Abstract
Background
Endometriosis-related infertility is a disease associated with significant morbidity and distress to the couple and requires timely, multidisciplinary, and often high-cost care involving assisted reproductive technologies (ART). Many health care systems in the Middle East do not provide coverage for ART. This study aims to describe the reproductive outcome in a form of a clinical pregnancy rate in women with endometriosis-related infertility in a health care system that does not provide coverage for ART.
Results
This is a retrospective observational cohort study on women who attended the gynecology clinic in a tertiary center in Oman with the diagnosis of endometriosis from January 2011 to December 2019. Women of reproductive age seeking pregnancy were included in the analysis. Out of total women with endometriosis, (144/262) 55.0% were included in the analysis with a mean age of 31.10 ± 5.73 years. The mean duration of follow-up was 30.18 months and 43/144 (29.9%) of our patients had a follow-up > 60 months. Based on surgical staging, 11.8% had mild disease, 70.1% had moderate to severe disease and 18.1% were not operated. After a thorough assessment, (30.2%) were advised to seek in vitro fertilization (IVF) as a primary treatment for infertility but 23.08% declined the advice. Of the 144, 24.3% achieved a clinical pregnancy. (16/144), 11% conceived spontaneously. 11/144) 7.6% conceived with ovulation induction ± intrauterine insemination (OI ± IUI) and the rest conceived with a self-sponsored IVF. The overall clinical pregnancy rate was not statistically different between those who had surgery and those did not have surgery (P value 0.474). The pregnancy rate based on the management plan were; surgery + IVF (7/25, 28.0%), surgery + OI/IUI (10/47, 21.3%), surgery alone (9/33, 27.3%). The pregnancy rate was not statistically different between the groups (P value 0.782).
In addition to endometriosis, a significant proportion (63/144, 43.8%) of these women have a coexisting gynecologic morbidity including 2.1% non-endometrioma ovarian cyst, 13.9% myomas, 4.2% adenomyosis, 8.3% Mullerian anomalies, 2.1% polycystic ovary syndrome, 6.3% pelvic inflammatory disease or tubo-ovarian abscess and 1.4% biopsy-proven endometritis.
Conclusion
The reproductive outcome of patients with endometriosis in this study population was generally poorer than what is reported in the literature with an overall pregnancy rate of 24.3% and a spontaneous pregnancy rate of 11%. Several causes can be noted for such an outcome, including advanced disease stage, coexisting gynecologic morbidities, and poor access to advance fertility management.
Similar content being viewed by others
Background
Endometriosis is a chronic disease characterized by the presence of endometrial glands and stroma outside the uterine cavity [1, 2]. It is classified into three histological phenotypes; superficial or peritoneal, ovarian endometrioma, and deep infiltrating endometriosis [3]. Its reported prevalence vary between 5 and 15% depending on the studied population and the method of diagnosis [1, 2, 4,5,6,7]. The literature on the prevalence of endometriosis was summarized in a systematic review describing the overall prevalence to be 18% [8]. Women with endometriosis may be asymptomatic or may have symptoms of pain or infertility. The pain syndromes are of the variable spectrum of dysmenorrhea, dyspareunia, dyschezia, dysuria, and or chronic pelvic pain [9, 10]. The reported prevalence of endometriosis in women with chronic pelvic pain is 23% and with infertility is 31% [8]. In Middle Eastern women, the prevalence of endometriosis with subfertility is 24% and in women undergoing laparoscopy for any indication is 12.9% [6, 11].
As recognized globally, fertility is a very important determinant of health. WHO defines infertility as a “disease characterized by the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse”. It is a disease that generates disability [12, 13]. It is also a social stigma that often has a negative impact on women socially, mentally, and physically [13, 14]. In the Middle East countries, infertility is a significant social stigma and endometriosis-related infertility can be an excuse for a man to divorce a woman or for polygamy [13].
The presence of infertility in women with endometriosis is estimated to be 35 to 50%. In our population, 45.4% of women with endometriosis presented with infertility [15].
The chronicity of endometriosis and its recurrent nature requires a lifelong cost-effective approach for preserving reproductive capacity for women [16,17,18,19]. Managing endometriosis-related infertility is a challenge to the patients, the health care systems, and the scientific community. As evidence accumulated over time, there has been a noticeable shift in the recommendations for the management of women with endometriosis from surgery being the gold standard for diagnosis and management to become selective and prioritized if medical management failed [19]. ART is becoming the best option for the management of endometriosis-related infertility in cases of severe disease, deep infiltrating endometriosis, and low endometriosis fertility index [19].
