Abstract
Background
Meiotic and mitotic errors often lead to aneuploidy and mosaicism. In this context, the self-correction mechanism enables the embryo to preferentially retain and preserve euploid cells through processes such as apoptosis, necrosis, or marginalization. This mechanism is thought to minimize the chance of genetic abnormalities during cell development.
Materials and methods
A literature search for articles written in English from January 2013 to October 2023 was conducted on PubMed, EBSCO, and Scopus, using the keywords “self-correction,” “self-repair,” “aneuploidy,” “mosaicism,” and “embryo.”
Results
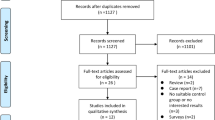
A total of 308 articles were collected, out of which 5 retrospective and 1 prospective study were selected based on inclusion criteria.
Discussions
Investigations showed that embryos remove chromosomally abnormal cells, supporting the self-correction mechanism. aCGH has been used in 4 studies to demonstrate the presence of self-correction in mosaic embryos. Furthermore, a higher relative viability of polyploidies than complex aneuploidies was observed, suggesting early discrimination against complex aneuploidy, particularly those arising from mitotic origins. However, there are doubts about the reliability of preimplantation genetic testing for aneuploidy at the blastocyst stage, as it may lead to a high rate of false positives and the discarding of "good" embryos.
Conclusions
Studies showed a self-correction mechanism in human embryos through the ability to expel abnormal cells. Further investigation is needed to elucidate the underlying mechanisms and determine optimal strategies for preimplantation genetic testing to fully understand and optimize the use of self-correction mechanisms in embryo assessment and selection.
Similar content being viewed by others
Background
Oocytes and sperm are subjected to a specialized cell division known as meiosis, where the chromosome count is reduced through two successive divisions, preparing for potential fertilization. In human female meiosis, there is a significant occurrence of chromosomal segregation error, leading to oocytes with an incorrect number of chromosomes. After fertilization, an embryo with an abnormal chromosome count is formed, often causing developmental issues. Meiotic errors typically lead to uniformly aneuploid embryos, while mitotic errors often cause mosaicism. As women age, errors in meiosis become more prevalent, increasing the risks of infertility, miscarriage, and congenital syndromes [1].
Self-correction mechanism manifests in embryos containing aneuploid cells. This comprises processes such as apoptosis, necrosis, or marginalization. The mechanism enables the embryo to preferentially retain and preserve euploid cells, thereby minimizing the chance of genetic abnormalities during cell development [2, 3]. Embryo with a normal cleavage rate is more prone to have chromosomally normal characteristics. In contrast, lagged or rapid cleavage results in higher rates of chromosomal abnormalities [4].
The prevailing approach for assessing chromosome status includes the implementation of Pre-implantation Genetic Screening (PGS), which detects abnormalities originating from both meiotic and post-zygotic events [5]. However, the self-correction mechanism may lead to inconsistencies in aneuploid trophectoderm biopsy results for euploid embryos, as the outcomes are solely influenced by the biopsied cells. This could lead to erroneous discarding of normal embryos [6]. This literature review aims to comprehensively understand the self-correction process in human embryos in response to aneuploidy. It presents the potential roles in enhancing the success rates of fertility procedures and reducing the chances of genetic anomalies during the early stage of embryonic development.
Materials and methods
A search was conducted for English-language studies sourced from literature repositories, including PubMed, EBSCO, and Scopus, published between January 2013 to October 2023. The keywords utilized were “self-correction,” “self-repair,” “aneuploidy,” “mosaicism,” and “embryo.” Excluded from the results were editorial letters and notes, conference papers, short surveys, and articles in the press.
Results
A total of 308 articles were collected from several databases, such as PubMed (n = 21), EBSCO (n = 23), and Scopus (n = 264) (Fig. 1). Subsequently, a total of 6 articles consisting of 5 retrospective and 1 prospective study were selected based on inclusion criteria. The characteristics of the study are summarized in Table 1.
