Abstract
Background
This prospective study was aimed at comparing phase contrast cardiac magnetic resonance imaging (PC-CMR) with 2D transoesophageal echocardiography (TEE) for determining potential candidature for transcatheter closure in ostium secundum ASD (OS-ASD) patients. We included consecutive adult patients with OS-ASD for the evaluation of feasibility for transcatheter closure using 2D-TEE and PC-CMR over a period of 2 years. Patients who fulfilled the conventional criteria for transcatheter closure, i.e. maximum ASD diameter ≤ 34 mm, adequate rims (≥ 5 mm, except for anterosuperior rim), and normal pulmonary venous drainage on both imaging modalities, were taken for device closure. In patients where there was discrepancy in the measurements of ASD diameter or rim size, making them eligible for device closure on one imaging modality and ineligible on the other hand, provisional device closure was attempted. All patients who underwent transcatheter closure were followed up to 6 months to rule out any complications.
Results
A total of 58 patients (mean age 35.93 ± 10.59 years) were enrolled in the study. Overall, there was significant positive correlation between 2D-TEE and CMR measurements of maximal ASD diameter and rim size (p < 0.001). However, TEE significantly underestimated maximal ASD diameter and posteroinferior rim size in comparison with CMR (p = 0.013 and p = 0.023, respectively). 46 (79.3%) patients were suitable for transcatheter closure on CMR, while 44 (75.9%) were eligible on TEE. Transcatheter closure was attempted in 48 patients based on imaging findings and was successful in 46 (95.8%) patients. Device closure was unsuccessful in 2 patients with defect size < 34 mm on TEE but > 34 mm on CMR. Among 7 patients with deficient posteroinferior rim on TEE, 5 had sufficient rim on CMR and underwent successful transcatheter closure. CMR detected anomalous pulmonary venous drainage in one patient which was missed on TEE, hence excluding the patient from transcatheter closure. Mean device size was 28.3 ± 7.4 mm and correlated more strongly with the defect dimensions on PC-CMR (r = 0.85, p < 0.001) compared to TEE (r = 0.71, p = 0.02).
Conclusions
PC-CMR may to be superior to 2D-TEE for the preprocedural planning and feasibility assessment for transcatheter closure in adult patients with ostium secundum ASD.
Similar content being viewed by others
Background
Atrial septal defect (ASD) is one of the most common forms of congenital heart diseases (CHD), accounting for 6–10% of all CHD cases and 30–40% of adult CHD patients [1]. Ostium secundum ASD is the most frequent type of ASD and comprises 70% of all such cases. First described by King et al. in 1976, transcatheter closure has become the procedure of choice for the treatment of secundum ASD patients with suitable morphology who are symptomatic or have hemodynamically significant shunts [2, 3]. Minimally invasive nature, shorter hospital stays, fewer complications, and greater cost effectiveness are the unequivocal advantages of this technique over conventional surgical closure [3, 4]. Meticulous preprocedural morphological evaluation for the feasibility of device closure forms the cornerstone of achieving successful results with this procedure [5, 6].
Transoesophageal echocardiography (TEE) is the most validated method of evaluating the feasibility of device closure in ASD patients [5,6,7]. Except for some paediatric patients with excellent acoustic windows where transthoracic echocardiography (TTE) may alone be sufficient for management decisions, TEE is recommended in all patients before contemplating transcatheter closure for detailed assessment of type, size, shape, number of defects, presence of adequate rims for device anchorage, and ruling out any associated anomalies that could potentially complicate or preclude device closure [7,8,9].
Cardiac magnetic resonance imaging (CMR) has dramatically evolved as an imaging modality for congenital heart disease over the last couple of decades [10]. It offers distinct advantages including superb spatial and temporal resolution, large field of view not restricted by body habitus or acoustic window, lesser operator dependence, and three-dimensional multiplanar imaging capability of various cardiac and extracardiac structures. Furthermore, CMR is a versatile investigative modality providing highly accurate and reproducible information about cardiovascular anatomy and physiology [11]. Phase contrast imaging represents a recent advance in CMR that relies on motion-induced phase shifts for the measurement of local flow velocities within cardiac or vascular structures [12, 13]. In patients with ASD, phase contrast CMR (PC-CMR) allows 3D en face visualisation of the defect and direct quantification of the left to right shunt [14, 15]. To date, there is limited data published in the literature where PC-CMR has been compared to TEE for the evaluation of feasibility for device closure in ASD patients [14,15,16,17,18,19,20]. The purpose of this study was to compare PC-CMR with 2D-TEE in secundum ASD patients for comprehensive morphological evaluation and determining potential candidature for transcatheter closure.
