Abstract
Background
Three or more consecutive pregnancy losses before the 20th week of gestation constitute recurrent pregnancy loss (RPL), and about half of these cases are still unsolved despite routine screening tests. The purpose of the current study was to identify the RPL-related placental decidual differential gene expression and to gain new knowledge about the biological mechanisms underlying RPL.
Methods
In the current work, we used RNA sequencing (RNA-seq) technology to identify the differentially expressed genes (DEGs) in placental decidua from patients of unexplained recurrent pregnancy loss (RPL). To conduct RNA-seq, two healthy unwanted medically terminated pregnancies (MTPs) and four RPL patients were enlisted.
Results
A total number of 96 significant differentially expressed genes (DEGs) were obtained which includes 73 up- and 23 downregulated genes between the RPL and MTP groups. Histocompatibility genes were significantly upregulated in the RPL. Interleukin 6 (IL-6), matrix metalloproteinase-10 (MMP10), and protein phosphatase 1 regulatory inhibitor subunit 11 (PPP1R11) genes which were significantly upregulated in RPL were further validated in an extended sample size. The validation results were consistent with the sequencing results. To find potential biological pathways connected to RPL, the Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were carried out.
The study indicates that arginine biosynthesis is significantly downregulated, while IL-17 signalling pathway is significantly upregulated in RPL.
Conclusion
In conclusion, the findings of the present study indicate involvement of arginine biosynthesis, immune regulatory pathways, and histocompatibility genes in the pathogenesis of recurrent pregnancy loss (RPL). However, to validate these observations, further investigations with a larger sample size are warranted.
Similar content being viewed by others
Background
Recurrent pregnancy loss (RPL) is defined as three or more successive pregnancy losses before the 20th week of gestation. About 50% of the RPL cases remains unexplained after routine screening tests, such as uterine anatomical abnormalities, chromosomal abnormalities, endocrine, and infectious and autoimmune screening. The placenta is the first organ to mature during mammalian development and is in charge of holding the embryo to the uterus and facilitating gas and nutrient exchange with the mother. Throughout the entire pregnancy, the placenta feeds the foetus, and placental defects are the cause of many pregnancy complications.
The completely decidualized endometrium, or decidua, or when a blastocyst implants into the mother’s uterus, come into intimate contact with the embryo and are expected to embrace it to allow for physical development throughout the whole pregnancy period. Negative pregnancy outcomes, such as pregnancy loss and hypertension, would be anticipated once the decidua begins to malfunction. High-throughput sequencing has developed into a potent technique for locating the gene alterations that might be responsible with RPL. In addition to coding for proteins, RNAs serve a variety of other purposes. For instance, they regulate transcription in many ways in the cytoplasm and nucleus. They regulate mRNA stability, translation, and post-translational modification and regulate nuclear architecture. Therefore, understanding the differential expression of variety of RNAs that are present helps us understand how cells and tissues function. Finding out the distinct transcriptome profile of the decidual cells from RPL patients is quite interesting given the crucial role that decidua plays in the process of maintaining pregnancy. In light of this, the current work sought to identify the placental decidual differential gene expression linked to RPL and provide fresh knowledge about the relevant molecular pathways of RPL.
Methods
Subjects under study
The participants in the current case-control study were recruited from the Government Modern Maternity Hospital in Petlaburj, Hyderabad. Four of the participants had unexplained recurrent pregnancy loss, and two had an unplanned, healthy pregnancy that was medically terminated. Women with unexplained recurrent pregnancy loss were deemed cases, and placental decidual tissue was collected. The study did not include pregnancy losses brought on by known conditions such chromosomal abnormalities, uterine anomalies, endocrine disorders, antiphospholipid syndrome, inherited thrombophilia, and infections. As control subjects, placental tissue from women who had their pregnancies medically ended and had successfully delivered at least two children without a history of pregnancy loss was used. A typical questionnaire was used to gather the study group’s clinical history. All of the study participants gave their consent. This study was approved by the Institutional Ethics Committee (ref. no. IEC/OMC/2022/M. no. (10)/Acad-99).
