Abstract
Background
Cardiovascular disease (CVD) is the most common cause of death in patients with renal diseases. Cardiac arrhythmia and sudden cardiac death are particularly important, and the burden is higher in patients on hemodialysis. The aim of this study is to compare specific ECG changes as markers of arrhythmias in patients with CKD and patients with end-stage renal disease (ESRD); all without clinically manifest heart disease, with normal control subjects.
Results
Seventy-five ESRD patients on regular hemodialysis, 75 patients with stage 3–5 CKD and 40 healthy control subjects were included. All candidates were subjected to thorough clinical evaluation and laboratory tests including serum creatinine, glomerular filtration rate calculation, serum potassium, magnesium, calcium, phosphorus, iron, parathyroid hormone, and total iron binding capacity (TIBC). Resting twelve-lead ECG was done to calculate P wave dispersion (P-WD), corrected QT interval, QTc dispersion, Tpeak-Tend interval (Tp-e), and Tp-e/QT. Patients with ESRD had a significantly higher QTc dispersion (p < 0.001) and P-WD (p = 0.001) when compared to the other 2 groups. In the ESRD group, males had a significantly higher P-WD (p = 0.045), insignificantly higher QTc dispersion (p = 0.445), and insignificantly lower Tp-e/QT ratio (p = 0.252) as compared to females. Multivariate linear regression analysis for ESRD patients showed that serum creatinine (β = 0.279, p = 0.012) and transferrin saturation (β = − 0.333, p = 0.003) were independent predictors of increased QTc dispersion while ejection fraction (β = 0.320, p = 0.002), hypertension (β = − 0.319, p = 0.002), hemoglobin level (β = − 0.345, p = 0.001), male gender (β = − 0.274, p = 0.009) and TIBC (β = − 0.220, p = 0.030) were independent predictors of increased P wave dispersion. In the CKD group, TIBC (β = − 0.285, p = 0.013) was an independent predictor of QTc dispersion while serum calcium (β = 0.320, p = 0.002) and male gender (β = − 0.274, p = 0.009) were independent predictors of Tp-e/QT ratio.
Conclusions
Patients with stage 3–5 CKD and those with ESRD on regular hemodialysis exhibit significant ECG changes that are considered substrates for ventricular as well as supraventricular arrhythmias. Those changes were more evident in patients on hemodialysis.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) is a serious worldwide health problem. Cardiovascular disease (CVD) is the commonest cause of death in CKD as it accounts for 43% of all-cause mortality in hemodialysis patients [1]. Arrhythmia is common in patients with CKD and sudden cardiac death accounts for 25% of deaths in dialysis patients [2].
Several electrocardiographic (ECG) methods can be used to assess the risk of arrhythmia including measurement of the P wave dispersion (P-WD), corrected QT interval (QTc), peak of T wave to end of T wave interval (Tp-e), and QTc dispersion on the standard 12-lead ECG. Limited studies have assessed the resting 12-lead ECG as a screening tool in intermediate risk populations [3].
This study aimed to evaluate P-WD, QTc interval, QTc dispersion, Tp-e and Tp-e/QT ratio in patients with stage 3–5 CKD not on renal replacement therapy (RRT) and end-stage renal disease (ESRD) patients on regular hemodialysis; all without clinically manifest heart disease; compared to values in normal control subjects.
Methods
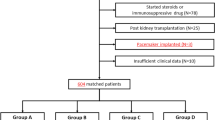
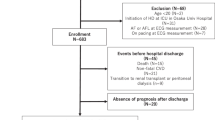
Seventy-five ESRD patients on regular hemodialysis, 75 stage 3–5 CKD patients (eGFR < 60 mL/min) without RRT and 40 healthy control subjects were included.
Patients excluded from the study were those on drugs that were known to prolong the QT interval (e.g., antibiotics as clarithromycin, antifungal as fluconazole, antiarrhythmics as amiodarone, antipsychotic as chlorpromazine), patients with structural heart diseases (e.g., significant valvular lesions), patients with impaired left ventricular systolic function (defined as ejection fraction < 50%) and patients with atrial fibrillation (because of the marked variabilities in the ECG intervals).