Methods
The present study was a prospective and comparative study conducted in the Department of Cardiology, Sher-i-Kashmir Institute of Medical sciences over a period of 2 years. We included consecutive adult patients (18 years or older) diagnosed with ostium secundum ASD on TTE for the evaluation of feasibility for transcatheter closure using 2D-TEE and PC-CMR. Patients with contraindication for CMR, other types of ASD, i.e. ostium primum or sinus venosus defects, atrial septal aneurysm (ASA), or multiple ASDs, were excluded from the study. An informed consent was obtained from each patient before enrolment in the study and the study protocol was approved by the Institutional Ethics Committee. All the eligible patients underwent evaluation by both PC-CMR and 2D-TEE within 2 weeks of initial diagnosis by TTE.
Transoesophageal echocardiography
2D-TEE of each patient was performed by a team of two experienced cardiologists who were blinded to the CMR details of the patient. After sedating the patient with 2–5 mg midazolam, the procedure was performed using Aloka, Prosound, SSD α-110 (South Korea) 2D TEE system with multiplanar 5 MHz transducer, while continuously recording a 1-lead ECG. The inter-atrial septum was visualised after intra-oesophageal transducer placement at different rotation angles (0°–130°) at the upper oesophageal, mid-oesophageal, and gastroesophageal junction levels. Maximum ASD size was measured at ventricular end-systole. Atrial septal margins were measured as per conventional definitions [7, 9]. The anterior inferior (AI) rim was measured from the defect to the mitral valve, the anterior superior (AS) rim from the defect to the aortic root, posterior inferior (PI) rim from the defect to the inferior vena cava and posterior superior (PS) rim from the defect to the superior vena cava. The TEE views for measurement of the rims were: the mid-oesophageal four-chamber view for AI rim, basal short axis view for AS rim, and biatrial views for PI and PS rims. All the rims were evaluated in at least three sequential related multiplane views in 15° increments. For the better visualisation of posteroinferior rim the TEE probe was advanced into the stomach, retroflexed, and slowly withdrawn to lower oesophagus, while imaging in the long axis at 90° ± 20°. This manoeuvre allows the ultrasound beam to be directed perpendicular to the PI rim thus profiling it more clearly. For the imaging of right pulmonary veins, the probe was placed at mid-oesophageal level at 45° ± 10°, rotated clockwise and gradually withdrawn till veins were visualised. Left pulmonary veins were visualised by placing the probe at mid-oesophageal level at 120° ± 10°, rotating counterclockwise with gradual withdrawal. Additional colour Doppler signal imaging (colour scale 35–40 cm/s) was used to outline the margins of the defect. All measurements were based on consensus opinion of both cardiologists.
Cardiac magnetic resonance imaging
CMR of each patient was performed by a team of two experienced radiologists who were blinded to the TEE details of the patient. All examinations were performed with a 1.5 T whole-body MR imaging unit (Magnetom Avanto, Siemens Medical Systems, Germany). The maximum gradient performance of this system was 45 mT/m amplitude with slew rate of 200 T/m/ms. A five-element cardiac phased-array coil was used for signal acquisition. After taking a HASTE localiser, 4 chamber views were obtained using a retrospectively gated cine MRI sequence (trufi/FLASH) during end-expiratory breath hold of 8–10 s. After getting a clear view of the ASD in 4 chamber planes, biatrial parasagittal cine sections were obtained through the defect with a section thickness of 6 mm and no intersection gap. Through-plane phase contrast sequences were planned on the 4 chamber and biatrial planes using an encoding velocity (Venc) of 60–80 cm/s, to ensure sensitivity to lower blood flow velocities close to the defect edge, thereby preventing underestimation of the diameter of large defects with low shunt flow velocities. One to three contiguous cine phase-contrast MR imaging sections were obtained in the atrial septal plane to provide an en face view of the spatial position of the ASD. From this projection, sections were obtained from the defect toward the SVC, the IVC, the aortic root, and the atrioventricular valves. Care was taken to match the TEE protocol as closely as possible. Maximal ASD diameter was assessed at the ventricular end-systole and the sizes of the four ASD rims (described above) were measured. Size measurements obtained from en face images were only accepted if the defect was truly located in plane, as suggested from a narrow rim of signal void in the magnitude images. Pulmonary and systemic venous return was assessed with two-dimensional time-of-flight MR angiography with transverse-plane acquisitions. To enhance signal from thoracic veins, a trigger delay was chosen so that images were acquired during early diastole, when venous flow is maximal. All measurements were based on consensus opinion of both radiologists.
