Abstract
Background
The transradial approach (TRA) has already become popular worldwide, but only recently has gained acceptance among Iraqi interventional cardiologists. The aim of this study is to document single operator experience with TRA and to test the benefit of assessing dual hand circulation before the TRA. It was an observational prospective study. Over a 2-year period (Jan 1, 2015, to Dec 31, 2016), 1561 patients underwent transradial coronary angiography (CAG) and/or percutaneous coronary intervention (PCI) by a single operator. Patients were divided into two groups: A (the first 450 patients), in which dual hand circulation was assessed by Allen’s test or plethysmography/oximetry test before TRA, and B (1111 patients) in which TRA was done without assessing dual hand circulation.
Results
A total of 1561 patients were included, 69.1% males and 30.9% females. The mean age was (57 ± 10.0) years. We performed 1684 procedures (1005 CAG and 679 PCIs). The total transradial success rate was 95.6%, and PCI procedural success rate was 96.5%. The crossover rate from radial to femoral access was 4.4%. The primary causes for crossover were severe tortuosity of the aorta and brachiocephalic trunk, radial artery spasm, puncture failure, and radial loop. The main complication was radial artery occlusion (RAO) (3.7%). There were no cases of hand ischemia or complications that need surgical repair or blood transfusion. No statistically significant difference between groups A and B was observed regarding hand ischemia, the incidence of RAO, or the crossover rate.
Conclusions
TRA is safe and can be applied in the majority of cases. The routine assessment of dual hand circulation before TRA might not be necessary.
Similar content being viewed by others
Background
Coronary angiography (CAG) and percutaneous coronary intervention (PCI) can be performed via the femoral, brachial, or radial arteries. The femoral approach had traditionally been the primary approach for most operators [1]. Following the first report of radial CAG by Campeau in 1989 and radial PCI by Kiemeneij et al. in 1993, there is an increase in use of transradial access around the world [2,3,4]. The major advantage of the TRA is the reduction in the incidence of complications related to the site of puncture associated with early ambulation, reduction in hospital stay, and consequently reduction in costs, making way for interventions in an outpatient care regimen [5,6,7]. The dual blood supply of the hand limits the potential for limb-threatening ischemia [8, 9]; therefore, assessment of dual hand circulation is considered essential before performing TRA.
Although TRA had been used as the preferred approach for CAG and PCI for more than two decades across the world, unfortunately, its use in Iraq is still limited. Only a few Iraqi cardiologists in few cardiac centers use the radial access as the default approach, while the majority of cardiologists still prefer the femoral access. In our center “Slemani Cardiac Hospital” (SCH), the TRA was started more than 6 years ago, and nowadays, more than 90% of coronary diagnostic and interventional procedures are done via the radial access. SCH is the first public cardiac center in Sulaymaniyah, Iraq.
The aim of this study is to document single operator experience with the TRA for CAG and PCI both in the elective and emergency setting, and to test the value of assessing dual hand circulation before the procedure.
Patients and methods
It was an observational prospective study conducted in Slemani Cardiac Hospital (SCH). The study was approved by the “Scientific and Ethical Committee” of SCH in December 2014. Informed written consent to participate in the study was provided by all participants. Over a 2-year period (Jan 1, 2015, to Dec 31, 2016), 1561 patients were admitted to SCH and underwent transradial CAG and/or PCI by the same operator (the author).
To investigate the benefit of assessing dual hand circulation before the TRA, we divided the study population into two groups: groups A (the first 450 cases) and B (all other patients). In group A, dual hand circulation was assessed by Allen’s test or plethysmography/oximetry test and patients with abnormal tests were excluded from the study. While in group B, TRA was done without any assessment for dual hand circulation.
