Abstract
Background
Endothelial dysfunction and no-reflow share microcirculatory obstruction as a common pathophysiological mechanism. This study evaluated the relationship between systemic peripheral endothelial dysfunction assessed by flow-mediated dilatation (FMD) of the brachial artery and no-reflow in patients with ST-segment elevation myocardial infarction (STEMI) who received successful fibrinolysis.
Results
This study included 150 patients managed by the percutaneous coronary intervention (PCI) after successful fibrinolysis. Patients were divided according to coronary angiographic success into normal flow versus no-reflow groups. According to FMD measured through brachial artery ultrasound, patients were divided based on their endothelial function into endothelial dysfunction versus normal endothelial function. No-reflow occurred in 44 patients (29.3%). No-reflow patients had longer pain to door time (6.52 ± 1.82 vs 5.19 ± 1.85 h), more Killip class II (36.4% vs 16%, p = 0.006), and lower FMD (7.26 ± 1.92 vs 8.23 ± 2.76%, p = 0.036). Also, they showed more endothelial dysfunction; however, this difference was statistically nonsignificant (97.7% vs 87.7%, p = 0.055). One hundred and thirty-six patients (90.7%) had endothelial dysfunction. They were older (57.51 ± 5.92 vs 50.86 ± 4.55 years, p value ≤ 0.001), more smokers (41.2% vs 14.3%, p = 0.04). Patients with normal endothelial function had a more myocardial blush grade (MBG) 3 (78.6% vs 26.5%, p value = 0.001) in comparison with more MBG 2 in those with endothelial dysfunction (41.9% vs 14.3%, p value = 0.001). Endothelial dysfunction patients had nonsignificant more no-reflow (31.6% vs 7.1%, p-value: 0.06). There was a significant weak positive correlation between thrombolysis in myocardial infarction (TIMI) flow and FMD (r = 0.174, p = 0.033) and a significant moderate positive correlation between MBG and FMD (r = 0.366, p < 0.001). Patients with TIMI I flow had significantly lower FMD compared with patients with TIMI II and TIMI III flow post-PCI. FMD ≤ 6% could predict post-procedural TIMI I flow.
Conclusions
In STEMI patients who underwent PCI within 24 h after successful fibrinolysis, those who had no-reflow showed worse peripheral systemic endothelial function as they had lower brachial artery FMD. Also, FMD showed a significant positive correlation with the post-procedural angiographic flow (TIMI flow and MBG). FMD ≤ 6% could predict TIMI I flow.
Similar content being viewed by others
Background
Normal endothelium regulates vascular motor tone through the release of nitric oxide. Also, it regulates vascular hemostasis and maintains blood flow by preventing platelet adhesion, leukocyte activation, and uncontrolled coagulation. On the other hand, dysfunctional endothelium plays a pivotal role in the development and progression of atherosclerosis. Also, it increases the vulnerability of atherosclerotic plaques and the risk of myocardial infarction [1,2,3,4]. Ruptured vulnerable atherosclerotic plaques show increased release and activity of vasoactive substances such as endothelin-1 [5]. Endothelin-1 could play a role in the pathogenesis of coronary no-reflow complicating coronary intervention through the mediation of functional microcirculatory obstruction [6].
No-reflow is related to increased cardiovascular morbidity and mortality. The pathophysiological mechanism of no-reflow is complex and multifactorial [7]. It includes mechanical microcirculatory obstruction due to distal thrombotic embolization and functional microcirculatory obstruction due to the release of vasoconstrictor mediators [6]. The link between endothelial dysfunction and no-reflow is still unclear. Theoretically, increased endothelin-1 may link endothelial dysfunction to no-reflow; however, clinical studies could not establish this relationship in ST-segment elevation myocardial infarction (STEMI) patients managed by the primary percutaneous intervention (PCI) [8].
This study evaluated the relationship between systemic peripheral endothelial dysfunction assessed by flow-mediated dilatation (FMD) of the brachial artery and no-reflow in patients with STEMI who received successful fibrinolysis.