Transcatheter device closure
Percutaneous device closure was performed by a team of two experienced interventional cardiologists. Patients who fulfilled the conventional criteria for transcatheter device closure, i.e. maximum ASD diameter ≤ 34 mm, adequate rims (≥ 5 mm, except for AS rim), and normal pulmonary venous drainage on both imaging modalities were taken for device closure [8]. Patients in whom there was discrepancy in the measurements of ASD diameter or rim size which made them eligible for device closure on one imaging modality and ineligible on the other hand were provisionally taken up for the procedure and device closure was attempted. All the defects were closed using an Amplatzer Septal Occluder (St. JudeMedical, St. Paul, MN, USA) under fluoroscopic and 2D-TEE guidance using standard techniques. The device size was chosen depending on the maximum ASD diameter and adequacy/floppiness of the rims. Procedural success was defined by standard criteria, i.e. stable device position after release from the delivery cable, absence of any residual shunt across the defect on colour flow imaging, and no impingement of important structures like ostia of coronary sinus or venae cavae, and mitral or tricuspid valves on post-procedural TEE [3, 4].
Follow-up
All the patients were observed for 24–48 h in the hospital for any acute complications (device embolisation, stroke/transient ischemic attack, pericardial effusion, etc.) and discharged after confirming satisfactory results on repeat TTE as per hospital protocol. Each patient received dual antiplatelet therapy (aspirin 75 mg and clopidogrel 75 mg per day), starting 24 h before the procedure and continued 1 month after the procedure. Aspirin monotherapy was continued for another 5 months. All patients were followed up at 1-week, 1-month, 3-month, and 6-month intervals on out-patient basis with clinical history taking and cardiovascular examination. A repeat 2D-TEE was performed at 6 months to rule out any late complications, i.e. device erosion.
Statistical analysis
Statistical analysis was performed by SPSS software package (version 20.0, SPSS Inc, Chicago, Illinois, USA). All continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were reported as frequency and percentages. Pearson’s correlation coefficients were used to assess the strength of relationship between measurements of defect and rim sizes on TEE and CMR. Differences in estimations were calculated using paired t-tests and expressed as mean difference (95% confidence interval). Bland–Altman comparative analysis was performed to demonstrate agreement between the TEE and CMR measurements. Statistical significance was defined as a p value of < 0.05.
Results
A total of 65 patients were initially screened for eligibility by TTE over a period of 2 years. Five patients were excluded from the study based on TTE findings (three patients with multiple ASDs and two with ASA). One patient had claustrophobia and another did not give consent for the study. Finally, 58 patients who fulfilled the eligibility criteria were enrolled in the study. The mean age of our patients was 35.93 ± 10.59 years (range 18–60 years). The majority (73%) of patients were in the age group of 18–40 years. Female patients outnumbered males in the present study [42 (72.4%) vs. 16 (27.6%)]. All the patients in the present study were in sinus rhythm.
Correlation between defect diameter and rim sizes on 2D-TEE and CMR are described in Tables 1 and 2. On Pearson correlation analysis there was significant positive correlation between TEE and CMR measurements of maximal ASD diameter, AS rim, PS rim, AI rim, and PI rims (p < 0.001). Paired t-tests demonstrated that maximal ASD diameter and PI rim size were consistently smaller on TEE in comparison with CMR (p = 0.013 and p = 0.023, respectively). There was no significant difference between mean measurements of other rims on either modality. Bland–Altman analysis revealed an overall good agreement between 2D-TEE and CMR measurements of ASD diameter (Fig. 1) and rim sizes (Fig. 2).