The patients were prepared for radial and femoral approaches. All the procedures were done through the right radial artery. Under local anesthesia (1–2 ml, Xylocaine 5%), radial punctures were performed using the transradial kit (Prelude, Merit Medical) which consisted of a 21-gauge needle, a 0.018″ guide-wire, and a short (7 cm long) sheath. Six-F sheath was used for all patients. After sheath insertion, a cocktail containing 200 μg nitroglycerin and 5000 IU unfractionated heparin (UFH) was injected into the radial artery.
For diagnostic CAG, the following catheters were used: 6F or 5F Tiger (TIG) catheter (Terumo, Japan) or 6F Ultimate catheter (Merit Medical) to cannulate both left and right coronary arteries or Judkin’s left (JL 6/3.5 and 6/4) and Judkin’s right (JR 6/4 and 6/3.5) catheters to cannulate the left and right coronary artery respectively.
For patients with PCI, Judkin’s guiding catheters (JL6/3.5 and JR 6/4) and extra back-up (EBU) guiding catheter (6/3.5) were the most widely used catheters for coronary engagement. All patients were loaded with dual antiplatelet drugs (300 mg aspirin and 600 mg clopidogrel for elective PCI, or 300 mg aspirin and 180 mg Ticagrelor for primary PCI). UFH (70_100 IU/kg) is the standard anticoagulation before the procedure. A drug-eluting stent (DES) (“Xience”, Abbott Vascular or “Resolute”, Medtronic) was used whenever stenting is indicated.
The radial sheath was removed immediately after the procedure and compression was performed proximal to puncture site for 2 h using radial compression device (Finale; Merit medical) (Fig. 1). Thereafter, a light pressure bandage was applied and removed in the next day.
Most of the elective PCI patients were discharged on the same day provided that no complications occurred in the first 6 h after the procedure. Patients with primary PCI were discharged after 48 h when they were stable. The site of radial puncture was examined before discharge and after 2 weeks, and radial artery patency was assessed by checking the radial pulse. Radial artery occlusion (RAO) was considered present in the absence of a radial pulse distal to the puncture site.
Statistical method
Statistical analyses were performed using the SPSS 23. Continuous variables were analyzed and presented as mean ± SD whereas categorical variables were given as numbers (percentages). The comparison between categorical variables was done by chi-square test. p < 0.05 was considered statistically significant.
Results
A total of 1561 consecutive patients were included. There were 1079 males (69.1%) and 482 females (30.9%). The age ranged from 26 to 95 years (mean of 57 ± 10.0). Baseline characteristics of the patients are summarized in Table 1.
During the study period, we performed (1684) procedures, which include 1005 CAG (59.7%) and 679 PCI (40.3%). One hundred eighty-nine patients had elective PCI, 274 patients had CAG and PCI in the same session (ad Hoc PCI), 123 patients had second transradial PCI as part of staged procedure, and 93 patients underwent primary PCI (PPCI) for ST-elevation myocardial infarction (STEMI) (Table 2).
Total transradial technical success rate was 95.6%, and PCI procedural success rate was 96.5%. The PCI procedures include single-vessel disease, multi-vessel disease, total occlusions, bifurcational lesions, left main stem (LMS) disease, and PPCI. Transradial PCI failed in 24 patients (3.5%), due to chronic total occlusion (CTO) in 22 patients and failure to cross the coronary lesion with balloon or stent in 2 patients.
Crossover from radial approach to femoral approach occurred in 69 (4.4%) patients. The main reasons for crossover were severe subclavian or aortic tortuosity in 16 (1.0%) patients, severe radial artery spasm which did not respond to multiple doses of intra-arterial nitroglycerin and IV analgesia in 14 (0.9%) patients, puncture failure in 11 (0.7%) patients, and radial loop in 9 (0.6%) patients. Other causes for crossover are shown in Table 3.
Crossover from radial to femoral access was higher in old-age patients (above 60 years) than younger patients (5.6% versus 3.5%); however, the difference between the two groups was statistically not significant (p value = 0.052) (Table 4).
