Abstract
Background
Treatment of endometrioma before in vitro fertilization (IVF) is challenging as it may affect ovarian response to induction.
Objective
A systematic review to search for the available optimal management of ovarian endometrioma before ovulation induction in IVF.
Search strategy
Screening of the MEDLINE, Web of Science, EMBASE, Cochrane database, and the clinical trial registration sites, covering the period from their inception up to June 2023 was done by two reviewers independently using the keywords ovarian endometrioma, ovarian endometriosis, endometrioma/surgery, endometrioma/hormonal treatment, randomized controlled trial(s), case-controlled studies, and cohort studies.
Selection criteria
All types of studies were included. Participants included were women with unilateral or bilateral ovarian endometriomas candidate for IVF/ICSI. We included 18 studies in the review. Three studies were randomized controlled parallel studies, six were prospective cohort, and nine were retrospective cohort studies.
Data collection and analysis
Data from all included studies were extracted by two authors (A. M., A. O.) independently. Data extracted included sample size, population characteristics including age, BMI, duration of infertility, ovarian reserve markers, cyst size, and bilaterality and induction protocol used.
Main results
We found 18 studies. Women with untreated endometrioma had significantly higher numbers of MII oocytes (the mean difference (MD) effect estimate was − 0.53 with [− 1.04, − 0.01] 95% CI and 0.04 P-value), higher number of obtained embryos (MD effect estimate was − 0.25 with [− 0.38, − 0.11] 95%CI and < 0.001 P-value), and required lower doses of gonadotropins for induction (MD effect estimate was 361.14 with [168.13, 5554.15] 95% CI and < 0.001 P-value) compared to those who had undergone surgical management of endometrioma. However, live birth (OR effect estimate was 0.79 with [0.54, 1.18] 95% CI and 0.25 P-value), clinical pregnancy (OR effect estimate was 0.95 with [0.72, 1.26] 95% CI and 0.73 P-value), miscarriage (OR effect estimate was 0.74 with [0.33, 1.63] 95% CI and 0.45 P-value), cancellation rates (OR effect estimate was 1.62 with [0.57, 4.66] 95% CI and 0.37 P-value), and the duration of stimulation (MD effect estimate was 0.19 with [− 0.42, − 0.81] 95% CI and 0.54 P-value) did not show any significant difference between the two groups of women. Hormonal treatment of endometrioma was associated with higher ongoing pregnancy rate (OR effect estimate was 3.39 with [1.83, 6.26] 95% CI and < 0.001 P-value), higher clinical pregnancy rate (OR effect estimate was 3.36 with [2.01, 5.63] 95% CI and < 0.001 P-value), and higher numbers of MII oocytes (MD effect estimate was 2.04 with [0.72, 3.36] 95% CI and 0.003 P-value) when compared to women who did not receive such therapy. These effects were evident in treatment with GnRH agonists, OCPs (oral contraceptive pills), and dienogest, while the miscarriage and cycle cancellation rates did not show these differences.
Conclusions
The optimal approach for treating endometrioma prior to IVF is not clear yet due to lack of well-designed randomized controlled trials.
Registration number
CRD42020151736.
Similar content being viewed by others
Introduction
Endometriosis is a benign gynecological pathology characterized by the existence of endometrium outside the uterine cavity and was commonly diagnosed by surgery [1]. Recently, ESHRE in 2022 stated that diagnosis of endometriosis with laparoscopy showed to be restricted to only to those with negative different imaging findings, and women with failed empirical treatment and the group stated that laparoscopy is no longer considered as the gold standard for diagnosis of endometriosis [2]. Endometriosis is an estrogen-dependent chronic inflammatory pathology that affects between 10 and 15% of women during their childbearing period. It is commonly associated with pain, infertility [3, 4], chronic stress, and anxiety [5]. Difficult conception is observed in 30 to 50% of women diagnosed with endometriosis [6], and many of them are trying to conceive through different assisted reproductive techniques [7].