Bland Altman plot for agreement of modes of measurement between TEE and CMR for Maximum ASD diameter and rim sizes. Note: ASD, Atrial Septal Defect; TEE, Transesophageal Echocardiography; MRI, Magnetic Resonance Imaging; AS, Anterosuperior; PS, Posterosuperior; AI, Anteroinferior; PI, Posteroinferior
Based on the conventional criteria for device closure, 46 (79.3%) patients were suitable for transcatheter closure on CMR, while 44 (75.9%) were eligible on TEE. Six patients had maximum defect size > 34 mm on CMR. Four of these were excluded from device closure as defect size on TEE was also > 34 mm. The other two (TEE defect size 28 mm and 30 mm vs. CMR defect size 35 mm and 37 mm, respectively) were provisionally taken up for transcatheter closure, but the device could not be deployed as the largest available Amplatzer ASO device did not achieve a stable position due to insufficient rim support. Seven patients had PI rim < 5 mm on TEE. Two of these were excluded from transcatheter closure as CMR also showed a deficient PI rim (< 5 mm). The other five had > 5 mm PI rim on CMR and thus were provisionally scheduled for device closure. In all of these patients, the procedure was completed successfully with total occlusion of the defect using an appropriately sized ASO device, which was released only after confirming a stable position by ‘Minnesota wiggle manoeuvre’ and ruling out impingement of important structures (especially protrusion into IVC hampering inflow to the right atrium). Two patients had deficient PS rim and one had deficient AI rim on both CMR and TEE making them ineligible for the procedure. CMR detected anomalous drainage of left upper pulmonary vein into innominate vein in one patient which was missed on 2D-TEE, hence excluding the patient from transcatheter closure.
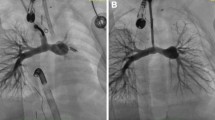
Transcatheter closure was attempted in 48 patients based on imaging findings, and a device could be successfully deployed in 46 (95.8%). Mean device size was 28.3 ± 7.4 mm and correlated more strongly with the defect dimensions on PC-CMR (r = 0.85, p < 0.001) as compared to 2D-TEE (r = 0.71, p = 0.02). None of the patients who underwent device closure had any complications on follow-up over a period of 6 months.
Discussion
The present study demonstrates that PC-CMR-derived measurements of ASD diameter and rim sizes show strong positive correlation with the corresponding values assessed by conventional 2D TEE in adult patents with ostium secundum ASD. PC-CMR also appears to be superior to 2D-TEE for evaluating the preprocedural feasibility for device closure in these patients, as 2D-TEE significantly underestimates maximal ASD diameter and PI rim size and can occasionally miss an anomalously draining pulmonary vein. In this study, all the patients deemed to be suitable for device closure on CMR ultimately underwent a successful transcatheter closure with a device whose size correlated strongly with the defect size measured on CMR.
TEE is the most widely available and validated method for evaluating the feasibility for device closure in adult ASD patients and is recommended for all patients who are being planned for this procedure [8, 9]. It provides a thorough insight into the defect morphology including type, size, shape, number of defects, presence of adequate rims for device anchorage, and ruling out any associated anomalies that could potentially complicate or preclude device closure [5,6,7,8,9]. TEE also serves as an invaluable tool for intra-procedural guidance and allows safe and accurate device deployment by ensuring stable device position, ruling out residual shunts and impingement of important structures before device release [3, 4, 21]. However, TEE is a semi-invasive imaging modality causing discomfort to the patients and requires sedation or occasional administration of general anaesthesia in uncooperative patients. Furthermore, although a relatively safe procedure, serious complications including oesophageal laceration or perforation, upper GI bleeding, pharyngeal tears, cardiac arrhythmias, laryngospasm, and hypoxia have been described in 0.1–0.9%. Mortality is rare, occurring in 0.01–0.02% cases [22, 23].