The frequency of various complications was as follow: five patients (0.3%) had forearm hematoma which was treated conservatively. Radial artery dissection with extravasation of contrast in one patient (0.06%), which was resolved conservatively. Radial artery occlusion (RAO) was observed in 57 patients (3.7%). There were no cases of hand ischemia, pseudoaneurysm, arteriovenous fistula, or bleeding complications that need surgical repair or blood transfusions (Table 5).
We found no statistically significant difference between groups A and group B regarding hand ischemia, the incidence of RAO, or crossover rate (p value = 0.052) (Table 6).
Discussion
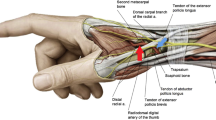
The radial approach is an attractive alternative to the classical femoral approach for CAG and PCI. The radial artery is very superficial, making it easy to puncture, and bleeding is controlled by compression. There are no major nerves or veins near the radial artery, thus minimizing the risk of nerve and vascular injuries [10, 11]. The benefits of TRA have been documented in many studies. These benefits include less bleeding [10,11,12,13,14,15,16,17], lower morbidity, early ambulation, lower total hospital costs [10, 18], patient preference and comfort, same-day discharge is possible, less chance of developing ischemia due to dual blood supply of the hand, and easy access for the patients with myocardial infarction (MI) and aortic aneurysm [10, 19, 20]. The approach is advantageous for people with severe occlusive aortoiliac disease or difficulty lying down (e.g., due to back pain, obesity, or congestive heart failure) [8, 21].
As has been shown in several studies, the radial access permits treatment of the same type of patients and lesions as femoral access provides [11, 17, 22,23,24,25,26,27]. The radial artery readily accommodates 6-F sheaths, and sheathless 7-F techniques have recently been described [28, 29]. Thus, there is no limitation to performing complex PCI successfully via the radial approach [30]. High-risk subsets such as unprotected left main coronary artery [31], bifurcational lesions, and chronic total occlusions [32] can all be readily addressed through radial access [30]. Results from our study show that the transradial PCI was associated with high procedural success rates (96.5%) and favorable clinical outcomes in all patients, both in the elective and emergency (STEMI) setting.
Patients with STEMI are the most intensely anticoagulated, and many had received thrombolytic therapy prior to arrival at the PCI center, so they have high risk of bleeding. Thus, the potential for access-site complications is highest in this group and the potential benefit from TRA is greatest [33, 10, 34]. Bleeding after an acute myocardial infarction (MI) is associated with worse short- and long-term outcomes and prolonged hospitalization [35, 36]. Many trials have proved that TRA has lower risk of bleeding in STEMI patients as compared to transfemoral approach (TFA). The RIVAL (radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes) study showed that TRA is associated not only with a lower rate of local vascular complications in the overall population, but also with a reduction in mortality in the setting of acute PCI [37, 38]. These results have been confirmed in another randomized study, the RIFLE-STEACS study (radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome). This trial specifically compared the TRA and the TFA for primary PCI, in which a relative reduction in access-site complications and in mortality of nearly 40% was found with TRA [38,39,40].
According to the latest (2018) European Society of Cardiology (ESC) guidelines on myocardial revascularization, the radial access should be the standard approach for coronary angiography and PCI in all clinical settings (class I, level of evidence A) [41].
Currently, it is well established that TRA nearly abolishes access-site complications in all patients. All studies comparing TRA versus TFA have demonstrated a reduction in major bleeding with TRA, both in the elective and the acute setting [38, 42, 43]. When access-site complications still occur after TRA, they usually have a benign course and do not influence the prognosis of patients [38]. Surgical intervention for the treatment of hematoma or arteriovenous fistulae has been rarely observed [5, 44]. The incidence of complications in our study matches the literature findings.
The advantages of TRA extend to the elderly patients as well. In a recent meta-analysis of 777,841 elderly patients by Alnasser et al [45], TRA compared to the TFA was associated with a significant reduction in vascular complications and stroke, but mortality benefit was seen only among patients presenting with STEMI.