Patients were chosen from the Internal Medicine inpatient department and outpatient clinic and the chronic hemodialysis unit at Cairo University Hospital. The protocol of the study was approved by the ethics committee of the Faculty of Medicine, Cairo University, Cairo, Egypt.
After obtaining written, informed consents, individuals were subjected to thorough clinical evaluation (age, sex, history of hypertension (HTN) or diabetes mellitus (DM)) and laboratory tests including complete blood count, urea, creatinine, glomerular filtration rate (eGFR) (calculated by CKD-EPI equation) [4], serum potassium, magnesium, calcium, phosphorus, parathyroid hormone (PTH), iron and total iron binding capacity (TIBC).
Twelve-lead ECG was performed by General Electric Prucka (GE Healthcare Technologies, WI, USA) electrophysiological study machine. The machine was set to draw cardiac electric waves at a standard voltage (10 mm) and speed (25 mm/s). Corrected QT (QTc), QTc dispersion, P-WD, Tp-e interval and Tp-e/QT ratio were measured as follows:
-
Corrected QT interval:
-
The QT interval was measured in each of the 12 leads, from QRS complex onset to the end of the T wave off-set, as defined by the return of the terminal T wave (upright or inverted) to isoelectric TP baseline.
-
The QT interval was then corrected for the heart rate (QTc) using Bazett's formula:\(QTc=\frac{QT}{\sqrt{RR}}\) [5]. The maximum and the minimum QTc values were reported.
-
The average of the 12-lead QTc intervals was calculated. Prolonged QTc was diagnosed when QTc > 450 ms in males and > 470 ms in females [6].
-
-
QTc dispersion was calculated as the difference between the maximum and the minimum QTc. QTc dispersion should be between 30 and 60 ms in normal subjects [7].
-
P-WD was calculated as the difference between the maximum and the minimum P wave durations [8]. The normal value of P-WD is 29 ± 9 ms. A maximum P-WD value is 36 ms [9].
-
T wave peak to end interval
-
Tp-e interval was measured in each of the 12 leads, as the distance from the peak to the end of the T wave.
-
The mean of the Tp-e intervals was then calculated.
-
-
The ratio between the mean of the Tp-e intervals and the mean of the QTc intervals was calculated [10]. The normal Tp-e/QT ratio should be ≤ 0.25 [11].
Statistical methods
The collected data were revised, coded, tabulated and introduced to a PC using statistical package for social science (SPSS 15.0.1 for windows; SPSS Inc, Chicago, IL, 2001). Data were presented as mean and standard deviation for quantitative parametric variables. Frequency and percentages were used to describe qualitative data. Student t-test, Mann Whitney and ANOVA tests were used to compare means of quantitative data while Chi square/Fisher exact test was used to compare qualitative data. Pearson correlation was used to correlate quantitative data. Regression analysis was used to detect independent predictors of ECG changes. P-value < 0.05 was considered statistically significant.
Results
Table 1 shows the basic clinical and laboratory findings in the 3 study groups. Subjects in the control group were younger, less anemic and had a lower prevalence of hypertension.
Patients with ESRD had significantly higher QTc max, QTc dispersion, and P-WD when compared to the other 2 study groups, Table 2.
Comparison between patients with ESRD and patients with CKD showed non-significant differences except for levels of urea (p = 0.002), creatinine (p < 0.001), PO4 (p = 0.016) and PTH (p = 0.002) which were higher in the ESRD group, while serum calcium level (p = 0.023) was significantly lower in the ESRD group.
Subgroup analysis of patients with ESRD showed that males had a significantly higher P-WD values (p = 0.045), insignificantly higher QTc dispersion (p = 0.445), and insignificantly lower Tp-e/QT ratio (p = 0.252) when compared to females. While in the CKD group, only the Tp-e/QT ratio was significantly higher in males, Table 3.