Sample collection and RNA isolation
Age of study subjects along with the duration of gestation is represented in Table 1. Placental decidua tissue was collected from both unexplained recurrent pregnancy loss and medically terminated pregnancies in RNA later solution (Invitrogen, Thermo Scientific Solutions). Approximately, 50-mg tissue was weighed, and RNA was isolated using RNeasy Mini Kit and followed by DNase treatment. Concentration of RNA was checked using Quantus RNA system. Samples quality was assessed by running on Agilent TapeStation 4200 using Agilent RNA ScreenTape.
Library preparation and QC
Ribosomal RNAs (rRNAs), which make about 80–90% of the total RNA extracted, are exceedingly prevalent. Effective rRNA removal is essential for the economical sequencing of RNA samples. When samples are eukaryotic and have intact poly(A) tails, oligo dT-based mRNA isolation is the best method for enriching mRNA and separating it from rRNA. To separate intact poly(A)+ RNA from previously isolated total RNA, the NEBNext Poly(A) mRNA Magnetic Isolation Module was used. In accordance with manufacturer guidelines, ribodepleted RNA samples were utilised to prepare libraries using the NEBNext® Ultra™ II Directional RNA Library Prep Kit for Illumina®.
In a nutshell, after being purified, the RNA was broken apart using divalent cations at a high temperature. The cDNA is subsequently created using reverse transcriptase and random hexamers in a subsequent phase called first-strand synthesis. The cDNA is then changed into double-stranded cDNA by substituting uracil for thymine. A USER enzyme-based digestion of the second strand preserves the strand specificity, leaving one functional strand that matches to the DNA strand from which it was transcribed. The USER digested single-strand molecules were enriched and indexed in a limited cycle PCR, followed by AMPure bead purification, to create the final cDNA library for sequencing. The prepared libraries were quantified using Qubit HS DNA kit, and library size distribution was assessed on Agilent TapeStation High Sensitivity D1000 ScreenTape®.
Sequencing and data quality
NextSeq 2000 platform was used for sequencing with sequencing chemistry 2 × 150 bp paired end. Using Illumina’s bcl2fastq Conversion Software, the data was demultiplexed and converted to fastq files. The quality check of the raw reads was performed using FASTQC. The low-quality bases and the adapters were trimmed using TrimGalore. Post-trimming QC was also performed.
Alignment and quantification
GRCh38 was used as the reference genome. The reference genome was indexed, and the reads were aligned in a splice-aware fashion using STAR. Quantification refers to the transcript-level counts for the aligned reads; in other words, the number of reads mapped to each gene. Feature counts from subread package was used to count the number of reads associated with each feature of interest using BAM as an input file for each sample.
Differential gene expression and enrichment
Differential expression analysis is carried out using DESeq2 R library. It is a pipeline for differential analysis in counted data using statistical estimates. Genes were identified as significant DE if their p-adj values < 0.05 and their log2FC>=|1|. MA and volcano plots were generated showing the differential genes. The significant differential genes were enriched for Gene Ontology and pathway using ShinyGO web server.
Real-time qPCR validation of differentially expressed genes
Three differentially expressed genes, interleukin 6 (IL-6), matrix metalloproteinase-10 (MMP10), and protein phosphatase 1 regulatory inhibitor subunit 11 (PPP1R11), were selected for qRT-PCR validation with an extended sample size of 16 RPL cases and 12 MTPs. The primers for the chosen genes were designed using the PrimerQuest programme from Integrated DNA Technologies (IDT), and the specificity of the primers was examined using BLAST. Eurofins India Pvt Limited synthesised and provided the primer sequences.