Despite the aforementioned advantages, there are potential disadvantages to the TRA [8]. The TRA is technically more complex than the TFA due to the greater difficulty in cannulating the artery, the possibility of spasm, anatomical variations in the arteries of the upper limb, and the change in manipulation of the catheters that is necessary to cannulate the coronary arteries [11, 46, 47]. All these difficulties result in an increase in the length of procedural time and the need for a significant learning curve [8, 11, 47]. Some interventions may be technically challenging via the radial route due to the size of the technology required, e.g., large bore rotational atherectomy [8, 48]. Moreover, TRA is usually more demanding and needs longer procedural time in elderly patients because of the frequent presence of specific vascular abnormalities such as tortuosity, calcifications, or arterial loops [4].
TRA has been associated with a greater access crossover rate, which was reported to be 4–7% in various studies [4, 49, 50]. Louvard et al. reported a crossover rate of 10% in the first 50 cases, and 3–4% after other 500 cases; then, it stabilizes at less than 1% after 1000 procedures [1, 51]. In our study, the crossover rate was 4.4%, with higher rate in older patients (≥ 60 years old) than younger patients (5.6% versus 3.5% respectively). However, the difference between the two groups was statistically not significant. In the meta-analysis of elderly patients by Alnasser et al., access site crossover rate was higher for TRA compared to the TFA (11% vs. 3%, p = 0.0003), but remains acceptably low [45].
While serious bleeding complications are uncommon, the TRA bears the risk of radial artery occlusion (RAO). The incidence of RAO varies between 3 and 10%, according to different studies and protocols [40, 52, 53]. RAO rarely results in serious adverse events nor is it symptomatic, but the artery is lost for future procedures [33, 40, 54]. Therefore, any effort should be taken to reduce the risk of RAO [40].
The occurrence of RAO is determined by one or more of the following three factors, and all of them are operator dependent and therefore preventable: incomplete anticoagulation, catheter- artery mismatch, and prolonged arterial compression [38, 55]. The incidence of RAO is directly related to the ratio between the sheath and artery size [1, 55, 56]. Therefore, smaller guiding catheters are potentially advantageous leading to less arterial spasm, pain, and post-procedural RAO [1]. Prolonged and forceful post-procedure radial artery compression is perhaps the most common cause of RAO [33, 55]. Patent or non-occlusive artery hemostasis—that is, applying enough pressure to the radial access site to achieve hemostasis and yet maintaining antegrade flow in the radial artery—has been shown to drastically reduce the incidence of RAO [38, 40, 55].
In our study, the rate of RAO was 3.7%. Moreover, 123 patients had second TRA (as part of staged PCI) reflecting the preserved patency of the radial artery after TRA, or it may indicate that RAO is temporary in some cases. The use of 6F radial sheath in all procedures, followed by routine administration of heparin (5000 IU) into the radial artery after sheath insertion, and the controlled pressure over the radial artery with “radial compression device” all resulted in the reduction of the incidence of RAO.
Since the introduction of TRA, it has been recommended to assess dual hand circulation before use [57]. This assessment is done by using Allen’s test which is subjective, or the more objective oximetry/plethysmography test. In most TRA studies, patients with abnormal tests were excluded from the studies. In our study, the majority of patients (71.2%) underwent transradial procedure without any assessment of dual hand circulation (group B); however, this did not result in worse outcomes such as hand ischemia or higher rates of access crossover or RAO. In an international transradial practice survey by Bertrand et al. [57] which included 1107 interventional cardiologists from 75 countries, 23.4–30.8% of operators did not assess dual hand circulation at all. Because the Allen test or the oximetry/plethysmography test have not been shown to be predictive of hand ischemia in case of RAO, it remains uncertain whether the assessment of dual hand circulation before TRA is required [57].