Unfortunately, the pathogenesis of endometriosis associated with infertility remains unclear. Endometriosis causes infertility through various mechanisms: distortion of the normal pelvic anatomy, scarring of the fallopian tubes, inflammation of different pelvic organs with adhesion formation, alteration of immune response and hormonal environment of ova, impairment of implantation of a pregnancy, and alteration of oocytes quality [8]. Various inflammatory changes have been proposed to be the reasons behind endometriosis-associated infertility including alteration of macrophages proliferation and its phagocytic activity, increasing the numbers of malfunctioning natural killer cells and T lymphocytes with enhancement of proinflammatory and angiogenic cytokines release [9, 10]. Ovarian endometrioma decreases the volume of functioning ovarian tissue through its space-occupying action and/or the local inflammatory and immune reactions or both. This reduction in functioning ovarian tissue is aggravated by surgery. Clinical examination has low sensitivity and specificity for diagnosis of endometriosis, and laparoscopy remains the gold standard for diagnosis; however, recent studies look promising for new sonographic and magnetic resonance imaging (MRI) techniques [11].
Endometrioma could be managed by either medical or surgical modalities, and both affect the reproductive potential of the women. Women should be counselled about behavioral changes that creates optimum patients’ characteristics and healthy lifestyle before they started medical and/or surgical treatments. One of these is the maintenance of ideal body weight to decrease the endometriosis cancer risk and to improve the success of IVF [2]. The best management of endometriomas before starting IVF/ICSI trial is unknown. Medical treatment for endometrioma includes mainly hormonal therapy with progestogens, combined oral contraceptives, aromatase enzyme inhibitors, and gonadotropin-releasing hormone analogues. Their mode of action depends on their ability to decrease ovarian activity [12]. Surgical treatment includes cyst aspiration, laparoscopic ovarian cystectomy, ovarian cystectomy via laparotomy, or robotic surgery. Laparoscopic management is currently the most accepted approach being associated with lower costs and faster recovery compared to other approaches [13]. The aim of current review is to evaluate the impact of medical and surgical interventions to endometriomas prior to IVF-ET as there was no similar review to assess the effects of different treatment modalities of ovarian endometriomas on IVF outcomes.
Methodology
This study protocol was prospectively registered following the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) with CRD42020151736 number.
Research question
-
Population: Women with unilateral or bilateral ovarian endometrioma
-
Intervention: Surgical (including cystectomy and aspiration) and medical (including GnRH, progesterone, and aromatase inhibitors) management of endometrioma before IVF
-
Comparison: Surgical treatment and medical treatment compared to no intervention
-
Outcome: Outcomes of IVF cycles
In this systematic review, the following databases: MEDLINE, Web of Science, Embase, Cochrane Library, and the clinical trial registry were searched from inception up to June 2023. The search used the keywords as follows: endometriosis; endometrioma, ovarian endometriosis, and ovarian endometrioma; ovarian endometriosis; endometriotic ovarian cyst; endometrioma/surgery; endometrioma/hormonal treatment, GnRH agonist, letrozole, aromatase inhibitors, OCPs (oral contraceptive pills)), IVF/ICSI, and in vitro fertilization; intracytoplasmic sperm injection; assisted reproductive technologies; ART; oocytes; live birth; fertilization; and live birth rate, pregnancy rate, miscarriage rate, number of oocytes, cancellation rate, randomized controlled trial(s), case-controlled studies, and cohort studies. As only few randomized controlled trials were available, the authors agreed to include other types of studies as case controlled and cohort studies whether prospective or retrospective. Review articles and case reports, editorial opinion, and commentary were excluded. We analyzed the references and citations of all available studies (both primary and secondary), narrative and systematic reviews, abstracts of gynecology, and infertility seminars. We emailed the authors directly for any missing or unclear information. If necessary, all studies written in English comparing different surgical and medical modalities against no treatment or other modalities were included. Surgical modalities include laparoscopic cystectomy, open cystectomy, or cyst aspiration. Medical modalities include progestogens, aromatase inhibitors, GnRH agonists, or oral contraceptive pills (OCPs).
Cystectomy is the stripping of the cyst wall away from the healthy ovarian tissues through either laparoscopy or laparotomy. Aspiration procedure is the transvaginal aspiration of the cyst content guided by ultrasound. Medical treatment acts through antagonizing estrogen secretion and/or action.