2D TEE is the most widely used mode for assessing ASD anatomy before transcatheter closure. Given the two dimensional nature, it is fraught with inherent limitations of comprehensively defining the shape, eccentricity and multiplicity of the defects, especially in complex secundum defects [24]. With the advent of real-time 3D matrix array TEE transducers in recent years, many of these limitations have been circumvented. Real-time 3D TEE allows more comprehensive interrogation of atrial septal anatomy and dynamic en face visualisation of ASD throughout the cardiac cycle. However, limited availability and lack of widespread expertise are the major stumbling blocks in its routine use in our part of the world. Furthermore, the posteroinferior part of the atrial septum may be inadequately visualised due to artefactual dropout, small fenestrations may escape detection due to limited spatial resolution, and patient movement during image acquisition can result in malalignment and reconstruction artefacts [25, 26].
Besides the obvious advantages of three-dimensional imaging and non-invasive nature, CMR generally outperforms TEE in terms of larger field of view, unlimited tomographic planes acquisition, and lesser operator dependence [10, 11]. Conventional CMR sequences including spin-echo technique and cine gradient-echo technique are often inaccurate in depicting the size and margins of secundum ASD, owing to septal thinning adjacent to the defect and low interatrial pressure gradient across the defect [16]. PC-CMR imaging, on the other hand, has been demonstrated to be superior to these techniques in reliably defining the size and shape of ASDs as it more sensitively detects localised low velocity phase shifts across the defect and provides a 3D en face view of the defect based on flow related signal enhancement [15,16,17,18,19,20]. In a study of 30 adult patients, Holmvang et al. demonstrated that ASD dimensions measured on PC-CMR showed an excellent correlation with balloon sizing of the defect during catheterisation as well as template standards measured during surgery. Spin-echo imaging overestimated the defect diameter by 48% in the same study that was attributed to signal dropout in the fossa ovalis region due to septal thinning [16]. Beerbaum et al. in a study of 65 paediatric patients, demonstrated good agreement between PC-CMR and TEE-derived ASD size (mean difference < 1 mm). Among 30 patients who were scheduled for transcatheter closure based on PC-CMR findings, 5 patients were found to have unexpectedly large defects on stretched balloon sizing and hence referred for surgery [17]. Consistent with the previous studies, there was a strong correlation between TEE and CMR vis a vis ASD diameter in the present study (r = 0.81, p < 0.001). However, TEE significantly underestimated the defect size compared to PC-CMR (mean difference − 1.6 mm; 95% CI − 2.8, − 0.4 mm). Furthermore, 2 patients with defect diameter < 34 mm on TEE versus > 34 mm on CMR could not undergo successful transcatheter closure as the largest available device size (40 mm) did not suffice in completely occluding the defect while achieving a stable position before release. We did not use balloon sizing technique in this study as contemporary data suggest that it is no longer necessary and may occasionally lead to device oversizing [27, 28]. Our results also demonstrated that the final device size that successfully occluded the defect correlated more strongly with the defect dimensions on PC-CMR (r = 0.85, p < 0.001) as compared to TEE (r = 0.71, p = 0.02). These findings are in sync with those published by Thomson et al. and Durongpisitkul et al. which demonstrated better correlation between device size and ASD diameter on CMR in comparison with intracardiac echocardiography (ICE) and TEE, respectively. [15, 20] The ability to visualise the defect en face on PC-CMR provides an unequivocal advantage over conventional echocardiography in clearly outlining the defect especially when the location or shape is eccentric.