cDNA synthesis and expression analysis by SYBR green real-time qPCR
The QuantiTect Reverse Transcription Kit from QIAGEN was used to create cDNA. As directed by the manufacturer, the reaction was incubated for 30 min at 420 °C and 3 min at 950 °C. Using quantitative real-time (qRT)-PCR and gene-specific oligonucleotides, the housekeeping gene GAPDH and target genes IL-6, MMP10, and PPP1R11 were amplified (Table 2). Three replicates of real-time PCR experiments were performed for each sample in 96-well plate using an ABI 7000 Sequence Detection System from Applied Biosystems (Applied Biosystems). A total volume of 20-µl reaction was performed with SYBR Select Master Mix of 10 µl (cat. no. 4472908; Thermo Fisher Scientific, Inc.), 1 µl of each primer (10 µM), and 4-µl template cDNA. The amplification protocol consisted of an initial denaturation step at 95 °C for 4 min, followed by two-step PCR for 40 cycles at 95 °C for 30 s and 60 °C for 30 s. A melting curve analysis was also performed to check no primer dimmers or false amplicons interfered with the result. The Ct value was extracted for both reference and target gene with auto baseline and manual threshold. The fold change of expression was calculated by ∆∆Ct method. The difference between the level of expression in IL-6, MMP10, PPP1R11 genes, and housing keeping gene was determined by Livak method in recurrent pregnancy loss cases and medically terminated pregnancies.
Results
Clinical characteristics of study subjects
Table 1 displays the clinical characteristics of the study group.
Cases and controls were carefully selected to ensure gestational age matching for comparison purposes, with both groups falling within the range of 11 to 12 weeks of gestation.
Sequencing and data analysis
Good quality RNA with RNA integrity numbers 6.9 and 7.1 for control group and 6.3, 6.0, 7.2, and 6.9, respectively, for RPL 1–4 were used for library preparation. Phred score of Q33 was obtained for the data. All the samples showed > 83% of uniquely mapped reads. The alignment statistics are given in Table 3. The differential gene expression was compared with MTP group against RPL group with p-adj values < 0.05 and their log2FC>=|1| as thresholds. A total number of 96 significant DEGs were obtained which includes 73 up- and 23 downregulated genes. The 20 genes that were most noticeably up and downregulated in placental decidual tissue between women with RPL and MTP are included in Table 4. The results are illustrated in Fig. 1 as volcano plot and Fig. 2 as MA plot. IL-6, MMP10, and PPP1R11 genes which were significantly upregulated in the RPL were further validated in extended sample size.
Gene Ontology and KEGG enrichment
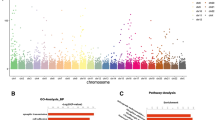
Gene Ontology enrichment and KEGG analysis were carried out to determine the pathways that contribute the most to the differentially expressed gene profiles between the RPL and MTPs.
The Gene Ontology results showed that the biological processes, neutrophil chemotaxis, and chemokine-mediated signalling pathway and molecular function — chemokine receptor binding are highly enriched. The arginine biosynthesis and IL-17 signalling pathways are thought to be enriched in the differentially expressed mRNAs, according to KEGG analysis.
Validation of differentially expressed genes by qPCR
To validate the RNA-sequencing results, quantitative RT-qPCR was carried out on an additional 28 samples (16 RPL cases and 12 MTPs). We have chosen the genes PPP1R11, MMP10, and IL-16 for validation. When compared to the control group, IL-10, MMP10, and PPP1R11 were all significantly upregulated in the RPL group. The validation results and the RNA-sequencing results were in agreement (Fig. 3).
Discussion
In order to effectively treat RPL, which is a complex condition with both short- and long-term effects on couples planning to have children, it is urgently necessary to identify novel mechanisms underlying its manifestation. Gene expression profiles and biological pathways associated with unexplained recurrent pregnancy loss can be captured, which may aid in the discovery of new biomarkers or therapeutic targets that may be useful in treating clinical conditions for RPL patients. In order to accurately measure the level of all protein-coding and non-coding (ncRNA) transcripts, RNA sequencing (RNA-Seq) offers a significantly higher level of resolution to capture the entire transcriptome. There are hardly any studies looking into the disruptions at the maternal-foetal interface, which might be causing abnormal placental development or reflecting the biological mechanisms underlying RPL.