ESRD hypertensive patients showed a significantly lower P-WD when compared to ESRD normotensive individuals, Table 3. On the other hand, patients with DM did not show any significant difference of the ECG parameters neither in the ESRD nor in the CKD patients as compared to non-diabetic patients.
Multivariate linear regression analysis of ESRD patients showed that serum creatinine (β = 0.279, p = 0.012) and transferrin saturation (β = − 0.333, p = 0.003) were independent predictors of increased QTc dispersion while ejection fraction (β = 0.320, p = 0.002), hypertension (β = − 0.319, p = 0.002), hemoglobin level (β = − 0.345, p = 0.001), male gender (β = − 0.274, p = 0.009) and TIBC (β = − 0.220, p = 0.030) were independent predictors of increased P-WD. On the other hand, age (β = − 0.296, p = 0.010) and serum creatinine (β = − 0.295, p = 0.010) were independent predictors of Tp-e/ QT ratio, Table 4.
In the CKD group, multivariate linear regression analysis showed that TIBC (β = − 0.285, p = 0.013) was an independent predictor of QTc dispersion while serum calcium (β = 0.320, p = 0.002) and male gender (β = − 0.274, p = 0.009) were independent predictors of Tp-e/QT ratio. However, no parameters predicted an increased P-WD in multivariate linear regression, Table 4.
The pattern of abnormal ECG changes in the study groups is shown in Table 5.
Calcium was significantly correlated to QTc average (r = − 0.344, p = 0.003) and Tp-e/QT (r = 0.263, p = 0.022), in CKD patients, Fig. 1, while creatinine level was significantly correlated to QTc dispersion (r = 0.247, p = 0.032), P-WD (r = 0.268, p = 0.020), Tp-e (r = − 0.239, p = 0.039) and Tp-e/QT (r = − 0.233, p = 0.044) in ESRD patients, Fig. 2.
Discussion
Chronic kidney disease (CKD) is defined as either kidney damage or a decreased glomerular filtration rate (eGFR) to less than 60 mL/min/1.73 m2 for at least 3 months. Whatever the underlying etiology, once the loss of nephrons and reduction of functional renal mass reaches a critical point, the remaining nephrons begin a process of irreversible sclerosis that leads to a progressive decline in the GFR [12].
Patients with CKD are vulnerable to cardiac arrhythmia and in many cases, renal dysfunction stimulates arrhythmia and arrhythmia exacerbates renal dysfunction [2]. In a recent study, the ARIC study, a 2-week cardiac monitoring of patients with CKD revealed a high prevalence of non-sustained ventricular tachycardia (30.2%) and AF (7.4%), while ventricular ectopy was present in more than 90% of patients [13]. The mechanism of arrhythmia in CKD may be due to electrolyte disturbances and/or the damage caused to kidneys and heart tissues by common comorbidities like hypertension and diabetes mellitus [2]. Arrhythmia causes premature death in the general population as well as in patients with CKD [2]. Sudden Cardiac Death (SCD) is common in CKD patients and it is the most common cause of death in dialysis patients [14].
Because most arrhythmias are intermittent and usually asymptomatic, documentation and diagnosis pose a challenge to the treating physician [2]. Several electrocardiographic (ECG) methods can be used to assess the risk of arrhythmia and this includes measurement of the P-WD, QTc interval, Tp-e interval, and QTc dispersion on the standard resting 12-lead ECG. P-WD is a non-invasive ECG marker for atrial remodeling and a predictor of atrial fibrillation (AF) [15]. Increased QTc dispersion indicates heterogeneity in ventricular repolarization, which is associated with an increased risk of ventricular arrhythmia and sudden cardiac death [16] while the Tp-e interval reflects the transmural dispersion of repolarization, and a relationship was found between Tp-e and ventricular arrhythmias, heart failure, and sudden cardiac death [17]. An increased Tp-e/QT was associated with arrhythmogenesis and the underlying mechanism was functional reentry [10].