These guidelines and practice trends are difficult to implement in an area like the Middle East which includes countries with different social and cultural contexts and health care systems. In these countries, the prevalence of endometriosis and endometriosis-related infertility are underestimated [2]. In addition to the cultural beliefs and social stigma, in many of these countries; infertility treatment is not prioritized as an important health issue, is not available in the public-funded health care services, and is not covered by health insurance; hence, access to ART for endometriosis patients is too limited.
The purpose of this study is to present real-world data on the reproductive outcome of women with endometriosis-related infertility in a Middle Eastern health care system that does not provide funding for ART in endometriosis-related fertility.
Methods
The sample
This is a retrospective observational cohort study on women who attended the gynecologic outpatient department at Sultan Qaboos University Hospital (SQUH) with endometriosis as a diagnosis over 9 years period from Jan 2011 to December 2019. This is a subgroup of our overall sample of endometriosis patients in the hospital described in Al Shukri et al. [15, 19]. SQUH is a tertiary referral center for primary and secondary health care institutions. The electronic medical record system of the hospital (TrackCare ®) mandates the treating gynecologist to enter the diagnosis for the international classification of Disease Version 10 (ICD-10). Hence, these women were identified by searching the system for the diagnosis of endometriosis as a keyword. The ethical approval for the study was granted by the Medical Research and Ethics Committee of the College of Medicine and Health Sciences at Sultan Qaboos University, Oman. Women with endometriosis and infertility were evaluated for endometriosis-related factors and assessed for surgical or medical management. The couple seeking fertility is further evaluated for ovarian reserve, uterine, tubal, and male factors. Those who were considered candidates for ovulation induction with or without intrauterine insemination were referred to the infertility team for assessment and further management. Those judged to be candidates for higher-level fertility management options like IVF/ICSI were advised to seek help in private health institutions that provide ART services and they were provided with the required documentation and reports to facilitate their care. Usually, those couples will seek the fertility center of their choice and at their own cost inside or outside the country. Women with poor ovarian reserve and candidates for surgical management are advised to seek fertility help by having ovulation induction and embryo freezing prior to the surgical management. Most of those women return for care after ART with or without pregnancy.
Data analysis
The data was collected and analyzed using Statistical Package for Social Sciences IBM- SPSS software, version 23. The sample demographics were presented with descriptive statistics. For continuous variables, mean and standard deviation were used to present the data. Levene’s test is used for continuous variables to test the difference in the mean when there is a significant difference in the size of the groups and they do not have a normal distribution.
For categorical variables, frequencies and percentages were reported. The chi-square test was used to test significance for categorical variables and a P value ≤ 0.05 was chosen for statistical significance.
Results
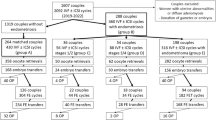
Out of the total number of women with endometriosis, (144/262) 55.0% were interested in pregnancy. For this analysis, we are including those in the reproductive age group as defined by the World Health Organization (15–45 years of age) with primary or secondary infertility and actively seeking infertility treatment. Figure 1 outlines the study population. For those who met these inclusion criteria, the youngest was 17 years of age and the eldest was 45 years with a mean age of 31.10 ± 5.73 years. Twenty-six entered our care, not in a relationship but interested in preserving fertility, and 11 of them got married and became actively seeking fertility treatment during follow-up so they were included in the primary infertility group to become 98 women. Of the 144 women, 68% had primary infertility, and 21.5% had secondary infertility. Of the 55.6% who attempted treatment, 22.5% got pregnant and about 44% of women though interested in pregnancy, they did not pursue treatment with us.
For these women, the mean age at first presentation to the clinic was 32.12 ± 7.25 years and a range of 15–45 years. There were 98 women in the primary infertility group with a mean age of 29.9 ± 5.28 years and a range of 17–45. In the secondary infertility group, there were 31 women, the mean age 33.32 ± 5.6, and the age range of 22–43. The mean duration of follow-up for these women was 30.18 months with a range of 0–92 months. Two women had a single visit to the clinic with no follow-up as they came for a second opinion. The follow-up duration was 6 months or less in 19.4%) of women and 29.9% of women had a follow-up duration of more than 5 years (> 60 months). Table 1 is showing the pregnancy rate in relation to the type of management patients received. The overall clinical pregnancy rate in these women was 34/144 (24.3%). Spontaneous conception occurred in 16 women, 11 got pregnant with OI ± IUI, and seven conceived with IVF in a private health institution in the country or abroad.