Methods
This prospective observational study was carried out in our institution in the period between September 2019 until August 2021. It included patients presented with STEMI within 12 h from chest pain who received successful fibrinolysis as the reperfusion modality when primary PCI is not available within 120 min; they were scheduled for invasive coronary angiography within 24 h from fibrinolysis according to European society of cardiology (ESC) guidelines [9]. Successful fibrinolysis was defined as (ST-segment resolution > 50% at 60–90 min; typical reperfusion arrhythmia; and disappearance of chest pain) [10]. The study excluded patients who refused to participate, patients with Killip class ≥ III, and patients referred for cardiothoracic surgical consultation after diagnostic angiography. The research ethics committee at Ain Shams University approved the study design (FMASU MD 352/2019), and all participants signed informed written consent. All patients received optimal medical therapy for STEMI as per ESC 2017 guidelines, unless any medication was contraindicated including loading anti-platelets (acetyl salicylic acid 300 mg and clopidogrel 300 mg) [9]. All participants were subjected to history taking to determine risk factors of coronary artery disease, established cardiovascular disease, and pain to door time in hours. Physical examination evaluated blood pressure and Killip class (where Killip class I showed no clinical signs of heart failure, Killip class II showed rales in the lungs, third heart sound (S3), and elevated jugular venous pressure, Killip class III showed acute pulmonary edema and Killip class IV had cardiogenic shock) [11]. Twelve-lead surface electrocardiogram (ECG) was done on admission to localize the territory of infarction (Anterior vs non-anterior) with the observation of baseline ST-segment elevation from J-Point. Our study included patients with successful fibrinolysis, so ECG was repeated 60–90 min after thrombolytic therapy to identify successful fibrinolysis (more than 50% ST segment resolution) [9, 10]. Transthoracic echocardiography excluded mechanical complications and evaluated left ventricular (LV) systolic function by the modified Simpson method. Blood samples were withdrawn on admission to measure creatine-kinase total (CK-total) and myocardial band (CK-MB).
Coronary intervention
Coronary angiography was done through femoral or radial access using a 6F arterial sheath under local anesthesia, and intravenous pre-medications were given as needed. Infarct-related artery (IRA) was identified (left anterior descending artery (LAD), left circumflex artery (LCX), or right coronary artery (RCA)), then thrombolysis in myocardial infarction (TIMI) thrombus grade [12] and pre-procedural TIMI flow were recorded [13]. The choice of guiding catheter was according to coronary anatomy and operator choice. Percutaneous transluminal coronary angioplasty (PTCA) wire crossing of the lesion was done after giving parenteral anticoagulation using 100 IU/kg of unfractionated heparin. Balloon dilatation was done if needed, and coronary stenting was done using drug-eluting stents (DES). No-reflow was defined as (TIMI flow < III or myocardial blush grade (MBG) ≤ 1 despite the mechanical reopening of the IRA) [14]. TIMI flow grading system was defined as TIMI 0: complete occlusion of the infarct-related artery, TIMI I: some penetration of the contrast material beyond the point of obstruction but without perfusion of the distal coronary bed, TIMI II: perfusion of the entire infarct vessel into the distal bed but with delayed flow compared with a normal artery, TIMI III: full perfusion of the infarct vessel with the normal flow [13]. Blush grades are defined as follows: 0, absence of blush or contrast density; 1, minimal contrast density; 2, moderate contrast density, but less than that obtained with angiography of an artery unrelated to the infarct; and 3, normal contrast density comparable to that obtained with angiography of an artery unrelated to the infarct [15]. According to the reperfusion success, they were classified into two groups, no-reflow versus normal flow.
Assessment of endothelial function
Assessment of endothelial function was done by measuring FMD before coronary angiography. Brachial artery duplex was performed using a General Electric (GE) vivid S5 machine using 8L-RS linear array transducer (4–13 MHz) with clear anterior and posterior intimal surfaces. Patients were lying in a supine position with their hands at the level of the heart, blood pressure cuff was placed over the antecubital fossa. Then the ultrasound probe was placed at a level just above the antecubital fossa and a baseline resting trans-sectional image of the brachial artery was obtained to measure its baseline diameter. After that, a blood pressure cuff was inflated to 40 mm Hg above systolic pressure for a standardized time (5 min), then the cuff was rapidly deflated to allow reactive hyperemia. Brachial artery diameter was recorded during the first minute after the pressure release to detect the maximal reactive hyperemia at the same point of the baseline diameter measurement. FMD is the percent change in diameter from baseline and was calculated by dividing the difference between hyperemic diameter and baseline diameter by the baseline diameter and then multiplying by 100. FMD% = (D2 − D1)/D1 × 100, Where D1 is baseline brachial artery diameter and D2 is hyperemic brachial artery diameter, and FMD% less than 10% was used to diagnose endothelial dysfunction [16]. The interventional cardiologists were blinded to brachial artery FMD% results. According to the presence of endothelial dysfunction, patients were classified into another two groups, endothelial dysfunction versus normal endothelial function.
