Abstract
Background
Changes in PR intervals after transcatheter pulmonary valve replacement (TCPVR) have not been thoroughly evaluated in children. This study evaluated the changes in PR and QRS intervals six months after TCPVR in children with congenital heart disease.
Results
This study included 41 patients who underwent TCPVR from 2010 to 2022. ECG of patients was reviewed before and six months after TCPVR, and the PR and QRS intervals were reported. Right ventricular systolic pressure (RVSP) was retrieved indirectly from echocardiography and compared pre- and 6-months after TPVR. The median age was 13 years (25th–75th percentiles: 11–16), and 61% were males. The preoperative diagnosis was tetralogy of Fallot (n = 29, 71%), transposition of great vessels (n = 4, 10%), common arterial trunk (n = 3, 7%), pulmonary valve stenosis (n = 3, 7%) and pulmonary atresia (n = 2, 5%). The Melody valve was used in 30 patients, and Edwards Sapien was used in 11 patients. RVSP was significantly reduced six months after the procedure (pre-RVSP 40 (30–55) mmHg vs. post-RVSP 25 (20–35) mmHg; P < 0.001). The PR interval was 142 (132–174) msec before TPVR and 146 (132–168) msec post-TCPVR (P = 0.442). Post-TPVR PR was positively related to the pre-PR (β: 0.79 (0.66–0.93), P < 0.001) and inversely related to the right ventricular outflow tract size (− 1.48 (− 2.76 to − 0.21), P = 0.023). The pre-TPVR QRS was 130 (102–146) msec, and the post-TPVR QRS was 136 (106–144) msec (P = 0.668).
Conclusions
In children undergoing TCPVR, the PR and QRS intervals did not change significantly during a 6-month follow-up.
Similar content being viewed by others
Background
Progressive pulmonary regurgitation (PR) can occur after surgical correction of several congenital cardiac lesions [1, 2]. Pulmonary regurgitation leads to progressive right ventricular (RV) dilatation and dysfunction, consequently leading to a progressive increase in PR degree [3]. Right ventricular dilatation is a major cause of ventricular arrhythmia, right-side heart failure, and sudden cardiac death [4]. Patients with corrected tetralogy of Fallot (TOF) and PR are prone to atrial arrhythmia, decreased quality of life, and survival [5]. Transcatheter pulmonary valve replacement (TCPVR) has emerged to manage pulmonary valve regurgitation after correction of tetralogy of Fallot and other conotruncal anomalies [6, 7]. A wide QRS interval is a risk factor for ventricular arrhythmia and sudden cardiac death in patients with repaired TOF, while its role in predicting atrial arrhythmia is limited [8]. Recent reports indicated that cardiac conduction abnormalities in the form of prolonged PR interval and first-degree heart block were associated with heart failure in patients with right ventricular cardiomyopathy [9, 10]. Studies on the changes in PR intervals after TCPVR in children are limited. Thus, we evaluated the changes in PR and QRS intervals six months after transcatheter pulmonary valve implantation in children with congenital heart disease.
Methods
Design
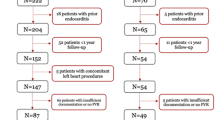
This retrospective cohort study included 41 patients with congenital cardiac lesions who underwent TCPVR from 2010 to 2020 in a single center. We included patients under 18 years of age who had pulmonary regurgitation after a prior correction of congenital heart disease. Patients with preprocedural heart block and those with permanent pacemakers were excluded from the study. Patients with missing baseline or follow-up ECG were also excluded. The study was approved by the local Institutional Review Board, and the need for the patient's (or guardian's) consent was waived.
Study data
We described the age, weight, height, and body surface area at the time of the procedure. Additionally, we reported the gender, associated genetic disease, diagnosis, and symptoms. Data related to the right ventricular outflow tract conduit, size, degree of pulmonary regurgitation, pulmonary artery pressure, and left ventricular function were recorded. Two types of transcatheter pulmonary valves were used during the study period: Melody (Medtronic Inc.) and Edwards Sapien (Edwards Lifesciences Inc.). The valve's choice depended on the diameter of the right ventricular outflow tract (RVOT). The Edwards Sapien valve was used in patients with large RVOT diameters because of the availability of large valve sizes.
ECG and outcomes
Twelve-lead ECG was reported before TCPVR and six months after the procedure. PR and QRS intervals were captured from the ECG, and right ventricular systolic pressure (RVSP) was recorded from the baseline and follow-up echocardiography. ECG measurements were reviewed by two cardiologists who were unaware of the patient's data. The measurements of only one physician were recorded, and the measurements from the second were used as confirmatory measures only. A third physician reviewed any controversy between the two measurements. The changes in these parameters were compared between the two measurements.