In so far as the assessment of various rims is concerned, we found a significant correlation between rim sizes measured on TEE and CMR (r = 0.68, p < 0.001 for AS rim; r = 0.64, p < 0.001 for PS rim; r = 0.54, p < 0.001 for AI rim; r = 0.56, p < 0.001 for PI rim). The mean difference of rim measurements was less than 1 mm for all rims except for PI rim. TEE significantly underestimated PI rim size compared to CMR (mean difference − 1.98; 95% CI − 3.68, − 0.28; p = 0.023). This was even after we used all the recommended TEE manoeuvres including retroflexion and withdrawal of the probe from stomach for adequate visualisation of PI rim. Among seven patients with insufficient PI rim on TEE, five had rim size > 5 mm on CMR and subsequently underwent a successful device closure. There are a few studies which have demonstrated that transcatheter closure, although difficult, may be safe and feasible in some patients with deficient PI rim on TEE [29, 30]. Given the facts that inadequate evaluation of the posteroinferior part of the atrial septum represents an inherent limitation of TEE, even with the latest 3D technology, and more than 70% patients with deficient PI rim on TEE actually had sufficient rim on CMR in our study, we can infer that CMR is superior to TEE in deciding the candidature for device closure based on PI rim size [13, 24,25,26]. In the present study, CMR picked up anomalous drainage of left upper pulmonary vein into innominate vein in one patient which was missed on TEE, hence excluding the patient from transcatheter closure. Previous studies have shown that CMR may identify additional cardiac or extracardiac anomalies in up to 20% patients that alter clinical management decisions [15, 17]. Meticulous echocardiographic evaluation and exclusion of patients with multiple defects or atrial septal aneurysms could have attributed to such lower proportion in the present study. Besides the lack of widespread availability, there are certain important limitations to CMR that need to be mentioned. Apart from prolonged scanning times and ability to hold breath, CMR has been shown to be inferior to TEE in detecting patent foramen ovale or small septal fenestrations [15, 31]. Furthermore, PC-CMR imaging does not reliably depict septal thickness because the method depends on flow imaging rather than on structural delineation of thin membranes. Therefore, “floppy” septa, which are often found to require a large closure device after balloon-sizing in the catheter laboratory, can sometimes be missed at PC-CMR imaging [17]. Finally, the utility of CMR is limited by the presence of metallic prostheses or claustrophobia in a small percentage of patients.
Limitations
There are some important limitations to the present study. First, this was a small single-centre study with limited sample size that included only adult patients. Therefore, extrapolation of these results to broader patient population would require validation from larger adequately powered multicentre studies including paediatric patients. Second, all the patients in this study were in sinus rhythm and had regular breathing patterns. Hence, our results do not apply to patients with arrhythmias or irregular breathing patterns in whom image quality may be distorted by motion artefacts. Third, we excluded patients with atrial septal aneurysms, multiple ASDs and multifenestrated ASDs from this study. Hence, we cannot comment on the utility and accuracy of CMR in these patient subgroups. Fourth, we did not use balloon sizing of the defect during cardiac catheterisation for comparison with TEE- or CMR-derived defect dimensions. The device size chosen for defect occlusion was based on operator’s discretion after integrated assessment of TEE and CMR results. Therefore, stronger correlation between CMR-derived defect dimensions and final device size could partly be a result of selection bias. Lastly, we did not use real-time 3D TEE in our study due to its non-availability at our centre. Whether the superiority of CMR persists despite using 3D TEE technology needs to be demonstrated in future studies.
Conclusions
PC-CMR may be superior to 2D-TEE for the preprocedural planning and feasibility assessment for device closure in adult patients with ostium secundum ASD. 2D-TEE significantly underestimates maximal ASD diameter and PI rim size as compared to CMR and can occasionally miss coexistent cardiac or extracardiac anomalies that may influence clinical management decisions. Whether the superiority of CMR persists despite using the latest real-