To the best of our knowledge, this is the first study from India to use RNA-Seq for transcriptomic profiling of placental decidua representing RPL cases in comparison with unwanted medically terminated healthy pregnancies. The significant DEGs were analysed, and human leukocyte antigen G (HLA-G), major histocompatibility complex, class II, DR beta 4 (HLA-DRB4), human leukocyte antigen class II histocompatibility, D-related beta chain (HLA-DRB1), CXCL8-C-X-C motif chemokine ligand 6 (CXCL6), and CXCL8-C-X-C motif chemokine ligand 8 (CXCL8) were observed to be significantly upregulated, and carbamoyl-phosphate synthase (CPS1) and nitric oxide synthase NOS1, NOS2, and NOS3 genes were significantly downregulated. Recurrent spontaneous abortions (RSA) have been associated with increased parental human leukocyte antigen (HLA) sharing, according to numerous reports. Parental HLA sharing raises the likelihood of feto-maternal histocompatibility and may have an impact on the mother’s ability to recognise the foetus during allorecognition. Endothelial nitric oxide synthase (NOS) genes, which are vasoactive mediators and encode enzymes crucial for generating vascular NO, are involved in the control of vascular activities at the feto-maternal interface and the development of the fetoplacental blood vessels. A gaseous molecule called NO plays a variety of physiological regulatory roles in the control of reproduction, including the development of new blood vessels, improvement of blood flow through the maternal arteries to the placenta, control of placental vessel tone, and immune protection of the developing foetus. All of these elements must be present for a pregnancy to be successful, and any disruption to these processes may raise the risk of miscarriage. A shift in the expression levels of the NOS genes, which are highly expressed by trophoblast cells during the first trimester of pregnancy, may cause RPL [1].
The Gene Ontology results showed that the biological processes, neutrophil chemotaxis, and chemokine-mediated signalling pathway and molecular function — chemokine receptor binding are highly enriched. The maternal-foetal interface contains trophoblast cells, decidual stromal cells (DSCs), and decidual immune cells (DICs), which are all members of the small-molecule cytokine superfamily known as chemokines. Chemokines and chemokine receptors play a crucial role in recruitment of immune cells, trophoblast invasion, and decidualization throughout pregnancy [2]. Furthermore, several pregnancy complications, such as pre-eclampsia (PE), recurrent spontaneous abortion (RSA), and preterm birth (PTB), have been linked to the functional abnormalities of chemokines [3].
KEGG pathway analysis showed downregulation of arginine biosynthesis and upregulation IL-17 signalling pathways. Semi-essential amino acid arginine is a precursor in the production of several compounds, including polyamines and nitric oxide (NO), which are crucial for pregnancy and foetal development. During pregnancy, maternal nutrition is essential for the placental and foetal development. Arginine is a crucial component of nutrition and metabolism because it acts as a precursor for the production of several physiologically significant compounds. Several biological processes, such as protein synthesis and the synthesis of ornithine and urea, glutamate, NO, creatine, proline, and polyamines, use arginine as a precursor. A growing body of research indicates that arginine is essential for reproduction, foetal development, wound healing, tissue integrity maintenance, immunological function, and the treatment of illnesses during pregnancy [4]. For the purpose of promoting protein synthesis, arginine may directly activate p70 S6 kinase and phosphorylate 4E-BP1 via the mTOR signalling pathway. The urea cycle, which eliminates ammonia from the liver and blood, also needs arginine. Through the activation of oxidative stress, rise in intracellular pH, reduction in ATP synthesis, and decrease in utero-placental blood flow and nutrient delivery, high amounts of ammonia are harmful to the developing foetus [5]. NO is a calming substance generated by the endothelium. The control of placental-foetal blood flow depends on NO. Therefore, NO may be essential for ensuring appropriate nutrition transfer from the mother to the foetus. Similarly, polyamines control several biological processes, including protein synthesis and gene expression, embryo/foetus proliferation, growth, and differentiation [6]. Preeclamptic pregnant women have lower plasma levels of arginine and placental eNOS abundance than healthy pregnant women [7]. In a disturbed pregnancy, both the synthesis and the catabolism of arginine are altered. This may affect foetal development, foetal programming, and increased risk for developing adult-onset diseases. Arginine-NO biogenesis dysregulation is implicated in a variety of vascular diseases, including cardiovascular disease and pre-eclampsia [8]. Vasculogenesis, angiogenesis, foetal growth, and survival may be negatively impacted by a change in the bioavailability of arginine and NO. The arginine-NO pathway is a crucial regulator of vascular development [9]. Arginine is considered to be abundant in protein-rich meals such as meats, dairy products, nuts, seeds, soy, and lentils. Between 3 and 15% of different proteins include arginine. Dietary patterns point towards the deficiency of arginine in pregnant women; arginine is considered to be nutritionally necessary for good health, fertility, and reproduction [10]. Since the arginine pathway restricts the amount of arginine that can be used for NO production, dysregulated arginine metabolic pathway may be implicated in the aetiology of RPL.