Two authors (A. O. and A. M.) independently assessed the titles, abstracts, and the full articles and then extracted the data of the included studies, and disagreements were discussed further with other authors. Extracted data included study type, settings, participants characteristics, details of intervention, and outcome parameters.
The risk of bias was assessed using the Cochrane Handbook of Systematic Reviews recommendations for the 3 RCTs [14]. These recommendations include random sequence generation, allocation concealment, participants and outcome assessors blinding, incomplete data of outcomes, selective reporting, and other biases.
The outcome parameters of this review included live birth, clinical pregnancy, miscarriage, cancellation rates, number of MII follicles, duration of stimulation, total dose of gonadotropin used for induction, and number of obtained embryos.
Statistical analysis
For analysis of continuous and dichotomous data, the mean difference and odd ratio with 95% confidence interval (CI) analysis were used, respectively. The random effect model was used to calculate the effect size, and I2 statistic and Cochran’s Q test were calculated to evaluate the studies heterogeneity. Significant P-value was considered when < 0.05, and significant I2 was considered when > 40%. Analyses were done using the Review Manager (RevMan) version 5.4.1 (The Nordic Cochrane Centre, Cochrane Collaboration, 2020, Copenhagen, Denmark).
Results
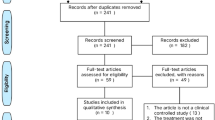
The PRISMA flow chart is shown in Fig. 1.
Study characteristics
Table 1 describes the characteristics of the included studies.
Eighteen studies were included in this review [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Five were conducted in Turkey [15, 16, 18, 25, 26], 3 in Japan [19, 27, 28], and 1 in each of the following countries: Canada [31], China [21], Denmark [22], Egypt [23], France [32], Italy [30], Russia [24], Spain [17], South Korea [20], and the USA [29]. Three studies were RCTs [16, 23, 26], six were prospective cohort [21, 22, 24, 25, 30, 32], and nine were retrospective cohort studies [15, 17,18,19,20, 27,28,29, 31]. All were single center except three trials [17, 31, 32] that were conducted in two centers. Six studies compared laparoscopic surgery to no treatment [16,17,18,19, 21, 27], five compared surgery to no treatment [15, 20, 25, 26, 29], and three compared cyst aspiration to no treatment [20, 25, 27]. Medical treatment was GnRH in two studies [23, 24], OCPs in one study [15], and dienogest in one study [24], while aromatase inhibitors were studied in two trials [22, 31].
Risk of bias of included studies
Risk of bias in the three RCTs is described in Table 2. The risk of bias for non-RCTs was evaluated using the Newcastle–Ottawa scale (Table 3).
Synthesis of results
Eighteen studies with 3063 participants were included in our review.
Surgical intervention versus no treatment
Live birth rate was evaluated in 4 studies (565 participants). The OR effect estimate was 0.79 with [0.54, 1.18] 95% CI and 0.25 P-value (Fig. 2).
Clinical pregnancy rate was evaluated in 11 studies (1447 participants). The OR effect estimate was 0.95 with [0.72, 1.26] 95% CI and 0.73 P-value (Fig. 3).
Miscarriage rate was evaluated in 6 studies (333 participants). The OR effect estimate was 0.74 with [0.33, 1.63] 95% CI and 0.45 P-value (Figure S1).
Cycle cancellation rate was evaluated in 4 studies (551 participants). The OR effect estimate was 1.62 with [0.57, 4.66] 95% CI and 0.37 P-value (Figure S2).
Number of MII oocytes was evaluated in 11 studies (1475 participants). The mean difference (MD) effect estimate was − 0.53 with [− 1.04, − 0.01] 95% CI and 0.04 P-value (Figure S3).
Number of obtained embryos was evaluated in 4 studies (569 participants). The MD effect estimate was − 0.25 with [− 0.38, − 0.11] 95% CI and < 0.001 P-value (Figure S4).
Duration of stimulation was evaluated in 6 studies (881 participants). The MD effect estimate was 0.19 with [− 0.42, 0.81] 95% CI and 0.54 P-value (Figure S5).
The total dose of gonadotropins was evaluated in 9 studies (1255 participants). The MD effect estimate was 361.14 with [168.13, 554.15] 95% CI and < 0.001 P-value (Figure S6).