Conclusions
The TRA for CAG and PCIs is effective and safe and can be applied in the majority of cases. It dramatically reduces access site complications. The routine assessment of dual hand circulation before TRA might not be necessary, however more studies are needed to confirm our results.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CAG:
-
Coronary angiography
- CTO:
-
Chronic total occlusion
- DES:
-
Drug-eluting stent
- EBU:
-
Extra back-up
- ESC:
-
European Society of Cardiology
- F:
-
French gauge
- IU:
-
International unit
- IV:
-
Intravenous
- JL:
-
Judkin’s left
- JR:
-
Judkin’s right
- LMS:
-
Left main stem
- MI:
-
Myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- PPCI:
-
Primary percutaneous coronary intervention
- RAO:
-
Radial artery occlusion
- SCH:
-
Slemani Cardiac Hospital
- SD:
-
Standard deviation
- SPSS:
-
Statistical Package for the Social Sciences
- STEMI:
-
ST elevation myocardial infarction
- TFA:
-
Transfemoral approach
- Tig:
-
Tiger
- TRA:
-
Transradial approach
- UFH:
-
Unfractionated heparin
References
Konstantinos Triantafyllou. Radial approach to percutaneous coronary intervention. hospital chronicles.2010; (suppl):128–136.
Campeau L (1989) Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn 16(1):3–7
Kiemeneij F, Laarman GJ (1995) Transradial artery Palmaz-Schatz coronary stent implantation: results of a single-center feasibility study. Am Heart J 130(1):14–21
Sinha SK, Mishra V, Afdaali N et al (2016) Coronary angiography safety between transradial and transfemoral access. Cardiol Res Pract 2016:1–7
Brito JC, Júnior AA, Oliveira A, Von Sohsten R, Filho AS, Carvalho H (2001) Transradial approach for coronary interventions. Arq Bras Cardiol. 76(5):374–378
Kiemeneij F, Laarman GJ, Slagboom T, Van der Wieken R (1997) Outpatient coronary stent implantation. J Am Coll Cardiol 29:323–327
Slagbomm T, Kiemeneij F, Laarman G, Wieken R. Actual same day discharge after coronary angioplasty. Eur Heart J.1996; (suppl):1973.
Kolkailah AA, Alreshq RS, Muhammed AM, ZahranME, Anas El-Wegoud M, Nabhan AF. Transradial versus transfemoral approach for diagnostic coronary angiography and percutaneous coronary intervention in people with coronary artery disease. Cochrane Database of Systematic Reviews.2018; Issue 4. Art. No.: CD012318. DOI:10.1002/14651858.CD012318.pub2.
Agostoni P, Biondi-Zoccai G, Benedictis M, Rigattieri S, Turri M, Anselmi M et al (2004) Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol 44(2):349–356
Anjum I, Khan M, Aadil M, et al. (June 03, 2017) Transradial vs. Transfemoral approach in cardiac catheterization: a literature review. Cureus 9(6): e1309. DOI 10.7759/cureus.1309.
Jorge Salgado Fernández a, Ramón Calviño Santos a, José M Vázquez Rodríguez a, et al. Transradial Approach to coronary angiography and angioplasty: initial experience and learning curve. Rev Esp Cardiol 2003; 56 (2):152-159.