The differentially expressed genes that are upregulated in the IL-17 signalling pathway are C-X-C chemokine ligands CXCL1, CXCL2, CXCL5, CXCL8, CCL20, and IL-6, granulocyte colony-stimulating factor (G-CSF), and S100 calcium-binding protein A8 (S100A8). Foetus represents an allograft to the maternal host. It has been documented that Th17 cells have a role in transplant rejection [11]. According to Wang et al., RSA (unexplained recurrent spontaneous abortion), which is a progressive stage of abortion, has a much larger number of Th17 cells in the peripheral blood and decidua than a typical early pregnancy. Furthermore, unexplained RSA patients had considerably greater levels of peripheral blood and decidual tissue expression of not only IL-17 but also the Th17-inducing cytokine, IL-23, and the Th17 transcription factor, retinoid orphan nuclear receptor (RORC) [12]. There has been evidence of reciprocal differentiation between Th17 and Treg cells, as well as a decline in Treg cells in RSA with no known cause [13]. In allogeneic murine pregnancy, Treg cell depletion in the early stages of pregnancy causes implantation failure and miscarriage [14]. The quantity of Th17 and Treg cells in the decidua and peripheral blood was found to be inversely correlated [12, 15]. According to recent research, IL-17 is secreted less frequently when oestrogen and placental protein 14 are present [16]. Proinflammatory cytokines like IL-6 and IL-1b promote the development of Th17 cells. The balance of these variables may have an impact on the Th17/Treg cell ratio during pregnancy [17]. CD4+CD25+ Tregs in healthy individuals prevented activated CD4+ T cells from secreting IL-17, and that this regulation was altered in cases with unexplained RSA [18].
Histocompatibility genes HLA-G, HLA-B, HLA-A, HLA-DRA, HLA-DPB1, and HLA-DQB1 were significantly upregulated in RPL cases compared to MTP. A successful pregnancy depends on the mother’s immune tolerance to the semi-allogeneic foetus. RPL, pre-eclampsia, and other pathological diseases are only a few of the potentially harmful pregnancy outcomes that could result from improper molecular cross-talk at the maternal-foetal interface [18]. The establishment of foetal-induced maternal immunological tolerance is regulated by the non-classical major histocompatibility complex class I (MHC-I) component known as human leukocyte antigen-G (HLA-G) [19]. At the foetal-maternal interface, extravillous trophoblasts (EVTs) of the placenta almost exclusively express HLA-G, which offers the semi-allogeneic embryo immune protection from attacks by the maternal NK cells [20]. Recent data suggests that the dysregulated expression levels of HLA-G have a role in the pathophysiology of RPL [21]. In placental tissue samples from RPL patients compared to healthy persons, HLA-G expression was downregulated [22]. According to reports, RPL patients’ levels of HLAG expression are lower than those of healthy controls [21]. These results, however, are in conflict with previous investigations [23]. Women who experience recurrent miscarriages have a higher frequency of the HLA-DRB1*07 allele [24]. Compared to controls, higher percentage of women with RPL was HLA DQ2/DQ8 positive [25].