Medical intervention versus no treatment
Ongoing rate was evaluated in 2 studies (270 participants). The OR effect estimate was 3.39 with [1.83, 6.26] 95% CI and < 0.001 P-value (Figure S7).
Clinical pregnancy rate was evaluated in 3 studies (330 participants). The OR effect estimate was 3.36 with [2.01, 5.63] 95% CI and < 0.001 P-value (Figure S8).
Miscarriage rate was evaluated in 2 studies (270 participants). The OR effect estimate was 1.31 with [0.46, 3.72] 95% CI and 0.61 P-value (Figure S9).
Cancellation rate was evaluated in 2 studies (270 participants). The OR effect estimate was 0.48 with [0.21, 1.10] 95% CI and 0.08 P-value (Figure S10).
Number of MII oocytes was evaluated in 3 studies (330 participants). The mean difference (MD) effect estimate was 2.04 with [0.72, 3.36] 95% CI and 0.003 P-value (Figure S11).
Subgroup analysis of all outcomes based on the type of surgical and medical intervention is shown in supplementary Table S1.
Candiani and colleagues (2020) [30] conducted a retrospective study on 142 women diagnosed with symptomatic endometrioma who were subjected to laparoscopic cyst stripping or CO2 cyst vaporization and then tried to conceive spontaneously and if failed through IVF. They reported pregnancy rates of 72.2% vs 74.3% (55.6% vs. 35.9% spontaneously;16.7% vs.38.5% through IVF) in stripping vs. laser respectively (P = 0.83), and 26.7% do not achieve pregnancy. They identified age and duration of infertility as independent indicators for pregnancy. They concluded that the pregnancy rate was not different between the two groups and suggest that CO2 laser one-step technique could be a good alternative to conventional cystectomy.
Cantor et al. in 2019 [31] conducted a retrospective study on 126 women with endometrioma and had a history of previous IVF cycle. Participants were subjected to either two doses of intramuscular 3.75-mg intramuscular depo-leuprolide with 1-month interval or daily 5-mg oral letrozole for 60 days along with the same leuprolide treatment before fresh IVF cycle. They reported a significantly higher AFC (10.3 ± 2.0 vs. 6.4 ± 2.5; P = 0.0001), lower required total doses of Gn (2079 ± 1119 versus 3716 ± 1314; P = 0.0001), higher number of mature oocytes (9.1 ± 2.4 versus 4.0 ± 1.7; P = 0.0001), clinical pregnancy rate (50% versus 22%, P = 0.003), and live birth rate (40% versus 17%, P = 0.008) in letrozole GnRH group compared to GnRH only group, respectively.
Lossl et al. in 2009 [22] conducted a prospective single arm study on 20 women candidate for IVF and diagnosed with endometrioma. They were subjected to three subcutaneous injections of 3.6 goserelin every 28 days in addition to daily oral tablet of 1-mg anastrozole for 70 days. They reported a significant decrease in endometriomal volume by 29% (P = 0.007) and serum CA125 by 61% (P = 0.001).
Discussion
In this review, women with untreated endometrioma had significantly higher numbers of MII oocytes (P = 0.04), higher number of obtained embryos (P < 0.001), and required lower doses of gonadotropins for induction (P < 0.001) compared to those who had undergone surgical management of endometrioma. However live birth (P = 0.25), clinical pregnancy (P = 0.73), miscarriage (P = 0.45), cancellation rates (P = 0.37), and the duration of stimulation (P = 0.54) did not show any significant difference between the two groups of women.
Our systematic review also confirmed that hormonal treatment of endometrioma was associated with higher ongoing pregnancy rate (P < 0.001), higher clinical pregnancy rate (P < 0.001), and higher numbers of MII oocytes (P = 0.003) when compared to women who did not receive such therapy. These effects were evident in treatment with GnRH agonists, OCPs, and dienogest, while the miscarriage (P = 0.61) and cycle cancellation rates (P = 0.08) did not show these differences.
Given the thorough search strategy and clear inclusion and exclusion criteria, this review provides a comprehensive overview of the current scientific evidence regarding surgical and medical treatment of endometriomas prior to IVF/ICSI. All 18 included studies included various comparisons, different study designs, and heterogenous reporting of data. This marked heterogeneity in study design, sample size, included population characteristics, intervention differences in type and timing, and different treatment modalities after intervention completion prevents the proper meta-analysis reporting of different outcome parameters. The review presented two different comparisons either surgical or medical treatment options.