This study aims to evaluate the substrates of arrhythmia (P-WD, QTc dispersion and Tp-e/QT ratio) in ESRD patients on regular hemodialysis (no. = 75) and in patients with CKD stages 3–5 without RRT (no. = 75) all without any clinically manifest heart disease as compared to 40 normal control subjects.
Baseline data showed that ESRD patients had a higher prevalence of hypertension and their laboratory data showed lower hemoglobin, and calcium levels and higher phosphorus and parathormone levels as compared to CKD patients and control subjects.
QTc dispersion was found to be significantly higher in ESRD and CKD patients as compared to normal controls. These data are in accordance with Kolluet al., who conducted their study on 133 patients with CKD stages 3–5 without RRT and 32 healthy controls and found that QTc dispersion values were higher in patients with CKD stages 3–5 on no RRT [11].
On the other hand, Covic et al., reported that hemodialysis increases QTc interval, but not QTc dispersion in 68 stable, non-diabetic dilaysis patients with ESRD without manifest cardiac disease [18].
The reason for the increased QTc dispersion could be explained by the study performed by Jaroszyński et al., who reported that the changes of serum calcium, phosphorus, potassium and extracellular volume during hemodialysis affected QTc dispersion and could promote ventricular arrhythmogenesis [19].
Tp-e/QT ratio was significantly higher in ESRD and CKD patients when compared to normal controls. These results are similar to the results found by Kollu et al., who found that Tp-e/QT is increased in CKD stages 3–5 patients without RRT [11] and Guclu et al. who found that Tp-e, Tp-e/QT, Tp-e/QT, and QTc were significantly higher in the hemodialysis and peritoneal dialysis patients [20]. The mechanism of ventricular repolarization prolongation in patients with ESRD was associated with traditional and CKD-related risk factors. Several CKD-related risk factors such as inflammation [21], hyperferritinemia, hyperparathyroidism, hyperphosphatemia [22], and structural changes in uremic myocardium are frequently present in patients with CKD. An increased prevalence of left ventricular dysfunction, autonomic dysfunction, myocyte dysfunction, altered electrolyte metabolism, and cardiac fibrosis may also contribute to arrhythmic risk in patients with kidney disease [23]. On the contrary, Karaagac et al. found that Tp-e/QT values were similar in ESRD patients receiving hemodialysis compared to healthy age and gender matched individuals [24].
P-WD was significantly higher in ESRD and CKD patients as compared to normal controls. This finding is similar to what was found by Kollu et al. who reported increased P-WD in CKD stages 3–5 patients without RRT [11]. Drighil et al. studied the impact of dilaysis on P-WD in 17 patients undergoing hemodialysis (HD). They found that P-WD decreased, and left atrial dimension decreased, after HD. The change in P-WD was correlated with fluid removed by HD and the subsequent decrease in LA dimensions [25].
Intracellular or intercellular factors may lead to site‐specific conduction differences [26]. P-WD increases with age and in patients with structural heart disease. Hypertension, coronary heart disease, valvular heart disease, pericarditis, dilated cardiomyopathy, ionic disturbances, and autonomic dysfunction are common underlying diseases associated with increased P-WD. Most patients with CKD have these abnormalities [27].
In the comparison of the ESRD group to the CKD group, we could not find a significant difference regarding prolonged QTc dispersion, prolonged P-WD duration or increased Tp-e/QT ratio.
P-WD values were significantly higher in males in the ESRD group and Tp-e/QT ratios were significantly higher in males in the CKD group. The studies which evaluated P-WD and Tp-e/QT in renal patients did not compare sex-related differences. Women are known to have a longer QTc interval, and a lower QTc dispersion than men. The mechanism for the longer QTc interval in women is not completely known but it does not appear to be related to the acute effects of estrogen or progesterone or differences in autonomic innervation [28].
Despite the fact that DM increases the risk of ventricular arrhythmogenesis [29], QTc dispersion, P-WD and Tp-e/QT in diabetic patients with ESRD and CKD were comparable to non-diabetic patients. This is consistent with Kollu et al. who found no difference between diabetic and non-diabetic population in terms of the duration of P-WD, QTc and Tp-e interval and also Tp-e/QT ratio [11]. On the contrary, a study published by Tokatli et al. reported that the values of Tp-e, Tp-e/QT ratio were higher in patients with type 2 DM [30].