After a thorough assessment, 39/129 (30.2%) were advised to seek IVF as a treatment for infertility. However, 11/39 (28%) declined that advice and opted to have OI + IUI which was carried out for a maximum of five attempts. None of these women achieved a pregnancy. The remaining 28 women did seek IVF services and 7/28 (25%) of them achieved a pregnancy.
Patients with primary infertility were more likely to undergo fertility treatment 64/96, (65.3%) compared to a patient with secondary infertility 15/30, (48.4%) with a p value of 0.009.
Figure 2 shows the proportion of women who has surgery per stage of disease. When it came to surgical intervention for those interested in pregnancy, 80.6% (116/144) had a fertility-preserving surgical intervention for endometriosis in the past. This surgical intervention was either at our center or before the presentation to our center. Twenty-eight women (19.4) did not have a surgical intervention for endometriosis. We have been selective in surgical intervention in our center and those who did not have surgery were they had their initial diagnosis of endometriosis at our center and continued to follow with us.
Table 2 shows the pregnancy rate per revised American Society Of Reproductive Medicine (rASRM) stage. The was no statistical difference between the different stages in pregnancy rate with a p value of 0.154. There was no difference in the mean age for those who were operated 31.01 years (± 5.18), and not 31.14 operated (± 7.72) for endometriosis. Also, the difference in the pregnancy rate between those who had surgery (26/116, 22.4%) and those who did not have surgery (8/28, 28.6%) was not significant with a p value of 0.474.
The pregnancy rate in the group of surgery and IVF was 7/25 (28.0%) in the group who had surgery and OI/IUI was 10/47 (21.3%) and in those who had surgery alone was 9/33 (27.3%). There is no significant difference between the groups with a p value of 0.782.
A significant proportion (63/144) 43.8% of those interested in pregnancy had another gynecologic morbidity as detailed in Table 3.
Discussion
In women with pelvic endometriosis aged 15–45 years, the pregnancy rate was 24.3% overall, spontaneous conception rate was 11.0%, 32.2% pregnancy was with OI ± IUI, and 25.0% pregnancy with ART. Our patients’ pregnancy rate is low compared to other endometriosis populations reported in the literature, overall, and in the subgroups of the type of management they received. The spontaneous pregnancy rate following surgery alone was reported to be 37.4% by Coccia and 40% by Vidal et al. [20, 21]. It is 27.3% of our study population. It is also reported that the rate of spontaneous pregnancy was significantly higher in the first 6 months following the surgical intervention compared to the later intervals [16, 22]. Our lower rate is can be explained by several reasons. The majority of our patients were having severe disease, with 71.6% rASRM stage III/IV. Many of the patient population in the study had surgical intervention outside our center and from our knowledge of the local and regional medical environment, we believe that the surgical intervention the patients had was of variable quality. They underwent surgery either in Oman or abroad and with variable levels of surgical expertise in moderate to severe endometriosis. Also, some women were advised ART, advice that they have declined.
Laparoscopic surgical intervention for endometriosis has been recommended as the gold standard for the management of endometriosis [17]. The proposed benefits are removal of visible disease lesions decreases disease burden and so the related inflammatory mediator [23].It restores the pelvic anatomy which results in improved endometriosis-related pain symptoms, and may improve sexual function and frequency of sexual relations [21]. Restoration of pelvic anatomy plays a role in tubal factor related to endometriosis [17, 24]. In 2020, a Cochrane review of randomized controlled trials for the treatment of endometriosis-related pain and infertility showed moderate quality evidence that laparoscopic intervention increases the chance of spontaneous pregnancy [21]. For patients planned for ART, surgical intervention prevented the risk of cyst rupture, allows transvaginal assessment of ovarian follicles, and decreases the difficulty of ovum pick-up. It is also reported that pathological examination of the removed endometriomas shows malignancy at a rate of 0.7% [25].