Barton M, Gruntzig J, Husmann M et al (2014) Balloon angioplasty - the legacy of Andreas Grüntzig, M.D. (1939–1985). Front Cardiovasc Med 1:15
Bourassa MG (2005) The history of cardiac catheterization. Can J Cardiol 21:1011–1014
Kedev S, Kalpak O, Dharma S et al (2014) Complete transitioning to the radial approach for primary percutaneous coronary intervention: a real-world single-center registry of 1808 consecutive patients with acute ST-elevation myocardial infarction. J Invasive Cardiol 26:475–482
Pasley TF, Khan A, Yen LY et al (2016) Left radial versus femoral access for coronary angiography in post-coronary artery bypass graft surgery patients. J Invasive Cardiol 28:81–84
Mann T, Cubeddu G, Bowen J et al (1998) Stenting in acute coronary syndromes: a comparison of radial versus femoral access sites. J Am Coll Cardiol 32:572–576
De Maria GL, Burzotta F, Trani C et al (2015) Trends and outcomes of radial approach in left-main bifurcation percutaneous coronary intervention in the drug-eluting stent era: A two-center registry. J Invasive Cardiol 27:125–136
Roussanov O, Wilson SJ, Henley K et al (2007) Cost-effectiveness of the radial versus femoral artery approach to diagnostic cardiac catheterization. J Invasive Cardiol 19:349–353
Cooper CJ, El-Shiekh RA, Cohen DJ et al (1999) Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J 138:430–436
Mann JT, Cubeddu MG, Schneider JE et al (1996) Right radial access for PTCA: a prospective study demonstrates reduced complications and hospital charges. J Invasive Cardiol 8(Suppl):40–44
Almany SL, O’Neill WW. Radial artery access for diagnostic and interventional procedures. Ann Arbor (MI). Accumed Systems, 1999.
Kiameneij F, Laarman GJ, De Melker E (1995) Transradial artery coronary angioplasty. Am Heart J 129:1–7
Fajadet J. Percutaneous transradial approach for coronary revascularization: what have we learned? J Invasive Cardiol.1996;8(Suppl D):8-13.
Schneider JE, Mann T, Cubeddu MG, Arrowood ME (1997) Transradial coronary stenting: a United States experience. J Invasive Cardiol 9:569–574
Saito S, Miyake S, Hosokawa G, Tanaka S, Kawamitsu K, Kaneda H et al (1999) Transradial coronary intervention in Japanese patients. Catheter Cardiovasc Interv 46:37–41
Lotan C, Hasin Y, Mosseri M, Rozenman Y, Admon D, Nassar H et al (1995) Transradial approach for coronary angiography and angioplasty. Am J Cardiol 76:164–167
Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, Van der Wieken R (1997) A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the Access Study. J Am Coll Cardiol 29:1269–1275
From AM, Bell MR, Rihal CS, Gulati R (2001) Minimally invasive transradial intervention using sheathless standard guiding catheters. Catheter Cardiovasc Interv 78:866–871
Mamas MA, Fath-Ordoubadi F, Fraser DG (2008) Atraumatic complex transradial intervention using large bore sheathless guide catheter. Catheter Cardiovasc Interv 72:357–364
Sunil V. Rao, Zoltan G. Turi, S. Chiu Wong, Sorin J. Brener, Gregg W. Stone. Radial versus femoral access. J Am Coll Cardiol. 2013;62(17) Suppl S: S11-S20.
Yang YJ, Kandzari DE, Gao Z et al (2010) Transradial versus transfemoral method of percutaneous coronary revascularization for unprotected left main coronary artery disease: comparison of procedural and late-term outcomes. J Am Coll Cardiol: Cardiovasc Intv 3:1035–1042
Rathore S, Hakeem A, Pauriah M, Roberts E, Beaumont A, Morris JL (2009) A comparison of the transradial and the transfemoral approach in chronic total occlusion percutaneous coronary intervention. Catheter Cardiovasc Interv 73:883–887
Olivier F (2010) Bertrand. Transradial approach for coronary angiography and intervention - ready for prime time? US Cardiology 7(1):81–84
Jolly SS, Amlani S, Hamon M et al (2009) Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J 157:132–140
Nardin M, Verdoia M, Barbieri L, Schaffer A, Suryapranata H, De Luca G (2017) Radial vs femoral approach in acute coronary syndromes: a meta analysis of randomized trials. Curr Vasc Pharmacol 16(1):79–92
Manoukian SV, Feit F, Mehran R et al (2017) Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 49(12):1362–1368
Jolly SS, Yusuf S, Cairns J et al (2011) RIVAL trial group. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet 377(9775):1409–1420
Amoroso G (2013) Transradial approach for percutaneous coronary interventions: the future is now. Interv. Cardiol 5(3):279–288
Romagnoli E, Biondi-Zoccai G, Sciahbasi A et al (2012) Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol 60(24):2481–2489
Lieuwe H (2016) Piers, Maarten A Vink and Giovanni Amoroso. Transradial approach in primary percutaneous coronary intervention: lessons from a high-volume centre. Interv cardiol review 11(2):88–92
The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018; 00:1–96.