time 3D TEE technology needs to be demonstrated in future studies.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- OS-ASD:
-
Ostium secundum atrial septal defect
- PC-CMR:
-
Phase contrast cardiac magnetic resonance imaging
- TEE:
-
Transoesophageal echocardiography
References
Hoffman JI, Kaplan S, Liberthson RR (2004) Prevalence of congenital heart disease. Am Heart J 147:425–439. https://doi.org/10.1016/j.ahj.2003.05.003
King TD, Thompson SL, Steiner C, Mills NL (1976) Secundum atrial septal defect: nonoperative closure during cardiac catheterization. JAMA 235:2506–2509. https://doi.org/10.1001/jama.1976.03260490024013
Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K (2002) Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol 39:1836–1844. https://doi.org/10.1016/S0735-1097(02)01862-4
Kazmouz S, Kenny D, Cao QL, Kavinsky CJ, Hijazi ZM (2013) Transcatheter closure of secundum atrial septal defects. J Invasive Cardiol 25:257–264
Savis A, Simpson J (2018) Echocardiographic approach to catheter closure of atrial septal defects: patient selection, procedural guidance and post-procedural checks. Echo Res Pract 5(2):R49–R64. https://doi.org/10.1530/ERP-18-0007
Reddy SC, Rao PS, Ewenko J, Koscik R, Wilson AD (1995) Echocardiographic predictors of success of catheter closure of atrial septal defect with the buttoned device. Am Heart J 129:76–82. https://doi.org/10.1016/0002-8703(95)90046-2
Vaidyanathan B, Simpson JM, Kumar RK (2009) Transesophageal echocardiography for device closure of atrial septal defects. JACC Cardiovasc Imaging 2:1238–1242. https://doi.org/10.1016/j.jcmg.2009.08.003
Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM et al (2019) 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 73(12):e81-192
Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, Hijazi ZM et al (2015) Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr 28:910–958. https://doi.org/10.1016/j.echo.2015.05.015
Knauth Meadows A, Ordovas K, Higgins CB, Reddy GP (2008) Magnetic resonance imaging in the adult with congenital heart disease. Semin Roentgenol 43:246–258. https://doi.org/10.1053/j.ro.2008.02.009
Kilner PJ, Geva T, Kaemmerer H, Trindade PT, Schwitter J, Webb GD (2010) Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur Heart J 31:794–805. https://doi.org/10.1093/eurheartj/ehp586
Nayak KS, Nielsen JF, Bernstein MA, Markl M, Gatehouse P, Botnar R et al (2015) Cardiovascular magnetic resonance phase contrast imaging. J Cardiovasc Magn Reson 17(1):71. https://doi.org/10.1186/s12968-015-0172-7
Markl M, Schnell S, Wu C, Bollache E, Jarvis K, Barker AJ et al (2016) Advanced flow MRI: emerging techniques and applications. Clin Radiol 71(8):779–795. https://doi.org/10.1016/j.crad.2016.01.011
Piaw CS, Kiam OT, Rapaee A, Khoon LC, Bang LH, Ling CW et al (2006) Use of non-invasive phase contrast magnetic resonance imaging for estimation of atrial septal defect size and morphology: a comparison with transesophageal echo. Cardiovasc Intervent Radiol 29:230–234. https://doi.org/10.1007/s00270-005-0003-6
Thomson LE, Crowley AL, Heitner JF, Cawley PJ, Weinsaft JW, Kim HW et al (2008) Direct en face imaging of secundum atrial septal defects by velocity-encoded cardiovascular magnetic resonance in patients evaluated for possible transcatheter closure. Circ Cardiovasc Imaging 1:31–40. https://doi.org/10.1161/CIRCIMAGING.108.769786
Holmvang G, Palacios IF, Vlahakes GJ, Dinsmore RE, Miller SW, Liberthson RR et al (1995) Imaging and sizing of atrial septal defects by magnetic resonance. Circulation 92:3473–3480. https://doi.org/10.1161/01.CIR.92.12.3473
Beerbaum P, Korperich H, Esdorn H, Blanz U, Barth P, Hartmann J et al (2003) Atrial septal defects in pediatric patients: noninvasive sizing with cardiovascular MR imaging. Radiology 228:361–369. https://doi.org/10.1148/radiol.2282020798
Teo KS, Disney PJ, Dundon BK, Worthley MI, Brown MA, Sanders P et al (2010) Assessment of atrial septal defects in adults comparing cardiovascular magnetic resonance with transoesophageal echocardiography. J Cardiovasc Magn Reson 12(1):44. https://doi.org/10.1186/1532-429X-12-44
Weber C, Weber M, Ekinci O, Neumann T, Deetjen A, Rolf A et al (2008) Atrial septal defects type II: noninvasive evaluation of patients before implantation of an amplatzer septal occluder and on follow-up by magnetic resonance imaging compared with TEE and invasive measurement. Eur Radiol 18:2406–2413. https://doi.org/10.1007/s00330-008-1033-7