There are contradictory observations regarding the impact of endometriosis on ovarian responsiveness to gonadotropins during controlled ovarian stimulation and IVF. Some studies reported diminished ovarian response to COH in women with unilateral endometriomas [33, 34]. On the other hand, a systematic review and meta-analysis of nine RCTs reported difference between the ovary with endometrioma compared to the contralateral nonaffected ovary regarding the numbers of retrieved oocytes, MII oocytes, and obtained embryos. All were reported to be lower from the affected ovaries. They suggested different mechanism for these findings including changes in immune markers as IL-6, VEGF, and oxidative stress markers with resultant decrease in density and diameters of primordial follicles. However, there was no differences regarding clinical pregnancy and live birth rates [35].
The impact of surgical treatment for endometrioma before IVF remains a controversy. In a retrospective trial that involved 292 women with existing endometrioma, no history of surgery who were candidate for IVF, they reported lower numbers of antral follicles and required higher doses of Gn in women with existing endometriomas compared to those with previous surgery for endometriomas and absent endometrioma at time of IVF without any significant difference in live birth rate between them [36].
In a recent meta-analysis, there was a significantly lower number of retrieved oocytes and higher rate of cycle cancellation in women with existing endometrioma during IVF cycle with similar clinical pregnancy and live birth rates when compared to women without endometriomas [37, 38]. Hamadan and colleagues suggested that the presence of ovarian endometrioma exerts a negative effect on the ovarian tissue with resultant decrease in number and quality of retrieved oocytes and increase in baseline FSH level [37].
On the other hand, a retrospective study reported a significantly higher clinical pregnancy and live birth rates trial in women who underwent laparoscopic cystectomy for endometrioma when compared to those underwent diagnostic laparoscopy without resection before IVF [39]. In a large observational study that involved 825 women diagnosed with endometriosis-related infertility, there was a significantly higher overall pregnancy rates in women who underwent endometrioma surgical resection followed by IVF compared to those who underwent surgical resection without IVF, IVF without prior resection, or no treatment [40].
Furthermore, several studies have reported the adverse effects of surgical treatment of endometrioma on ovarian reserve markers as the reduction of serum AMH levels after surgery [41]. After surgery, the level of AMH is reduced by 34% 1 week after surgery and gradually improves to reach 65% of its preoperative level 3 months after the operation. Measurement of AMH after 1 year of surgery revealed similar level to that reported after 1 month of surgery. Bilateral ovarian cystectomy is associated with more damage to ovarian reserve [42].
Regarding the medical treatment before IVF, a systematic review included 19 studies with 1709 participants compared with dydrogesterone to other hormonal therapies. It concluded that dydrogesterone caused better relieve of dysmenorrhea and improved the pregnancy rate compared to gestrinone with less side effects. They also concluded that dydrogesterone decreased the recurrence rate compared to with GnRH-a treatment. There are insufficient data to compare the efficacy of dydrogesterone, letrozole and leuprolide acetate, and the traditional Chinese medicine remains [43]. A Cochrane overview concluded that a 3-month regimen with GnRH agonist before IVF improves the pregnancy rates [44].
Strengths and limitations
This review is the first systematic review to study the clinical parameters of the effects of both surgical and medical treatment of endometrioma before IVF cycles. We included all the available studies that included all types of interventions. Adequate data extractions and proper meta-analysis when possible was done. The main limitation of this review is the low quality of evidence because of the absence of adequate numbers of RCTs and the marked heterogeneity among the included studies.
Conclusion
In conclusion, the optimal approach for treating endometrioma prior to IVF is not clear yet due to lack of well-designed randomized controlled trials.
Availability of data and materials
All data used are available within the manuscript itself.
Data used and/or analyzed during the study are available from the corresponding author upon request.