Quantitative real-time PCR (qRT-PCR) validation of a subset of differentially expressed genes showed significant increased expression of IL-6, PPP1R11, and MMP10 genes in 16 placental decidua samples of unexplained recurrent pregnancy loss cases compared to 12 unwanted medically terminated healthy pregnancies. Cytokines, the immune system’s intercellular messengers, play a crucial role in many aspects of pregnancy. A multipurpose cytokine, IL-6, is essential for the inflammatory response as well as for controlling T-cell development in adaptive immunity. Widespreadly expressed in the female reproductive tract and gestational organs, IL-6 regulates immunological adjustments necessary for pregnancy tolerance as well as embryo implantation and placental development [26]. An extensive range of studies indicate the role of IL6 among the array of cytokines and growth factors that control the events of placental morphogenesis, through coordinating trophoblast proliferation, migration, differentiation, and secretory function [27].
Siim et al. (2016) performed the first transcriptome analysis of RPL cases, and the differential expression analysis found that 51 (27%) transcripts had increased expression, and 138 (73%) had decreased expression when compared to electively terminated pregnancy (ETP) cases. In RPL, transcript levels for histones, regulatory RNAs, and genes involved in telomere, spliceosome, ribosomal, mitochondrial, and intracellular signalling processes were significantly lower. Several upregulated genes such as activating transcription factor 4 (ATF4), complement component 3 (C3), Pleckstrin homology-like domain family A member 2 (PHLDA2), glutathione peroxidase 4 (GPX4), intercellular adhesion molecule 1(ICAM1), and solute carrier family 16 member 2 (SLC16A2) were linked to placental function and pregnancy complications. Surprisingly, E2F transcription factors bind to approximately two-thirds of differentially expressed genes, coordinating mammalian endocycle and placental development [28]. Other studies on transcriptome analysis of RPL reported that the abnormal expression of some specific immunoregulatory genes involved in T-cell activation may play a key role in regulating maternal immune response [29]. Women with RPL had significantly higher levels of the tumour necrosis factor (TNF) and type I interferon signalling pathways [30]. Even our study is in agreement with the earlier studies, which all pointed to an enrichment of dysregulated genes involved in immune and inflammatory response pathways in recurrent pregnancy loss. Further, the present study also indicates the role of arginine biosynthesis pathway in the pathogenesis of RPL.
Conclusion
To summarise, this is the first case series of its kind in India. The present study predicts a role of arginine biosynthesis pathway along with IL-17 signalling pathway in the pathogenesis of RPL. The key genes and functional pathways identified in the current study should be validated in larger sample size to provide new insights into the molecular mechanisms involved in RPL pathogenesis and provide potential diagnostic and therapeutic targets. Management strategies for RPL could be established by further evaluating the arginine-derived molecules, supplement dosage, and treatment time frame during pregnancy.
Limitation of the study
Limitation of the present study is that only six cases are included.
Availability of data and materials
The raw data generated from the two MTP control group and the four RPL group is submitted to SRA database in NCBI.