Techniques of TCPVR
All patients underwent trans-femoral pulmonary valve replacement. Melody and Sapien valves are balloon-expandable valves. Both valves were delivered using the manufacturer-specific delivery system. The choice of valve type was made according to the discretion of the treating physician.
Statistical analysis
Descriptive analysis was used to present our results. Normally distributed variables are presented as the mean and standard deviation, and nonnormal variables are presented as the median and interquartile limits. Categorical variables were described as numbers and percentages. The pre- and postprocedure PR and QRS intervals and right ventricular systolic pressure were compared using the Wilcoxon signed rank test for matched pairs. The measures were compared between patients with Melody vs. Sapien valves using the Mann‒Whitney test. Spearman correlation was used to evaluate the relationship between right ventricular pressure and ECG intervals at follow-up. Quantile regression was used to study factors affecting the postprocedural PR interval. All preprocedural variables were included in a stepwise regression model with a forward selection method, and a stay P value was required to retain the variables in the final model. Stata 17 (Stata Corp-College Station-TX-USA) was used for all analyses. A P value of less than 0.05 was considered statistically significant.
Results
Preprocedural data
The median age was 13 years (25th–75th percentiles: 11–16), and 61% were males. Two patients had trisomy 21, and one patient had DiGeorge syndrome. The preoperative diagnosis was tetralogy of Fallot (n = 29, 71%), transposition of great vessels (n = 4, 10%), common arterial trunk (n = 3, 7%), pulmonary valve stenosis (n = 3, 7%) and pulmonary atresia (n = 2, 5%). RVOT was patched in 16 patients (39%), replaced with Contegra (Medtronic's Contegra, Medtronic, Inc.) in 15 patients (37%), a homograft in two patients (5%), or a bioprosthetic valve in four patients (10%) and was native in four patients (10%). Thirty-three patients (80%) had severe pulmonary regurgitation, and 31 (76%) had normal left ventricular function. The Melody valve was used in 30 patients, and Edwards Sapien was used in 11 patients (Table 1).
There were no differences in the baseline PR intervals [142 (130–180) vs. 146 (134–156) msec; P > 0.99] and QRS interval [125 (102–146) vs. 142 (92–150); P = 0.576] between Melody and Sapien valves, respectively. Patients with Sapien valves had significantly lower RVSP than those with Melody valves [45 (30–60) vs. 30 (30–40) mmHg; P = 0.021].
Changes in PR, QRS, and right ventricular pressure
The RVSP was significantly reduced six months after the procedure (pre-RVSP 40 (30–55) mmHg vs. post-RVSP 25 (20–35) mmHg; P < 0.001) (Fig. 1). The PR interval was 142 (132–174) msec before TCPVR and 146 (132–168) msec post-TCPVR (P = 0.442) (Fig. 2). The postprocedural PR was positively related to the pre-PR (β: 0.79 (0.66–0.93), P < 0.001) and inversely related to RVOT size (− 1.48 (− 2.76 to − 0.21), P = 0.023). The preprocedural QRS was 130 (102–146) msec, and the postprocedural QRS was 136 (106–144) msec (P = 0.668). The only factor affecting postprocedural QRS was the preprocedural QRS interval. There was no correlation between the PR interval and RVSP (r = − 0.22, P = 0.160) or between the QRS interval and RVSP (r = 0.185, P = 0.246) at six months after TPVR. There were no differences between both valves regarding the six-month PR interval [146 (132–172) vs. 148 (132–160) msec; P = 0.669), QRS interval [133 (106–144) vs. 138 (92–148); P = 0.648] and RVSP [30 (20–40) vs. 20 (20–30) mmHg; P = 0.06] in Melody vs. Sapien valves, respectively (Fig. 3).
Discussion
Right ventricular dilatation and dysfunction are commonly associated with pulmonary valve regurgitation [11]. Both can lead to right-side heart failure, conduction abnormalities, arrhythmia, and sudden cardiac death [12]. PR interval prolongation is an indication of abnormal conduction. In recent studies, progressive PR prolongation could be an ominous sign of right ventricular dysfunction and poor outcomes in patients with pulmonary regurgitation [9]. Transcatheter pulmonary valve replacement is increasingly used for managing pulmonary regurgitation in pediatric patients after correcting several congenital cardiac defects [13]. TCPVR has become a feasible and safe option to avoid open heart surgery and interrupt the vicious cycle of pulmonary regurgitation and right ventricular dilatation [14]. Progressive PR prolongation after TCPVR was evaluated in adult patients. This study evaluated the changes in PR and QRS intervals and RVSP six months after TCPVR in children with congenital heart disease. Despite the significant reduction in the right ventricular systolic pressure, we did not report significant changes in PR and QRS intervals.