References
Acién P, Velasco I (2013) Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol 17(2013):242149
Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, King K, Kvaskoff M, Nap A, Petersen K, Saridogan E, Tomassetti C, van Hanegem N, Vulliemoz N, Vermeulen N (2022) ESHRE Endometriosis Guideline Group ESHRE guideline: endometriosis. Hum Reprod Open 2022(2):hoac009
Fauconnier A, Chapron C (2005) Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update 11(6):595–606
Giudice LC (2010) Clinical practice Endometriosis. N Engl J Med 362(25):2389–2398
Garalejić E, Bojović-Jović D, Damjanović A, Arsić B, Pantić I, Turjacanin-Pantelić D, Perović M (2010) Hamilton Anxiety Scale (HAMA) in infertile women with endometriosis and its correlation with magnesium levels in peritoneal fluid. Psychiatr Danub 22(1):64–67
Macer ML, Taylor HS (2012) Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am 39(4):535–549
Stephansson O, Kieler H, Granath F, Falconer H (2009) Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Hum Reprod 24(9):2341–2347. https://doi.org/10.1093/humrep/dep186. (Epub 2009 May 12 PMID: 19439428)
Pellicer A, Oliveira N, Ruiz A, Remohi J, Simon C (1995) Exploring the mechanism(s) of endometriosis-related infertility: an analysis of embryo development and implantation in assisted reproduction. Hum Reprod 10(Suppl 2):91–97
SukhikhG T, Sotnikova NY, Antsiferova YS, Posiseeva LV, Veryasov VN, Vanko LV (2004) Cytokine production by immunocompetent cells of peritoneal fluid in women with external genital endometriosis. Bull Exp Biol Med 137(6):568
de Ziegler D, Borghese B, Chapron C (2010) Endometriosis and infertility: pathophysiology and management. Lancet 376(9742):730–738
Bazot M, Daraï E (2017) Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril 108(6):886–894
Hughes E, Brown J, Collins JJ, Farquhar C, Fedorkow DM, Vandekerckhove P (2007) Ovulation suppression for endometriosis. Cochrane Database Syst Rev 3:CD000155
Ahmad G, O’Flynn H, Duffy JM, Phillips K, Watson A (2012) Laparoscopic entry techniques. Cochrane Database Syst Rev 2:CD006583
Higgins, J.P.T. and Green, S. (2011) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration. http://handbook-5-1.cochrane.org
Demirdag E, Guler I, Selvi I, Cevher Akdulum MF, Canan S, Erdem A, Erdem M (2021) Analysis of 2438 cycles for the impact of endometrioma and its surgery on the IVF outcomes. Eur J Obstet Gynecol Reprod Biol 263:233–238. https://doi.org/10.1016/j.ejogrb.2021.06.034. (Epub 2021 Jun 29 PMID: 34242932)
Demirol A, Guven S, Baykal C, Gurgan T (2006) Effect of endometrioma cystectomy on IVF outcome: a prospective randomized study. Reprod Biomed Online 12(5):639–643. https://doi.org/10.1016/s1472-6483(10)61192-3. (PMID: 16790114)
Garcia-Velasco JA, Arici A (2004) Surgery for the removal of endometriomas before in vitro fertilization does not increase implantation and pregnancy rates. Fertil Steril 81(5):1206. https://doi.org/10.1016/j.fertnstert.2003.10.032. (PMID: 15136078)
Guler I, Erdem A, Oguz Y, Cevher F, Mutlu MF, Bozkurt N, Oktem M, Erdem M (2017) The impact of laparoscopic surgery of peritoneal endometriosis and endometrioma on the outcome of ICSI cycles. Syst Biol Reprod Med 63(5):324–330. https://doi.org/10.1080/19396368.2017.1332114. (Epub 2017 Jun 13 PMID: 28609124)
Kuroda K, Kitade M, Kikuchi I, Kumakiri J, Matsuoka S, Kuroda M, Takeda S (2009) The impact of endometriosis, endometrioma and ovarian cystectomy on assisted reproductive technology. Reprod Med Biol 8(3):113–118. https://doi.org/10.1007/s12522-009-0021-1. (Erratum.In:ReprodMedBiol.2009Sep19;8(4):181.PMID:29699316;PMCID:PMC5907135)