On multivariate linear regression analysis in the ESRD group, transferrin saturation was the only independent predictor of an increased QTc dispersion while hemoglobin and male gender were the independent predictors of an increased P-WD. In the CKD group, the serum calcium and male gender were the independent predictors of an increased TP-e/ QT ratio.
Conclusions
Patients with stage 3–5 chronic kidney disease and those with end-stage renal disease on regular hemodialysis exhibit significant ECG changes that could be substrates for ventricular and supraventricular arrhythmias. Those changes were more evident in patients on hemodialysis. Larger and more comprehensive studies are required for the assessment of ECG changes evolution from chronic kidney disease to hemodialysis and to evaluate the value of these ECG abnormalities in predicting cardiac arrhythmias.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CKD:
-
Chronic kidney disease
- CVD:
-
Cardiovascular diseases
- DM:
-
Diabetes mellitus
- ECG:
-
Electrocardiogram
- eGFR:
-
Estimated Glomerular filtration rate
- ESRD:
-
End-stage renal disease
- HD:
-
Hemodialysis
- HTN:
-
Hypertension
- P-WD:
-
P wave dispersion
- QTc:
-
Corrected QT interval
- RRT:
-
Renal replacement therapy
- SCD:
-
Sudden Cardiac Death
- TIBC:
-
Total iron binding capacity
- Tp-e:
-
T wave peak to end interval
References
Baigent C, Burbury K, Wheeler D (2000) Premature cardiovascular disease in chronic renal failure. Lancet 356(9224):147–152
Akhtar Z, Leung LW, Kontogiannis C, Chung I, Bin Waleed K, Gallagher MM (2022) Arrhythmias in chronic kidney disease. European cardiology 17:e05
Bozbas H, Atar I, Yildirir A, Ozgul A, Uyar M, Ozdemir N et al (2007) Prevalence and predictors of arrhythmia in end stage renal disease patients on hemodialysis. Ren Fail 29(3):331–339
Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M et al (2010) Comparative performance of the CKD epidemiology collaboration (CKD-EPI) and the modification of diet in renal disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56(3):486–495
Bazett HC (1920) An analysis of the time relations of electrocardiograms. Heart 7:353–370
Antoniou CK, Dilaveris P, Manolakou P, Galanakos S, Magkas N, Gatzoulis K et al (2017) QT prolongation and malignant arrhythmia: how serious a problem? European Cardiol 12(2):112–120
Malik M, Batchvarov VN (2000) Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol 36(6):1749–1766
Dilaveris PE, Gialafos EJ, Sideris SK, Theopistou AM, Andrikopoulos GK, Kyriakidis M et al (1998) Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J 135(5 Pt 1):733–738
Aytemir K, Ozer N, Atalar E, Sade E, Aksöyek S, Ovünç K et al (2000) P wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol PACE 23(7):1109–1112
Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT et al (2008) T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol 41(6):567–574
Kollu K, Altintepe L, Duran C, Topal M, Ecirli S (2018) The assessment of P-wave dispersion and myocardial repolarization parameters in patients with chronic kidney disease. Ren Fail 40(1):1–7
Schnaper HW (2014) Remnant nephron physiology and the progression of chronic kidney disease. Pediatric Nephrol 29(2):193–202
Kim ED, Soliman EZ, Coresh J, Matsushita K, Chen LY (2021) Two-Week burden of arrhythmias across CKD severity in a large community-based cohort: the ARIC study. J Am Soc Nephrol 32(3):629–638
Chiu DY, Sinha S, Kalra PA, Green D (2014) Sudden cardiac death in haemodialysis patients: preventative options. Nephrology 19(12):740–749
Pérez-Riera AR, de Abreu LC, Barbosa-Barros R, Grindler J, Fernandes-Cardoso A, Baranchuk A (2016) P-wave dispersion: an update. Indian Pacing Electrophysiol J 16(4):126–133