Several studies evaluated the changes in PR intervals after TCPVR. Kimura and colleagues reported temporal prolongation of PR intervals in adult patients with corrected TOF, which was correlated with right ventricular volumes and function [9]. Massin and coworkers reported a progressive increase in PR and QRS intervals after correcting TOF in 35 patients. The increase in PR interval was more evident in patients with a transannular patch or pulmonary homograft [15]. Sherptong and associates reported a negative correlation between QRS duration before and after pulmonary valve replacement and ventricular arrhythmia, heart failure, and death [16]. Additionally, they found that patients with prolonged QRS intervals before the procedure were less prone to QRS duration reduction after the procedure. Bokma and coworkers reported that QRS fragmentation is superior to QRS duration in predicting mortality after TOF correction [17]. On the other hand, Oosterhof and colleagues showed that the beneficial effect of pulmonary valve replacement on QRS duration was transient, followed by a steady increase in QRS duration only in patients with a preoperative QRS duration of more than 150 ms [18]. In our study, only four patients had QRS durations above 160 ms before TCPVR.
We reported nonsignificant changes in PR and QRS intervals after TCPVR in patients with corrected congenital lesions, mainly TOF. Similarly, Nguyen and coworkers reported no significant difference in QRS duration before and after TCPVR [19]. Additionally, we reported no correlation between ECG intervals and RVSP. These negative results and the difference between our study and other studies could be attributed to several factors. The duration of follow-up may not be sufficient to detect changes in ECG intervals. We followed the patients for six months; however, three months was enough in other studies to detect changes in PR intervals after TOF repair [9]. Furthermore, we included several types of congenital lesions, which could create heterogeneity in our population. However, we performed multivariable analysis for factors affecting the postprocedural PR intervals, and the diagnosis had no association with the postprocedure PR interval. Last, the published studies reported changes in PR intervals in adults with corrected TOF. Our study included pediatric patients only; the changes in PR intervals could require longer follow-up to adulthood. As reported by Oosterhof, the need for a longer follow-up period, even in adulthood, could be essential to show if the reported beneficial effect of TCPVR on QRS duration is transient [18]. The temporal changes in ECG intervals before TPVR are unknown, and TCPVR could have halted the progression of PR or QRS intervals that was reported by kimura and colleagues [9].
Study limitations
The study has several limitations. The study is a retrospective study with inherent selection bias. The study included patients with TCPVR only, which may not accurately reflect the population with corrected TOF. The small number of patients also limits the study, and we did not correlate the changes in ECG intervals with complications, such as heart failure, ventricular arrhythmia, and mortality.
Conclusions
In children undergoing TCPVR, the PR and QRS intervals did not change significantly during a 6-month follow-up.
Availability of data and materials
Un-identified data are available upon request with the corresponding author.