Lee KH, Kim CH, Lee YJ, Kim SH, Chae HD, Kang BM (2014) Surgical resection or aspiration with ethanol sclerotherapy of endometrioma before in vitro fertilization in infertilie women with endometrioma. Obstet Gynecol Sci 57(4):297. https://doi.org/10.5468/ogs.2014.57.4.297. (Epub 2014 Jul 15. PMID: 25105103; PMCID: PMC4124091)
Liang Y, Yang X, Lan Y, Lei L, Li Y, Wang S (2019) Effect of endometrioma cystectomy on cytokines of follicular fluid and IVF outcomes. J Ovarian Res 12(1):98. https://doi.org/10.1186/s13048-019-0572-7. (PMID:31639028;PMCID:PMC6802315)
Lossl K, Loft A, Freiesleben NL, Bangsbøll S, Andersen CY, Pedersen AT, Hartwell D, Andersen AN (2009) Combined down-regulation by aromatase inhibitor and GnRH-agonist in IVF patients with endometriomas-a pilot study. Eur J Obstet Gynecol Reprod Biol 144(1):48–53. https://doi.org/10.1016/j.ejogrb.2009.02.001. (Epub 2009 Mar 3 PMID: 19261371)
Maged AM, Rashwan H, Mahmoud M, El-Mazny A, Farouk M, Belal DS, Marie HM (2018) Effect of prolonged GnRH agonist downregulation on ICSI outcome in patients with endometriomas of less than 5 cm: a randomized controlled trial. Reprod Sci 25(10):1509–1514. https://doi.org/10.1177/1933719118756753. (Epub 2018 Feb 13 PMID: 29439618)
Muller V, Kogan I, Yarmolinskaya M, Niauri D, Gzgzyan A, Aylamazyan E (2017) Dienogest treatment after ovarian endometrioma removal in infertile women prior to IVF. Gynecol Endocrinol 33(sup1):18–21. https://doi.org/10.1080/09513590.2017.1415676. (PMID: 29264985)
Pabuccu R, Onalan G, Goktolga U, Kucuk T, Orhon E, Ceyhan T (2004) Aspiration of ovarian endometriomas before intracytoplasmic sperm injection. Fertil Steril 82(3):705–711. https://doi.org/10.1016/j.fertnstert.2004.02.117. (PMID: 15374718)
Pabuccu R, Onalan G, Kaya C (2007) GnRH agonist and antagonist protocols for stage I-II endometriosis and endometrioma in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril 88(4):832–839. https://doi.org/10.1016/j.fertnstert.2006.12.046. (Epub 2007 Apr 10 PMID: 17428479)
Suganuma N, Wakahara Y, Ishida D, Asano M, Kitagawa T, Katsumata Y, Moriwaki T, Furuhashi M (2002) Pretreatment for ovarian endometrial cyst before in vitro fertilization. Gynecol Obstet Invest 54(Suppl 1):36–40. https://doi.org/10.1159/000066293. (discussion 41-2 PMID: 12441659)
Takashima A, Takeshita N, Otaka K, Kinoshita T (2013) Effects of bipolar electrocoagulation versus suture after laparoscopic excision of ovarian endometrioma on the ovarian reserve and outcome of in vitro fertilization. J Obstet Gynaecol Res 39(7):1246–1252. https://doi.org/10.1111/jog.12056. (PMID: 23803008)
Wong BC, Gillman NC, Oehninger S, Gibbons WE, Stadtmauer LA (2004) Results of in vitro fertilization in patients with endometriomas: is surgical removal beneficial? Am J Obstet Gynecol 191(2):597–606. https://doi.org/10.1016/j.ajog.2004.05.079. (discussion 606-7 PMID: 15343246)
Candiani M, Ferrari S, Bartiromo L, Schimberni M, Tandoi I, Ottolina J (2021) Fertility outcome after CO2 laser vaporization versus cystectomy in women with ovarian endometrioma: a comparative study. J Minim Invasive Gynecol 28(1):34–41. https://doi.org/10.1016/j.jmig.2020.07.014. (Epub 2020 Jul 24. PMID: 32712323)
Cantor A, Tannus S, Son WY, Tan SL, Dahan MH (2019) A comparison of two months pretreatment with GnRH agonists with or without an aromatase inhibitor in women with ultrasound-diagnosed ovarian endometriomas undergoing IVF. Reprod Biomed Online 38(4):520–527. https://doi.org/10.1016/j.rbmo.2018.12.028. (Epub 2018 Dec 22 PMID: 30935663)