Statistical analysis
Data were collected, coded, and entered into IBM Statistical Package for Social Science (SPSS) version 23. Parametric numerical data were described by mean and standard deviation, while nonparametric numerical data was described by median and interquartile range (IQR). Non-numerical variables were presented as frequency and percentage. Analyses of qualitative variables were performed by chi-square. Parametric variables were analyzed by independent t-test, and nonparametric variables were analyzed by Mann–Whitney U test. Using receiver operating characteristic curves (ROC), we determined the prediction utility of brachial artery FMD% for no-reflow. The margin of error accepted was set to 5% by setting the confidence interval to 95%, so the p-value was considered significant if p < 0.05.
Results
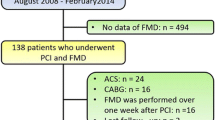
The study flowchart is represented in Fig. 1. In the current study, one-hundred and fifty patients were managed by PCI after successful fibrinolysis and divided according to the success of reperfusion into two groups (Normal flow and no-reflow) with Table 1 showing the comparison between the two groups. No-reflow occurred in 44 patients (29.3%). There was no significant difference in comparison age, gender, risk factors for coronary artery disease, and previous cardiovascular disease. No-reflow group had significantly more anterior myocardial infarction, while those with normal flow had more non-anterior infarction. No-reflow patients showed significantly longer pain to door time (6.52 ± 1.82 vs 5.19 ± 1.85 h), more incidence of Killip class II (36.4% vs 16%, p = 0.006), higher CK-MB (136 (78.5–230.5) vs 69.5 (54–96) IU, p < 0.001), and lower ejection fraction (EF) (46.57 ± 9.93 vs 53.49 ± 8.99%, p < 0.001). On comparing the findings of brachial artery ultrasound, the no-reflow group had significantly lower FMD (7.26 ± 1.92 vs 8.23 ± 2.76%, p = 0.036). Also, they showed more endothelial dysfunction; however, this difference was statistically nonsignificant (97.7% vs 87.7%, p = 0.055).
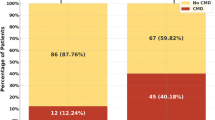
Patients were divided according to FMD into one-hundred and thirty-six patients (90.7%) with endothelial dysfunction, while 14 patients (9.3%) had a normal endothelial function with Table 2 showing the comparison between the two groups. There was no significant difference in comparing gender, hypertension, diabetes mellitus, Killip class, pain to door time, and EF between both groups, while those with endothelial dysfunction were significantly older (57.51 ± 5.92 vs 50.86 ± 4.55 years, p value ≤ 0.001), more smokers (41.2% vs 14.3%, p = 0.04), and had a significantly higher incidence of family history of premature coronary artery disease (19.9% vs 42.9%, p = 0.04). On comparing angiographic findings between both groups, there was no significant difference in baseline coronary TIMI flow and thrombus burden before PCI or post-PCI TIMI flow. However, on comparing MBG between both groups, patients without endothelial dysfunction had a higher incidence of MBG 3 (78.6% vs 26.5%) in comparison with a higher incidence of MBG 2 in those with endothelial dysfunction (41.9% vs 14.3%). However, on comparing the success of myocardial perfusion, endothelial dysfunction patients showed more no-reflow; however, this was not statistically significant (31.6% vs 7.1%, p-value: 0.06).
There was no significant correlation between FMD and age (r = − 0.101, p = 0.217) or pain to door (r = 0.019, p = 0.821), while there was significant weak positive correlation between post-procedural TIMI flow and FMD (r = 0.174, p = 0.033) (Fig. 2) in addition to significant moderate positive correlation between MBG and FMD (r = 0.366, p < 0.001) (Fig. 3).