Durongpisitkul K, Tang NL, Soongswang J, Laohaprasitiporn D, Nanal A (2004) Predictors of successful transcatheter closure of atrial septal defect by cardiac magnetic resonance imaging. Pediatr Cardiol 25:124–130. https://doi.org/10.1007/s00246-003-0481-8
Taniguchi M, Akagi T (2011) Real-time imaging for transcatheter closure of atrial septal defects. Interv Cardiol 3:679–694. https://doi.org/10.2217/ica.11.73
Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D’Ambra MN, Eltzschig HK (2010) Safety of transesophageal echocardiography. J Am Soc Echocardiogr 23(11):1115–1127. https://doi.org/10.1016/j.echo.2010.08.013
Freitas-Ferraz AB, Rodés-Cabau J, Vega LJ, Beaudoin J, O’Connor K, Turgeon PY et al (2020) Transesophageal echocardiography complications associated with interventional cardiology procedures. Am Heart J 221:19–28. https://doi.org/10.1016/j.ahj.2019.11.018
Magni G, Hijazi ZM, Pandian NG, Delabays A, Sugeng L, Laskari C et al (1997) Two- and three-dimensional transesophageal echocardiography in patient selection and assessment of atrial septal defect closure by the new DAS-angel wings device. Initial clinical experience. Circulation 96:1722–1728. https://doi.org/10.1161/01.CIR.96.6.1722
Roberson DA, Cui W, Patel D, Tsang W, Sugeng L, Weinert L et al (2011) Three-dimensional transesophageal echocardiography of atrial septal defect: a qualitative and quantitative anatomic study. J Am Soc Echocardiogr 24:600–610. https://doi.org/10.1016/j.echo.2011.02.008
Faletra FF, Ramamurthi A, Dequarti MC, Leo LA, Moccetti T, Pandian N (2014) Artifacts in three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr 27:453–462. https://doi.org/10.1016/j.echo.2014.02.003
Gupta SK, Sivasankaran S, Bijulal S, Tharakan JM, Harikrishnan S, Ajit K (2011) Trans-catheter closure of atrial septal defect: balloon sizing or no balloon sizing—single centre experience. Ann Pediatr Cardiol 4:28–33. https://doi.org/10.4103/0974-2069.79619
Rigatelli G, Dell’avvocata F, Cardaioli P, Giordan M, Dung HT, Nghia NT et al (2012) Safety and long-term outcome of modified intracardiac echocardiography-assisted “no-balloon” sizing technique for transcatheter closure of ostium secundum atrial septal defect. J Interv Cardiol 25:628–634. https://doi.org/10.1111/j.1540-8183.2012.00755.x
Mathewson JW, Bichell D, Rothman A, Ing FF (2004) Absent posteroinferior and anterosuperior atrial septal defect rims: factors affecting nonsurgical closure of large secundum defects using the amplatzer occluder. J Am Soc Echocardiogr 17:62–69. https://doi.org/10.1016/j.echo.2003.09.018
Kijima Y, Akagi T, Takaya Y, Taniguchi M, Nakagawa K, Kusano K et al (2016) Deficient surrounding rims in patients undergoing transcatheter atrial septal defect closure. J Am Soc Echocardiogr 29(8):768–776. https://doi.org/10.1016/j.echo.2016.04.010
Nusser T, Hoher M, Merkle N, Grebe OC, Spiess J, Kestler HA et al (2006) Cardiac magnetic resonance imaging and transesophageal echocardiography in patients with transcatheter closure of patent foramen ovale. J Am Coll Cardiol 48:322–329. https://doi.org/10.1016/j.jacc.2006.03.036
Acknowledgements
None.
Funding
No source of funding.
Author information
Authors and Affiliations
Contributions
Conception and design of study: Dr. TRS, Dr. JRB, Dr. NAC, Dr. VMJ. Literature review: Dr. TRS, Dr. JRB, Dr. FAR. Data acquisition: Dr. TRS, Dr. NAC, Dr. FAR, Dr. IY. Data analysis and interpretation: Dr. TRS, Dr. JRB, Dr. NAC, DR. IY. Drafting of manuscript: Dr. JRB, Dr. TRS, Dr. NAC, Dr. FAR. Revising and editing the manuscript: Dr. JRB, Dr. TRS, Dr. IY. Coordination and supervision of research: Dr. VMJ, Dr, JRB, Dr. NAC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Approval obtained from SKIMS Institutional Ethics Committee on 17-05-2016. A written informed consent was obtained from each for the participation in this study.
Consent for publication
A written informed consent was obtained from each patient for the participation in this study and for publication of their imaging findings with disclosing patient details.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shah, T.R., Beig, J.R., Choh, N.A. et al. Phase contrast cardiac magnetic resonance imaging versus transoesophageal echocardiography for the evaluation of feasibility for transcatheter closure of atrial septal defects. Egypt Heart J 74, 27 (2022). https://doi.org/10.1186/s43044-022-00269-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-022-00269-7