotronik, Abbott, and Boston Scientific. Dr Huang has received speaker fees/consulting fees from Boston Scientific, Bayer, Boehringer-Ingelheim, and Abbott. Dr Liew has received speaker fees and honorarium from Medtronic and Boston Scientific. Brian Van Dorn, Dr Holmes, and Amy Thompson are employees of Medtronic Inc. Dr Zhang has received speaker fees/consulting fees from Boston Scientific, Medtronic, Abbott, and Biotronik and has received steering committee fees from Medtronic. All other authors have reported that there are no relationships relevant to the contents of this paper to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, D., Bardooli, F., Chen, WJ. et al. Risk factors for mortality in post-myocardial infarction patients: insights from the improve SCA bridge study. Egypt Heart J 76, 72 (2024). https://doi.org/10.1186/s43044-024-00505-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-024-00505-2