Abbreviations
- PR :
-
Progressive pulmonary regurgitation
- RVSP:
-
Right ventricular systolic pressure
- TCPVR:
-
Transcatheter pulmonary valve replacement
- RV :
-
Right ventricular
- TOF :
-
Tetralogy of Fallot
- RVOT :
-
Right ventricular outflow tract
References
Senthilnathan S, Dragulescu A, Mertens L (2013) Pulmonary regurgitation after tetralogy of fallot repair: a diagnostic and therapeutic challenge. J Cardiovasc Echogr 23(1):1–9
Warnes CA (2006) Transposition of the great arteries. Circulation 114(24):2699–2709
Kato A, Drolet C, Yoo S-J, Redington AN, Grosse-Wortmann L (2016) Vicious circle between progressive right ventricular dilatation and pulmonary regurgitation in patients after tetralogy of Fallot repair? Right heart enlargement promotes flow reversal in the left pulmonary artery. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson 18(1):34
Zeppenfeld K, Schalij MJ, Bartelings MM, Tedrow UB, Koplan BA, Soejima K et al (2007) Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation 116(20):2241–2252
Wu M-H, Lu C-W, Chen H-C, Chiu S-N, Kao F-Y, Huang S-K (2015) Arrhythmic burdens in patients with tetralogy of Fallot: a national database study. Hear Rhythm 12(3):604–609
Wagner R, Daehnert I, Lurz P (2015) Percutaneous pulmonary and tricuspid valve implantations: an update. World J Cardiol 7(4):167–177
Ismail MF, Elmahrouk AF, Arafat AA, Hamouda TE, Edrees A, Bogis A, Arfi AM, Dohain AM, Alkhattabi A, Alharbi AW, Shihata MS, Al-Radi OO, Al-Ata JA, Jamjoom AA (2020) Bovine jugular vein valved xenograft for extracardiac total cavo-pulmonary connection: The risk of thrombosis and the potential liver protection effect. J Card Surg 35(4):845–853. https://doi.org/10.1111/jocs.14484
Valente AM, Gauvreau K, Assenza GE, Babu-Narayan SV, Schreier J, Gatzoulis MA et al (2014) Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart 100(3):247–253
Kimura Y, Fukuda K, Nakano M, Hasebe Y, Fukasawa K, Chiba T et al (2018) Prognostic significance of PR interval prolongation in adult patients with total correction of tetralogy of fallot. Circ Arrhythm Electrophysiol 11(11):e006234
Kimura Y, Noda T, Matsuyama T-A, Otsuka Y, Kamakura T, Wada M et al (2017) Heart failure in patients with arrhythmogenic right ventricular cardiomyopathy: What are the risk factors? Int J Cardiol 241:288–294
Cheng S, Li VW-Y, So EK-F, Cheung Y-F (2022) Right ventricular-pulmonary arterial coupling in repaired tetralogy of fallot. Pediatr Cardiol 43(1):207–217
Akazawa Y, Fujioka T, Ide H, Yazaki K, Honjo O, Sun M et al (2021) Impaired right and left ventricular function and relaxation induced by pulmonary regurgitation are not reversed by tardive antifibrosis treatment. Am J Physiol Heart Circ Physiol 321(1):H38-51
Tatewaki H, Shiose A (2018) Pulmonary valve replacement after repaired Tetralogy of Fallot. Gen Thorac Cardiovasc Surg 66(9):509–515
MecaAguirrezabalaga JA, Silva Guisasola J, Díaz Méndez R, EscaleraVeizaga AE, Hernández-Vaquero PD (2020) Pulmonary regurgitation after repaired tetralogy of Fallot: surgical versus percutaneous treatment. Ann Transl Med 8(15):967
Massin MM, Malekzadeh-Milani SG, Schifflers S, Dessy H, Verbeet T (2011) Long-term electrocardiographic follow-up after repair of tetralogy of Fallot. Ann noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiol 16(4):336–343
Scherptong RWC, Hazekamp MG, Mulder BJM, Wijers O, Swenne CA, van der Wall EE et al (2010) Follow-up after pulmonary valve replacement in adults with tetralogy of Fallot: association between QRS duration and outcome. J Am Coll Cardiol 56(18):1486–1492
Bokma JP, Winter MM, Vehmeijer JT, Vliegen HW, van Dijk AP, van Melle JP et al (2017) QRS fragmentation is superior to QRS duration in predicting mortality in adults with tetralogy of Fallot. Heart 103(9):666–671
Oosterhof T, Vliegen HW, Meijboom FJ, Zwinderman AH, Bouma B, Mulder BJ (2007) Long-term effect of pulmonary valve replacement on QRS duration in patients with corrected tetralogy of Fallot. Heart 93(4):506–509
Nguyen HH, Shahanavaz S, George F et al (2016) Percutaneous pulmonary valve implantation alters electrophysiologic substrate. J Am Heart Assoc 5:e004325
Acknowledgements
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AMH, AFE, HAB and MHM contributed to Conducted the literature search analysis and interpretation of data. AMH, AFE contributed to Conducted the statistical analysis. AMH,HAB and MHM ontributed to Designed the study, AMH and MHM contributed to performed data collection, Analysis and interpretation of data, AFE, AMH drafted the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and Consent to participate
Institutional Review Board from King Faisal Specialist Hospital & Research Center-Jeddah (KFSH&RC-J). Reference # (IRB # 2023-42) Date: April 2023., Consent to participate is not Applicable due to the retrospective nature of the study.
Consent for publication
Waived for the retrospective nature of the study.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Helal, A.M., Baho, H.A., Elmahrouk, A.F. et al. PR and QRS interval changes after transcatheter pulmonary valve replacement in children. Egypt Heart J 75, 66 (2023). https://doi.org/10.1186/s43044-023-00394-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-023-00394-x