de Ziegler D, Gayet V, Aubriot FX, Fauque P, Streuli I, Wolf JP, de Mouzon J, Chapron C (2010) Use of oral contraceptives in women with endometriosis before assisted reproduction treatment improves outcomes. Fertil Steril 94(7):2796–2799. https://doi.org/10.1016/j.fertnstert.2010.05.056. (Epub 2010 Jul 21 PMID: 20663495)
Somigliana E, Infantino M, Benedetti F, Arnoldi M, Calanna G, Ragni G (2006) The presence of ovarian endometriomas is associated with a reduced responsiveness to gonadotropins. Fertil Steril 86:192–196
Benaglia L, Somigliana E, Santi G, Scarduelli C, Ragni G, Fedele L (2011) IVF and endometriosis-related symptom progression: insights from a prospective study. Hum Reprod 26:2368–2372
Yang C, Geng Y, Li Y, Chen C, Gao Y (2015) Impact of ovarian endometrioma on ovarian responsiveness and IVF: a systematic review and meta-analysis. Reprod Biomed Online 31(1):9–19. https://doi.org/10.1016/j.rbmo.2015.03.005. (Epub 2015 Mar 19)
Dong X, Wang R, Zheng Y, Xiong T, Liao X, Huang B, Zhang H (2014) Surgical treatment for endometrioma does not increase clinical pregnancy rate or live birth/ongoing pregnancy rate after fresh IVF/ICSI treatment. Am J Transl Res 6(2):163–168
Hamdan M, Dunselman G, Li TC, Cheong Y (2015) The impact of endometrioma on IVF/ICSI outcomes: a systematic review and metaanalysis. Hum Reprod Update 21:809–825
Hong SB, Lee NR, Kim SK, Kim H, Jee BC, Suh CS et al (2017) In vitro fertilization outcomes in women with surgery induced diminished ovarian reserve after endometrioma operation: comparison with diminished ovarian reserve without ovarian surgery. Obstet Gynecol Sci 60:63–68
Opøien HK, Fedorcsak P, Byholm T, Tanbo T (2011) Complete surgical removal of minimal and mild endometriosis improves outcome of subsequent IVF/ICSI treatment. Reprod BioMed Online 23(3):389–395
Barri PN, Coroleu B, Tur R, Barri-Soldevila PN, Rodríguez I (2010) Endometriosis-associated infertility: surgery and IVF, a comprehensive therapeutic approach. Reprod BioMed Online 21(2):179–185
Iwase A, Hirokawa W, Goto M (2010) Serum anti-Müllerian hormone level is a useful marker for evaluating the impact of laparoscopic cystectomy on ovarian reserve. Fertil Steril 94(7):2846–49
Sugita A, Iwase A, Goto M, Nakahara T, Nakamura T, Kondo M, Osuka S, Mori M, Saito A, Kikkawa F (2013) One-year follow-up of serum antimüllerian hormone levels in patients with cystectomy: are different sequential changes due to different mechanisms causing damage to the ovarian reserve? Fertil Steril 100(2):516–22.e3
Peng C, Huang Y, Zhou Y. Dydrogesterone in the treatment of endometriosis: evidence mapping and meta-analysis. Arch Gynecol Obstet. 2021 Jan 4. https://doi.org/10.1007/s00404-020-05900-z. Epub ahead of print. PMID: 33398505.
Brown J, Farquhar C (2014) Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev 2014(3):D009590. https://doi.org/10.1002/14651858. (PMID:24610050;PMCID:PMC6984415)
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study concept and design, MK and WSR. Analysis and interpretation of data, AM and AO. Drafting of the manuscript, MK. Critical revision of the manuscript for important intellectual content, all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors give their consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Katta, M., Maged, A.M., Ogila, A.I. et al. Impact of treatment interventions of endometriomas prior to in vitro fertilization: a systematic review and meta-analysis. Middle East Fertil Soc J 29, 27 (2024). https://doi.org/10.1186/s43043-024-00189-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-024-00189-3