On comparing FMD percentage between TIMI flow grades, post hoc analysis showed that the patients with TIMI I flow post-PCI had significantly lower FMD compared with patients with TIMI II and TIMI III flow post-PCI, and there was no significant difference between FMD values between patients with TIMI II and TIMI III flow post-PCI as shown in Table 3. Furthermore, post hoc analysis comparing FMD percentage between MBG showed that patients who had MBG 3 had significantly higher FMD percentage compared to patients who ended up with MBG 2, MBG 1 and MBG 0 as shown in Table 4. ROC curve analysis showed that FMD ≤ 6% is the best cutoff value to predict post-procedural TIMI I flow as shown in Table 5.
Discussion
In the current study, patients who presented with STEMI within 12 h from chest pain onset and had PCI after successful fibrinolysis showed two key findings. The no-reflow group had a significantly lower brachial artery FMD measured before invasive angiography within 24 h from STEMI, and there was a significant positive correlation between brachial artery FMD percentage and post-procedural angiographic flow (TIMI flow and MBG). Also, FMD ≤ 6% could be able to predict TIMI I flow.
In the current study, the assessment of systemic endothelial function was done by measuring brachial arterial FMD which correlates with coronary endothelial function [17]. Endothelial dysfunction results in decreased production of nitric oxide with increased vascular tone and microvascular resistance due to increased activity of vasoactive substances such as endothelin-1. Coronary endothelial dysfunction shares in the pathophysiology of atherosclerosis and increases the vulnerability of atherosclerotic plaques increasing the risk of an acute coronary syndrome [1,2,3,4,5]. In return, myocardial infarction-induced endothelial damage aggravates endothelial dysfunction resulting in functional microvascular obstruction through increased microvascular resistance that increases the risk of no-reflow [2, 7]. Pathogenesis of no-reflow is multifactorial and several mechanisms are involved including high thrombus burden with distal thromboembolism, prolonged duration of ischemia, and reperfusion injury [7]. This may explain a worse post-procedural TIMI flow and MBG with declining brachial artery FMD. However, in comparing the presence of systemic endothelial dysfunction diagnosis between the no-reflow and normal flow groups, the difference was not statistically significant. Some factors could explain this finding. First, only a small percentage of myocardial infarction patients have normal endothelial dysfunction as in the current study (nearly only 10%). Second, despite the correlation between systemic and coronary endothelial function, local endothelial damage with myocardial infarction increases coronary endothelial dysfunction [2]. Third, the multifactorial pathogenesis of no-reflow includes endothelial dysfunction as a contributing factor among other variables. One of these factors is the prolonged ischemic time as in the current study; however, FMD did not correlate with ischemic time.
In comparison with the current study, Levi et al. [8] study did not show a difference in endothelial dysfunction between reflow and no-reflow patients as assessed by measuring peripheral arterial tonometry 2–3 days after primary PCI. However, this does not rule out this relationship as the number of patients in this study was small. On the other side, Rashed et al. [18] study concluded that FMD < 11% could predict no-reflow. Also, the study by Vasilieva et al. [19] showed that the incidence of spontaneous thrombolysis was more associated with better FMD. Moreover, the study by Bravo Baptista et al. [20] showed that endothelial dysfunction was associated with microvascular obstruction and larger infarction size.
The clinical importance of the findings of the current study may be summarized in two points. First, although endothelial dysfunction is not the only determinant of no-reflow, it is correlating with post-procedural angiographic success. So, measures taken to improve endothelial function may have a beneficial effect on angiographic success in the setting of acute myocardial infarction. Many studies evaluated the effect of different drugs on improving endothelial function [3, 21,22,23]. Among these drugs, statins and angiotensin-converting enzyme inhibitors have proven beneficial effects [24]. Second, as the current guidelines recommend invasive angiography within 2–24 h, there is enough time to measure brachial artery FMD that could predict TIMI I flow if ≤ 6 which may direct future research to study the preventive measures against no-reflow in this particular group.
Study limitations
One of the limitations of the study is that STEMI patients mostly have endothelial dysfunction. In the current study, nearly 10% only showed normal endothelial dysfunction. This small number of patients could explain that despite more no-reflow in those with endothelial dysfunction, this finding was statistically non-significant. The limitations of endothelial dysfunction assessment using this technique include the need for standardized protocols by trained personnel to maintain reproducibility, accuracy, and reliability [25, 26].