For those who had surgical intervention followed by OI ± IUI, the pregnancy rate was 21.3% in our group of patients. A study from the Cleveland clinic reported a pregnancy rate of 10% for severe disease (rASRM III/IV) with OI + IUI [23]. The role of OI + IUI in the management of endometriosis-related infertility did not have a significant focus in the literature compared to other treatment modalities. Also, studies had contradicting results. Some studies showed that OI + IUI increased the live birth rate significantly [26, 27]. For women with moderate to severe endometriosis following a surgical intervention and having at least one patent tube, the reported pregnancy rate is 40% [28]. However, the aforementioned study from the Cleveland clinic, compared the fertility outcome for 2 subsets of patients, the mild disease (rASRM I/II) and the more severe disease (rASRM III/IV). The spontaneous pregnancy rate in stage I/II was 45% and 42% with OI + IUI, and in stage III/IV was 20% for spontaneous pregnancy rate and 10% for OI + IUI [23]. In both subsets of disease severity, OI + IUI did not improve the pregnancy rates compared to the chance of spontaneous pregnancy. The most widely quoted guidelines for the management of endometriosis-related infertility; European Society of Human Reproduction and Embryology (ESHRE) in their most recent update (ESHRE 2022) is endorsing the use of OI + IUI for mild disease compared to expectant management as it improves the chances of a pregnancy [19]. However, they do acknowledge that its role in severe disease with patent tubes is controversial, and they leave that to the discretion of the treating team and the couple for it to be considered [19].
In our study, the combined approach laparoscopy-IVF resulted in a pregnancy rate of 28.0%. It is reported that the integrated laparoscopy-IVF treatment approach achieved a pregnancy rate of 56.1% [20, 21]. Currently, IVF is considered the most effective treatment for endometriosis-related infertility [18, 24]. There are two major issues with this treatment approach. The first is the availability and affordability of IVF in a country. Unlike in many European countries; in many health care systems including our system, at the time of writing this article, ART is not provided in any government health care institution, nor is sponsored by the government for any indication except for pre-implantation genetic diagnosis (IVF-PDG) for proven genetic disease. It is also not covered by any health insurance company. This places a significant financial burden on the couple and many cannot afford it [29,30,31]. This results in delays in seeking timely and appropriate treatment resulting in decreasing chances of pregnancy even if they pursue IVF later. This also might explain the couple resorting to desperate measures of attempting OI + IUI when it is not the best choice. The other issue with IVF is that our community like any other; has its own set of traditional beliefs around childlessness and treatment of infertility[14, 32]. Like in many cultures, there is difficulty accepting the diagnosis of infertility, there is a lack of awareness resulting in the assumption that IVF babies are unnatural, seeking IVF is a social stigma and there is difficulty accepting it as a first-line treatment without spending a long time trying for spontaneous pregnancy or trying other less invasive measures like IUI [13, 14]. There is also the IVF-associated emotional strain, cultural myths, social stigma, and moral, and ethical dilemmas associated with it that might make the couple avoid it or drop out from treatment [14, 33, 34]. Secondary infertility usually is in older women, who have difficulty accepting the fact that they need help to get pregnant because they have conceived spontaneously previously.
As described in Table 2, 43.8% of our study population of women with endometriosis seeking fertility had a co-existing gynecologic condition that has also an impact on the reproductive outcome. These conditions are also common among women in general. Endometriosis, adenomyosis, uterine myomas, PCOS, premature ovarian insufficiency, endometritis, Mullerian anomalies and endometrial hyperplasia are all associated with compromised fertility potential. Endometriosis, adenomyosis, uterine myomas, PCOS, and endometrial hyperplasia are characterized as estrogen-dependent conditions that affect the reproductive tract of women in the reproductive age group [35]. Endometriosis, adenomyosis, and uterine myomas may present in the same woman [36]. They were also likely to share environmental, genetic, dietary, and inflammatory factors that play a role in their development [36]. Although there is no hypo- or hyper- secretion of estrogen in these disorders, hypersensitivity of estrogen receptors with genetic predisposition is proposed to play a significant role and they respond similarly upon treatment with GnRH agonist [36,37,38].
The risk of endometriosis was significantly increased in women with uterine leiomyoma to about 3-folds [39]. The literature describes that myomas are present in 12 to 20% of women with endometriosis. Twenty percent of women undergoing surgery for uterine myomas are found to have a concomitant endometriosis [40]. Women with endometriosis being evaluated by ultrasound found that 3.1% had uterine myomas, 21.2% had adenomyosis and 14.6% had the 3 conditions of endometriosis, adenomyosis, and uterine myomas [41]. The coexistence of endometriosis and uterine myoma has significant surgical and reproductive implications.
Adenomyosis is considered to be a closer relative of endometriosis in that they both share the pathology of the existence of endometrial glands and stroma outside the endometrial cavity [42]. They do frequently co-exist especially deep infiltrating endometriosis and adenomyosis of the outer myometrium in more than 50%women [43,44,45,46,47]. We do strongly believe that adenomyosis is underdiagnosed in our group of endometriosis patients which reflects the clinical ignorance of the disease and the lack of agreed criteria for diagnosis [48].