Agostoni P, Biondi-Zoccai GG, de Benedictis ML et al (2004) Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; systematic overview and meta analysis of randomized trials. J Am Coll Cardiol 44(2):349–356
Mamas MA, Ratib K, Routledge H et al (2012) Influence of access site selection on PCI-related adverse events in patients with STEMI: meta-analysis of randomised controlled trials. Heart 98(4):303–311
Barbeau G, Carrier G, Ferland S, Letourneau L, Gleeton O, Larivière M (1996) Right transradial approach for coronary procedures: Preliminary results. J Invas Cardiol 8:19D–21D
Sami M. Alnasser, Akshay Bagai, Sanjit S. Jolly et al. Transradial approach for coronary angiography and intervention in the elderly: a meta-analysis of 777,841 patients. Int J Cardiol2017; 228:45-51.
Kim J, Yoon J (2011) Transradial approach as a default route in coronary artery interventions. Korean Circ J 41(1):1–8
Hess C, Peterson E, Neely M, Dai D, Hillegass W, Krucoff M et al (2014) The learning curve for transradial percutaneous coronary intervention among operators in the United States. Circulation 129:2277–2286
Watt J, Oldroyd KG (2009) Radial versus femoral approach for high-speed rotational atherectomy. Catheter Cardiovasc Interv 74(4):550–554
Pristipino C, Pelliccia F, Granatelli A et al (2007) Comparison of access-related bleeding complications in women versus men undergoing percutaneous coronary catheterization using the radial versus femoral artery. Am J Cardiol 99:1216–1221
Pristipino C, Trani C, Nazzaro MS et al (2009) Major improvement of percutaneous cardiovascular procedure outcomes with radial artery catheterisation: results from the PREVAIL study. Heart 95(6):476–482
Louvard Y, Benamer H, Garot P et al (2004) Comparison of transradial and transfemoral approaches for coronary angiography and angioplasty in octogenarians (the OCTOPLUS study). Am J Cardiol 94:1177–1180
Zwaan EM, Koopman AG, Holtzer CAJ et al (2015) Revealing the impact of local access-site complications and upper extremity dysfunction post transradial percutaneous coronary procedures. Neth Heart J 23:514–524
Sanmartin M, Gomez M, Rumoroso JR et al (2007) Interruption of blood flow during compression and radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv 70:185–189
Valgimigli M, Campo G, Penzo C et al (2014) Transradial coronary catheterization and intervention across the whole spectrum of Allen test results. J Am Coll Cardiol 63:1833–1841
Pancholy S, Coppola J, Patel T et al (2008) Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv 72:335–340
Saito S, Ikei H, Hosokawa G, Tanaka S (1999) Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv 46:173–178
Olivier F. Bertrand, Sunil V. Rao, Samir Pancholy et al. Transradial approach for coronary angiography and interventions, Results of the first international transradial practice survey. J Am Coll Cardiol: cardiovasc interv.2010;3(10):1022-1031.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
JA designed the study, did all the diagnostic and interventional procedures, collected the data, reviewed the literature, wrote the manuscript, and revised the final draft. AI participated in the design of the study, performed the statistical analysis, prepared the tables, and revised the final draft. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the “Scientific and Ethical Committee” of SCH in December 2014. Informed written consent to participate in the study was provided by all participants.
Consent for publication
Not applicable as no identifiable information was present.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Aldoori, J.S., Mohammed, A.I. Transradial approach for coronary angiography and percutaneos coronary intervention: personal experience. Egypt Heart J 71, 10 (2019). https://doi.org/10.1186/s43044-019-0006-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-019-0006-2