References
Parveen F, Faridi RM, Alam S, Agrawal S (2011) Genetic analysis of eNOS gene polymorphisms in association with recurrent miscarriage among North Indian women. Reprod Biomed Online 23:124–131. https://doi.org/10.1016/j.rbmo.2011.03.022
Ashley RL, Runyan CL, Maestas MM, Trigo E, Silver G (2021) Inhibition of the C-X-C motif chemokine 12 (CXCL12) and its receptor CXCR4 reduces utero-placental expression of the VEGF system and increases utero-placental autophagy. Front. Vet. Sci 8:650687. https://doi.org/10.3389/fvets.2021.650687
Zheng Z, Chen H, Zhu S, Hu Y (2021) CXCR4/CXCR7 protein expression levels in placentas of patients with preeclampsia. Med. Sci. Monit 27: e931192. https://doi.org/10.12659/msm.931192
Rodrigues-Krause J, Krause M, Rocha IMGD, Umpierre D, Fayh APT (2018) Association of l-arginine supplementation with markers of endothelial function in patients with cardiovascular or metabolic disorders: a systematic review and meta-analysis. Nutrients 11:15. https://doi.org/10.3390/nu11010015
Herring CM, Bazer FW, Johnson GA, Wu G (2018) Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp. Biol. Med 243:525–533. https://doi.org/10.1177/1535370218758275
Hussain T, Tan B, Ren W, Rahu N, Kalhoro DH, Yin Y (2017) Exploring polyamines: functionsin embryo/fetal development. Anim. Nutr 3:7–10. https://doi.org/10.1016/j.aninu.2016.12.002
Kim YJ, Park HS, Lee HY, Ha EH, Suh SH, Oh SK, Yoo HS (2006) Reduced L-arginine level and decreased placental eNOS activity in preeclampsia. Placenta 27:438–444. https://doi.org/10.1016/j.placenta.2005.04.011
Boger RH, Diemert A, Schwedhelm E, Luneburg N, Maas R, Hecher K (2010) The role of nitric oxide synthase inhibition by asymmetric dimethylarginine in the pathophysiology of preeclampsia. Gynecol Obstet Invest 69(1):1–13. https://doi.org/10.1159/000245940.13
Krause BJ, Hanson MA, Casanello P (2011) Role of nitric oxide in placental vascular development and function. Placenta 32(11):797–805. https://doi.org/10.1016/j.placenta.2011.06.025
Hou Y, Yin Y, Wu G (2015) Dietary essentiality of “nutritionally non- essential amino acids” for animals and humans. Exp Biol Med 240(8):997–1007. https://doi.org/10.1177/1535370215587913
Chadha R, Heidt S, Jones ND, Wood KJ (2011) Th17: contributors to allograft rejection and a barrier to the induction of transplantation tolerance? Transplantation 91(9):939–945. https://doi.org/10.1097/TP.0b013e3182126eeb
Wang WJ, Hao CF, Yi-Lin Yin GJ, Bao SH, Qiu LH, Lin QD (2010) Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J. Reprod. Immunol 84(2):164–170. https://doi.org/10.1016/j.jri.2009.12.003
Yang H, Qiu L, Chen G, Zhao A, Chen G, Hu K, Lin Q (2008) Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil. Steril. 89(3):656–661. https://doi.org/10.1016/j.fertnstert.2008.04.068
Shima T, Sasaki Y, Itoh M, Nakashima N, Ishii K, Sugamura K, Saito S (2010) Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol 85(2):121–129. https://doi.org/10.1016/j.jri.2010.02.006
Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou GX, Luo LH, Luan HB (2011) Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am. J. Reprod. Immunol 65(5):503–511. https://doi.org/10.1111/j.1600-0897.2010.00921.x
Ochanuna Z, Geiger-Maor A, Dembinsky- Vaknin A, Karussis D, Tykocinski ML, Rachmilewitz J (2010) Inhibition of effector function but not T cell activation and increase in FoxP3 expression in T cells differentiated in the presence of PP14. PLoS ONE 9:e12868. https://doi.org/10.1371/journal.pone.0012868
Saito S, Nakashima A, Shima T, Ito M (2010) Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol 63(6):601–610. https://doi.org/10.1111/j.1600-0897.2010.00852.x
Rackaityte E, Halkias J (2020) Mechanisms of fetal T cell tolerance and immune regulation. Front Immunol 9(11):588. https://doi.org/10.3389/fimmu.2020.00588
Zhuang B, Shang J, Yao YHLA-G (2021) An important mediator of maternal-fetal immune-tolerance. Front Immunol 29(12):744324. https://doi.org/10.3389/fimmu.2021.744324