A recent study by Wang et al. [7] examines the process by which blastocysts expel arrested cells or cellular debris. The process entailed analyzing arrested cells/cellular debris and trophectoderm (TE) cells using NGS (next-generation sequencing) from the selected blastocysts. Among the examined blastocysts, 47.6% (10 out of 21) showed aneuploidies or mosaicism. It is important to note that chromosomal rearrangements and the number of abnormal chromosome fragments increased in 85.7% of the 21 cases where blastocysts had arrested cells or expelled cellular debris. Aneuploid arrested cells or cellular debris were discovered to be expelled from 9 aneuploid and 9 euploid blastocysts.
A total of 63.6% of 11 paired blastocysts released cellular debris containing additional chromosomal rearrangements, according to a study by Orvieto et al. [8]. Approximately 5 of euploid blastocysts (55.5% of the total) released material containing aneuploid cells. From the 18 blastocysts derived from partially compacted morula obtained from TE biopsies, 13 were euploid, while 5 were aneuploid, according to a study by Lagalla et al. [9]. After further cytogenetic analysis, 2 of the 13 euploid blastocysts were observed to have pure euploidy. In contrast, 5 had aneuploidy (38.5%), 6 had no amplification (46.2%), and 3 had fragmented or degraded DNA (15.4%). The array comparative genomic hybridization (aCGH) results for the excluded cells of 5 aneuploid blastocysts were positive for aneuploidy, with increased complexity in 4 of the 5 cases (80%).
Balakier et al. [10] provided strong evidence in support that the human embryo has the ability for self-correction based on morphokinetic and PGS evaluations of multinucleated (MN) embryos. Approximately 56.8% of 2-cell MN embryos developed into high-quality blastocysts, with 50% being euploid. During the initial 4 to 5 cell divisions of MN2 and MN4 embryos, morphokinetic evidence suggested that repair mechanisms were active.
A study by McCoy et al. [11] compared blastomere and TE samples by counting the number of chromosomes affected and calculating the percentage difference in aneuploidy rates. This index measured the proportion of embryos that either did not progress between the 2 sampling points or underwent self-correction. Since the data from blastomere and TE biopsies were collected independently, the study design did not permit distinguishing between embryonic arrest and self-correction. The incidence of errors comprising a growing number of chromosomes became less prevalent in TE biopsies when compared to blastomeres, with the decline stabilizing at around 11 affected chromosomes. However, the difference was less extreme when more than 18 chromosomes were affected.
Mertzanidou et al. [12] compared 13 embryos, comprising 4 fresh embryos with good quality and 9 that had been frozen on day 3. Among the fresh embryos, 1 had a normal chromosome, while the other 3 showed mosaic patterns with abnormal cells ranging from 16 to 75%. From the cryopreserved embryos, 1 had a normal chromosomal complement in two-thirds of the blastomeres examined, 4 had chromosomal abnormalities between 50 and 75% of the blastomeres, and 1 showed aneuploidy in all cells. Meiotic abnormalities were observed in the remaining 3 embryos, with 2 having mitotic problems in several cells. The combination of PCR (polymerase chain reaction) results and log2 ratios provided strong evidence that the observed losses in these cells were nullisomic. In addition, chromosome 19 and X uniparental isodisomy were observed in cells 11, 12, 13, and 14.
Discussion
Meiotic and mitotic errors often lead to aneuploidy and mosaicism. Self-correction mechanism enables the embryo to preferentially retain and preserve euploid cells, thereby minimizing the chance of genetic abnormalities during cell development in the form of apoptosis, necrosis, or marginalization [2, 3]. Existing literature was reviewed to provide more information regarding embryo response to aneuploidy through self-correction or self-repair mechanism. A study by Wang et al. [7] focuses on comparing the results of NGS analysis of TE biopsies with the genetic composition of the expelled cells or debris and determining discrepancies or similarities. The results supported the hypothesis that removing chromosomally abnormal cells in early embryonic development could be the self-correction mechanism. Expelled cells may contain genetic abnormalities or errors recognized by the embryo, leading to elimination and contributing to the reduction of abnormal cell proportions in subsequent embryos [7].