Lee KW, Okin PM, Kligfield P, Stein KM, Lerman BB (1997) Precordial QT dispersion and inducible ventricular tachycardia. Am Heart J 134(6):1005–1013
Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R et al (2011) Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol 4(4):441–447
Covic A, Diaconita M, Gusbeth-Tatomir P, Covic M, Botezan A, Ungureanu G et al (2002) Haemodialysis increases QT(c) interval but not QT(c) dispersion in ESRD patients without manifest cardiac disease. Nephrol Dial Transplantat Off Publ European Dial Transplant Assoc European Renal Assoc 17(12):2170–2177
Jaroszyński AJ, Załuska WT, Ksiazek A (2005) Effect of haemodialysis on regional and transmural inhomogeneities of the ventricular repolarisation phase. Nephron Clin Pract 99(1):c24-30
Guclu A, Sipahioglu MH, Icli A, Alpay MF, Narman S (2016) Tp-e/qt and tp-e/qtc ratio in hemodialysis and peritoneal dialysis patients
Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE et al (2008) The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 74(10):1335–1342
Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK (2001) Association of elevated serum PO4, Ca× PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12(10):2131–2138
Glassock RJ, Pecoits-Filho R, Barberato SH (2009) Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol CJASN 4(Supplement 1):S79–S91
Karaagac K (2016) Tp-e Interval and Tp-e/QT Ratio in Chronic Renal Failure Patients Requiring Hemodialysis. J Clin Anal Med. https://doi.org/10.4328/jcam.3858
Drighil A, Madias JE, El Mosalami H, El Badaoui N, Mouine B, Fadili W et al (2007) Impact of hemodialysis on P-wave amplitude, duration, and dispersion. Indian Pacing Electrophysiol J 7(2):85–96
Rombola G, Colussi G, De Ferrari ME, Frontini A, Minetti L (1992) Cardiac arrhythmias and electrolyte changes during haemodialysis. Nephrol Dial Transplant Off Publ European Dial Transplant Assoc European Renal Assoc 7(4):318–322
Szabó Z, Kakuk G, Fülöp T, Mátyus J, Balla J, Kárpáti I et al (2002) Effects of haemodialysis on maximum P wave duration and P wave dispersion. Nephrol Dial Transplant 17(9):1634–1638
Larsen JA, Kadish AH (1998) Effects of gender on cardiac arrhythmias. J Cardiovasc Electrophysiol 9(6):655–664
Ninkovic VM, Ninkovic SM, Miloradovic V, Stanojevic D, Babic M, Giga V et al (2016) Prevalence and risk factors for prolonged QT interval and QT dispersion in patients with type 2 diabetes. Acta Diabetol 53(5):737–744
Tokatli A, Kilicaslan F, Alis M, Yiginer O, Uzun M (2016) Prolonged Tp-e interval, Tp-e/QT ratio and Tp-e/QTc ratio in patients with type 2 diabetes mellitus. Endocrinol Metab 31(1):105–112
Acknowledgements
Authors acknowledge the staff of the Cardiovascular and the Internal Medicine departments for the unlimited support they offered during the study.
Funding
None.
Author information
Authors and Affiliations
Contributions
H.Y and M.G proposed the idea, M.E collected the data, K.S and G.Y wrote the first draft of the manuscript and H.Y, M.G, K.S, M.E and G.Y finalized the draft and prepared the paper in its final form. All authors critically revised the manuscript and approved its final form.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of the Faculty of Medicine, Cairo University in March 2019. All participants gave written informed consent for participation in the study and for publication of data.
Consent for publication
A verbal consent for publication was taken from the study participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yehia, H., Youssef, G., Gamil, M. et al. Electrocardiographic substrates of arrhythmias in patients with end-stage and chronic kidney diseases: a case–control study. Egypt Heart J 75, 13 (2023). https://doi.org/10.1186/s43044-023-00338-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-023-00338-5