Conclusions
In STEMI patients who underwent PCI within 24 h after successful fibrinolysis, those who had no-reflow showed worse peripheral systemic endothelial function as they had lower brachial artery FMD. Also, FMD showed a significant positive correlation with the post-procedural angiographic flow (TIMI flow and MBG). FMD ≤ 6% could predict TIMI I flow on PCI to IRA.
These findings emphasize the relationship between endothelial dysfunction and no-reflow. Also, they may point to a potential reduction of no-reflow through improving endothelial dysfunction.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under curve
- CABG:
-
Coronary artery bypass graft
- CK:
-
Creatine kinase
- CK-MB:
-
Creatine kinase myocardial band
- DBP:
-
Diastolic blood pressure
- DES:
-
Drug-eluting stents
- ECG:
-
Electrocardiography
- ESC:
-
European society of cardiology
- EF:
-
Ejection fraction
- FMD:
-
Flow-mediated dilatation
- GE:
-
General electric
- IQR:
-
Interquartile range
- IRA:
-
Infarcted related artery
- LAD:
-
Left anterior descending artery
- LCX:
-
Left circumflex artery
- LV:
-
Left ventricle
- MBG:
-
Myocardial blush grade
- PCI:
-
Percutaneous intervention
- PTCA:
-
Percutaneous transluminal coronary angioplasty
- RCA:
-
Right coronary artery
- ROC:
-
Receiver operating characteristic curves
- SBP:
-
Systolic blood pressure
- STEMI:
-
ST-segment elevation myocardial infarction
- SPSS:
-
Statistical package for social science
- TIMI:
-
Thrombolysis in myocardial infarction
References
Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A et al (2010) From endothelial dysfunction to atherosclerosis. Autoimmun Rev 9(12):830–834. https://doi.org/10.1016/j.autrev.2010.07.016
Bhagat K (1998) Endothelial function and myocardial infarction. Cardiovasc Res 39(2):312–317. https://doi.org/10.1016/s0008-6363(98)00138-2
Matsuzawa Y, Lerman A (2014) Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coron Artery Dis 25(8):713–724. https://doi.org/10.1097/MCA.0000000000000178
Oikonomou E, Siasos G, Tsigkou V, Bletsa E, Panoilia ME, Oikonomou IN et al (2020) Coronary artery disease and endothelial dysfunction: novel diagnostic and therapeutic approaches. Curr Med Chem 27(7):1052–1080. https://doi.org/10.2174/0929867326666190830103219
Mayyas F, Al-Jarrah M, Ibrahim K, Mfady D, Van Wagoner DR (2015) The significance of circulating endothelin-1 as a predictor of coronary artery disease status and clinical outcomes following coronary artery catheterization. Cardiovasc Pathol 24(1):19–25. https://doi.org/10.1016/j.carpath.2014.08.004
Lerman A, Holmes DR, Herrmann J, Gersh BJ (2007) Microcirculatory dysfunction in ST-elevation myocardial infarction: Cause, consequence, or both? Eur Heart J 28(7):788–797. https://doi.org/10.1093/eurheartj/ehl501
Rezkalla SH, Kloner RA (2002) No-reflow phenomenon. Circulation 105(5):656–662. https://doi.org/10.1161/hc0502.102867
Levi Y, Sultan A, Alemayehu M, Wall S, Lavi S (2016) Association of endothelial dysfunction and no-reflow during primary percutaneous coronary intervention for ST-elevation myocardial infarction. Cardiovasc Revasc Med 17(8):552–555. https://doi.org/10.1016/j.carrev.2016.08.013
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC). Eur Heart J 39(2):119–177
Anderson RD, White HD, Ohman EM, Wagner GS, Krucoff MW, Armstrong PW et al (2002) Predicting outcome after thrombolysis in acute myocardial infarction according to ST-segment resolution at 90 minutes: a substudy of the GUSTO-III trial. Global use of strategies to open occluded coronary arteries. Am Heart J 144(1):81–88. https://doi.org/10.1067/mhj.2002.123319
Killip III T, Kimball JT (1967) Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol 20(4):457–464
Sianos G, Papafaklis MI, Serruys PW (2010) Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol 22(10 Suppl B):6B-14B
Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J et al (1987) Thrombolysis in myocardial infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 76(1):142–154. https://doi.org/10.1161/01.cir.76.1.142
Henriques JPS et al (2003) Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation 107(16):2115–2119
Van ’t Hof AWJ, Liem A, Suryapranata H, Hoorntje JCA, de Boer M-J, Zijlstra F (1998) Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction. Circulation 97(23):2302–2306