A total of 4 studies used array-based comparative genomic hybridization as cytogenetic methods, with each having different observative aspects to prove embryo self-correction mechanism. A study by Lagalla et al. [13] found a higher prevalence of aneuploidies in the excluded cells compared to the corresponding trophectoderm cells collected by biopsies, suggesting support for a potential self-correction mechanism in mosaic embryos. Another study examined multinucleated embryos, resulting in more than half of euploid outcomes. Furthermore, it observed an extension of the first cleavage division and a prolonged duration of the 2-cell and 4-cell stages in multinucleated embryos. The observations suggest a repair mechanism, potentially contributing to the ability of self-correction during the early cleavage divisions. This leads to the development of euploid blastocysts and, consequently, the birth of healthy infants [10].
Compared to blastomeres, TE biopsies had a lower frequency of errors comprising an increasing number of chromosomes. This implied that polyploidies had a higher relative viability than complex aneuploidies. The results prove early discrimination against complex aneuploidy, especially those of mitotic origin [11]. The report is in line with a recent study showing rates of 52.9% and 68.5% (p 0.001) for aneuploidy in embryo without and with blastomere exclusion at any stage [14].
In another study, the progression of aneuploidy was tracked up to day 4 of embryonic development. No evidence of self-correction was detected, suggesting the process will not commence until a later stage. Furthermore, researchers discovered a significant pattern that may prove that a single cell was subjected to DNA replication without cell division (endoreduplication) and divided using a tetrapolar spindle. During the process of division, many chromosomes were disorganized and detached from the spindles. In some instances, nondisjunction of sister chromatids would cause the observed isodisomy. In other cases, nullisomy occurred, in which one of the cells received no chromosomes. These results suggest that chromosome disorders and mosaicism are common in the earliest stages of embryonic development, thereby affecting implantation and pregnancy outcomes [12].
While evidence supports the self-correction mechanism in embryos, one study raised doubts about the efficacy and reliability of preimplantation genetic testing for aneuploidy at the blastocyst stage. The assumption that non-invasive preimplantation genetic testing aneuploidy (niPGT-A) can accurately identify euploid embryos by analyzing cell-free DNA in spent culture media is challenged by the ability to self-correct and expel abnormal blastomeres as cell debris. Because of that, many “good” embryos can be discarded due to a high false-positive rate in niPGT-A. Mislabeling of cell-free DNA from expelled cell debris as aneuploid or mosaic blastocysts undermines the validity of niPGT-A. Different chromosomal abnormalities were found in the cell debris of euploid blastocysts expelled during niPGT-A, disproving the hypothesis that more DNA would leak out of euploid cells than from apoptotic aneuploid cell [8].
Conclusion
In conclusion, the human embryo has a self-correction mechanism by expelling abnormal cells, which may be classified as a physiological phenomenon. However, additional studies are required to clarify the mechanisms underlying embryonic self-correction and identify the most effective approaches for preimplantation genetic testing. Therefore, an embryo with a higher chance of implantation and healthy development can be selected based on knowledge of its genetic makeup.