Manaster I, Goldman-Wohl D, Greenfield C et al (2012) MiRNA-mediated control of HLA-G expression and function. PLoS One 7(3):e33395. https://doi.org/10.1371/journal.pone.0033395
Mosaferi E, Alizadeh Gharamaleki N, Farzadi L, et al. (2019) The study of HLA-G gene and protein expression in patients with recurrent miscarriage. Adv Pharm Bull 9(1):70-5. https://doi.org/10.15171/apb.2019.009
Khani H, Feizi MA, Safaralizadeh R, Mohseni J, Haghi M (2023) Altered expression of the HLA-G and IL10RB genes in placental tissue of women with recurrent pregnancy loss. Clinical Laboratory 1:69(5). https://doi.org/10.7754/Clin.Lab.2022.220822
Eskicioglu F, Ozdemir AT, Ozdemir RB, Turan GA, Akan Z, Hasdemir SP (2016) The association of HLA-G and immune markers in recurrent miscarriages. J Matern Fetal Neonatal Med 29(18):3056–60. https://doi.org/10.3109/14767058.2015.1114085
Thomsen CK, Steffensen R, Nielsen HS, Kolte AM, Krog MC, Egerup P, Larsen EC, Hviid TV, Christiansen OB (2021) HLA-DRB1 polymorphism in recurrent pregnancy loss: new evidence for an association to HLA-DRB1*07. Journal of Reproductive Immunology 145:103308. https://doi.org/10.1016/j.jri.2021.103308
Silvia D'Ippolito, Antonio Gasbarrini, Roberta Castellani, Rocchetti S, Sisti LG, Scambia G, Di Simone N (2016) Human leukocyte antigen (HLA) DQ2/DQ8 prevalence in recurrent pregnancy loss women. Auto Immunity Reviews 638-643. https://doi.org/10.1016/j.autrev.2016.02.009
Prinsa Jelmer R, b, Nardhy Gomez-Lopeza, Sarah, (2012) Robertsona interleukin-6 in pregnancy and gestational disorders. Journal of Reproductive Immunology 95:1–14. https://doi.org/10.1016/j.jri.2012.05.004
Bowen JM, Chamley L, Mitchell MD, Keelan JA (2002) Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta 23:239–256. https://doi.org/10.1053/plac.2001.0781
Siim Sober, Kristiina Rull1, Mario Reiman, Piret Ilisson, Pirkko Mattila, Maris Laan (2016) RNA sequencing of chorionic villi from recurrent pregnancy loss patients reveals impaired function of basic nuclear and cellular machinery. Scientific Reports 6:38439. https://doi.org/10.1038/srep38439
Gu H, Li L, Du M, Xu H, Gao M, Liu X, Wei X, Zhong X (2021) Key gene and functional pathways identified in unexplained recurrent spontaneous abortion using targeted RNA sequencing and clinical analysis. Front. Immunol 12:717832. https://doi.org/10.3389/fimmu.2021.717832
Li Y, Wang R, Wang M, Huang W, Liu C, Fang Z, Liao S, Jin L (2021) RNA sequencing of decidua reveals differentially expressed genes in recurrent pregnancy loss. Reproductive Sciences. https://doi.org/10.1007/s43032-021-00482-w
Acknowledgements
We would like to thank Dr. P. Malathi, Superintendent, Modern Government Maternity Hospital, Petlaburj, Hyderabad, for providing samples. We acknowledge the support of National Genomics Core, Centre for DNA Fingerprinting and Diagnostics, Hyderabad, for the sequencing and data analysis services.
Funding
This study was funded by the Department of Science and Technology, New Delhi, India, under the WOS-A scheme to Dr. Shehnaz Sultana with grant number SR/WOS-A/LS-53/2018.
Author information
Authors and Affiliations
Contributions
SS conceptualised, designed, and performed the experiments and prepared the manuscript. DB performed the data analysis and prepared manuscript. AV approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee (Ref. No. IEC/OMC/2022/M. No. (10)/Acad-99). Prior consent was obtained from all the study subjects.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sultana, S., Bhanu, B.D. & Ananthapur, V. Identification of key functional pathways: arginine biosynthesis and IL-17 signalling in placental decidua of unexplained recurrent pregnancy loss through RNA sequencing—a case series. Middle East Fertil Soc J 29, 31 (2024). https://doi.org/10.1186/s43043-024-00190-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-024-00190-w