Onkelinx S, Cornelissen V, Goetschalckx K, Thomaes T, Verhamme P, Vanhees L (2012) Reproducibility of different methods to measure the endothelial function. Vasc Med 17(2):79–84
Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A et al (1998) Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 82(12):1535–1539. https://doi.org/10.1016/s0002-9149(98)00702-4
Rashed MI, Saleh MA, Elfekky EM, Elmahmoudy AM (2022) CHA2DS2 VASc score and brachial artery flow-mediated dilation as predictors for no-reflow phenomenon in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Egypt Heart J 74(1):13. https://doi.org/10.1186/s43044-022-00249-x
Vasilieva E, Urazovskaya I, Skrypnik D, Shpektor A (2009) Total occlusion of the infarct-related coronary artery correlates with brachial artery flow-mediated dilation in patients with ST-elevation myocardial infarction. Acute Card Care 11(3):155–159. https://doi.org/10.1080/17482940902763372
Baptista SB, Faustino M, Brizida L, Loureiro J, Augusto J, Abecasis J et al (2017) Early peripheral endothelial dysfunction predicts myocardial infarct extension and microvascular obstruction in patients with ST-elevation myocardial infarction. Rev Port Cardiol (English, Portuguese) 36(10):731–742. https://doi.org/10.1016/j.repc.2017.01.006
Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X et al (2021) Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev 73(3):924–967. https://doi.org/10.1124/pharmrev.120.000096
Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, González-Garrido A, Villarreal-Molina T, Jacobo-Albavera L (2021) Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci 22(8):3850. https://doi.org/10.3390/ijms22083850
Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I et al (2017) Targeting vascular (endothelial) dysfunction. Br J Pharmacol 174(12):1591–1619. https://doi.org/10.1111/bph.13517
Novo García E, Balaguer J, Jiménez E, GarcíaLledó A, Caballero M, Chaparro M (2003) Anális de las diferencias encontradas en la dilatación mediada por flugo según la terapia seguida en pacientes con enfermedad coronaria [Analysis of differences in flow-mediated dilation in relation to the treatment of coronary patients]. Rev Esp Cardiol (Spanish) 56(2):128–136. https://doi.org/10.1016/s0300-8932(03)76836-5
Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA et al (2011) Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300(1):H2-12. https://doi.org/10.1152/ajpheart.00471.2010
Thijssen DH, Bruno RM, van Mil ACCM, Holder SM, Faita F, Greyling A et al (2019) Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40(30):2534–2547. https://doi.org/10.1093/eurheartj/ehz350
Acknowledgements
Not applicable.
Funding
No funds were received to fulfill this work.
Author information
Authors and Affiliations
Contributions
MAE took part in the conception and design of the study, performing echocardiography, catheter intervention, interpretation of the data, drafting of the manuscript. MAS took part in conception and design of the study, interpretation of data, drafting of the manuscript revision of the final manuscript. SSS took part in the conception and design of the study, interpretation of data, drafting of the manuscript, revision, and approval of the final manuscript. IB took part in the conception and design of the study, performing echocardiography, catheter intervention, interpretation of data, drafting of the manuscript, revision, and approval of the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study design was revised and approved by the ethical committee at Ain Shams University (FMASU MD 352/2019) following declaration of Helsinki last updated 2008. The patients were consented by an informed written consent as regard participation and they accepted.
Consent for publication
Written informed consents have been obtained from all study participants to publish data and they accepted.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elbendary, M.A.W., Saleh, M.A., Sabet, S.S. et al. Correlation between endothelial dysfunction and occurrence of no-reflow in patients undergoing post-thrombolysis early invasive percutaneous intervention for ST-elevation myocardial infarction. Egypt Heart J 74, 70 (2022). https://doi.org/10.1186/s43044-022-00309-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-022-00309-2