Availability of data and materials
The datasets used and analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- aCGH:
-
Array comparative genomic hybridization
- DNA:
-
Deoxyribonucleic acid
- MN:
-
Multinucleated
- NGS:
-
Next-generation sequencing
- niPGT-A:
-
Non-invasive preimplantation genetic testing aneuploidy
- PCR:
-
Polymerase chain reaction
- PGS:
-
Pre-implantation genetic screening
- SNP-array:
-
Single nucleotide polymorphism array
- TE:
-
Trophectoderm
References
Capalbo A, Poli M, Rienzi L, Girardi L, Patassini C, Fabiani M et al (2021) Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial. Am J Hum Genet 108(12):2238–2247. https://doi.org/10.1016/j.ajhg.2021.11.002
Coticchio G, Barrie A, Lagalla C, Borini A, Fishel S, Griffin D, Campbell A (2021) Plasticity of the human preimplantation embryo: developmental dogmas, variations on themes and self-correction. Hum Reprod Update 27(5):848–865. https://doi.org/10.1093/humupd/dmab016
Tobler KJ, Zhao Y, Ross R, Benner AT, Xu X, Du L et al (2015) Blastocoel fluid from differentiated blastocysts harbors embryonic genomic material capable of a whole-genome deoxyribonucleic acid amplification and comprehensive chromosome microarray analysis. Fertil Steril 104(2):418–425. https://doi.org/10.1016/j.fertnstert.2015.04.028
Sachdev NM, Maxwell SM, Besser AG, Grifo JA (2017) Diagnosis and clinical management of embryonic mosaicism. Fertil Steril 107(1):6–11. https://doi.org/10.1016/j.fertnstert.2016.10.006
McCoy RC (2017) Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet 33(7):448–463. https://doi.org/10.1016/j.tig.2017.04.001
Esfandiari N, Bunnell ME, Casper RF (2016) Human embryo mosaicism: did we drop the ball on chromosomal testing? J Assist Reprod Genet 33(11):1439–1444. https://doi.org/10.1007/s10815-016-0797-y
Wang X, Zhao J, Yao Z, Xia Q, Chang T, Zeng J et al (2023) Arrested cells/cellular debris expelled from blastocysts is self-correction phenomenon during early embryonic development. Reprod Sci 30(7):2177–2187. https://doi.org/10.1007/s43032-022-01159-8
Orvieto R, Shimon C, Rienstein S, Jonish-Grossman A, Shani H, Aizer A (2020) Do human embryos have the ability of self-correction? Reprod Biol Endocrinol 18(1):98. https://doi.org/10.1186/s12958-020-00650-8
Lagalla C, Coticchio G, Sciajno R, Tarozzi N, Zacà C, Borini A (2020) Alternative patterns of partial embryo compaction: prevalence, morphokinetic history and possible implications. Reprod Biomed Online 40(3):347–354. https://doi.org/10.1016/j.rbmo.2019.11.011
Balakier H, Sojecki A, Motamedi G, Librach C (2016) Impact of multinucleated blastomeres on embryo developmental competence, morphokinetics, and aneuploidy. Fertil Steril 106(3):608–614.e2. https://doi.org/10.1016/j.fertnstert.2016.04.041
McCoy RC, Demko ZP, Ryan A, Banjevic M, Hill M, Sigurjonsson S, Rabinowitz M, Petrov DA (2015) Evidence of selection against complex mitotic-origin aneuploidy during preimplantation development. PLoS Genet 11(10):e1005601. https://doi.org/10.1371/journal.pgen.1005601
Mertzanidou A, Spits C, Nguyen HT, Van de Velde H, Sermon K (2013) Evolution of aneuploidy up to Day 4 of human preimplantation development. Hum Reprod 28(6):1716–1724. https://doi.org/10.1093/humrep/det079
Lagalla C, Tarozzi N, Sciajno R, Wells D, Di Santo M, Nadalini M et al (2017) Embryos with morphokinetic abnormalities may develop into euploid blastocysts. Reprod Biomed Online 34(2):137–146. https://doi.org/10.1016/j.rbmo.2016.11.008
Shenoy CC, Bader A, Walker DL, Fredrickson JR, Weaver AL, Zhao Y (2023) Embryo blastomere exclusion identified in a time-lapse culture system is associated with embryo ploidy. Reprod Sci 30(6):1911–1916. https://doi.org/10.1007/s43032-022-01141-4
Acknowledgements
The authors would like to express gratitude to the Department of Obstetrics and Gynecology, Faculty of Medicine Universitas Indonesia, for supporting this study.
Funding
The authors receive no funding for this study.
Author information
Authors and Affiliations
Contributions
A.K.H.: conceptualization, methodology, validation, writing—review and editing, visualization, funding administration. A.F.A.: formal analysis, investigation, data curation, writing—original draft. A.S., N.U., A.R.P., P.N.: visualization, writing—review and editing. B.W.: supervision, writing—review and editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia, under No. KET-1856/UN2.F1/ETIK/PPM.00.02/2023.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harzif, A.K., Andyra, A.F., Sayogo, A. et al. Embryo response to aneuploidy through self-correction mechanism: a literature review. Middle East Fertil Soc J 29, 16 (2024). https://doi.org/10.1186/s43043-024-00176-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-024-00176-8