In our group of endometriosis patients, 8.1% of them had associated Mullerian anomalies. The strong association between endometriosis and Mullerian anomalies is well established in gynecologic history as it is the bases for retrograde menstruation theory for the development of endometriosis [36, 49].
There is an element of inflamed endometrium seen in endometriosis, adenomyosis and chronic endometritis. This inflammation affects endometrial receptivity resulting in infertility or recurrent pregnancy loss [50, 51]. Literature also shows an association between endometriosis and chronic endometritis where in about 52.9% of women with endometriosis, there is histological evidence of chronic endometritis [52]. The cause of endometritis in endometriosis is not fully understood and it might be independent pathology [52]. However, some speculated that some humoral and cellular factors produced by the endometriosis can be transmitted to the endometrial cavity through the fallopian tubes resulting in an inflamed environment [50, 52].
There was 6.8% of our endometriosis patients had concomitant PID/TOA. Lin K et al. in a study from Taiwan demonstrated that tubo-ovarian infection is a significant comorbidity in patients with endometriosis with an adjusted hazards ratio of 2.86 compared to patients without endometriosis [53]. Studies also suggested that tubo-ovarian abscesses occur not only more often, but also more severe in patients with endometriosis compared to those without endometriosis [51]. Plausible mechanisms are that endometriosis is associated with changes in the immunological environment resulting in impaired ability to clear infection, the blood content of the endometrioma is an ideal culture medium for bacterial growth, and endometriosis is associated with an increased risk of bacterial contamination increasing the risk of PID [53].
Endometriosis and PCOS co-existed in 2.1% of our study population. The association between these 2 common disorders has been described for a long time [54]. Endometriosis is present in 11.8–16.5% of women with PCOS [54, 55]. Women with PCOS have 2.86 times the risk of endometriosis compared to women without PCOS. Mostly, these cases are diagnosed by laparoscopy and both are recognized causes of infertility [56].
The strengths of this study are that it provides real-world pooled reproductive outcome data of patients with endometriosis in a setting with limited access to IVF. The management and outcomes in this study are not optimized for research but reflect a clinical set-up. It also provides important information over a significant follow-up period. This information is much needed for the physicians involved in the care of women with endometriosis and health policy makers taking into consideration the presence of other gynecologic morbidities with endometriosis. However, the fact that it is a retrospective study is a significant limitation. Although the total sample size is adequate, the number of patients for subgroup analysis of treatment methods or gynecologic comorbidities is small and not sufficient to draw confirmatory conclusions. The results are also biased towards gloomier results than what is reported in the literature reflecting the fact that these are more severe cases, failed management of symptoms in another institution, and many had multiple surgical interventions.
In conclusion, the reproductive outcome of patients with endometriosis in this study is generally poorer than what is reported in the literature with an overall pregnancy rate of 24.3% and a spontaneous pregnancy rate of 11.2%. Several causes can be noted for such an outcome, most of these patients have severe disease, and do not have a timely access to advanced fertility treatment. Also, a significant number of these women with endometriosis (43.8%) have co-existing gynecologic morbidity that is likely to play a role in impairing fertility.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Missmer SA (2004) Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 160:784–796. https://doi.org/10.1093/aje/kwh275
Mousa, M., Al-Jefout, M., Alsafar, H., Becker, C.M., Zondervan, K.T. and Rahmioglu, N. (2021) Impact of endometriosis in women of Arab ancestry on: health-related quality of life, work productivity, and diagnostic delay. Frontiers in global women’s health, Frontiers Media SA, 2, 708410. https://doi.org/10.3389/fgwh.2021.708410
Chapron, C., Pietin-Vialle, C., Borghese, B., Davy, E., Foulot, H.E. and Chopin, N. Endometriosis associated ovarian endometrioma is a marker for greater severity of deeply infiltrating endometriosis. https://doi.org/10.1016/j.fertnstert.2008.06.003
Houston DE (1984) Evidence for the risk of pelvic endometriosisi by age, race and socioecomomic status. Epidemiol Reviews Oxford Academic 6:167–191. https://doi.org/10.1093/OXFORDJOURNALS.EPIREV.A036270
De Oliveira R, Adami F, Mafra FA, Bianco B, Vilarino FL, Barbosa CP (2016) Causes of endometriosis and prevalent infertility in patients undergoing laparoscopy without achieving pregnancy. Minerva Ginecol 68:250–258
Mousa M, Al-Jefout M, Alsafar H, Kirtley S, Lindgren CM, Missmer SA, Becker CM, Zondervan KT, Rahmioglu N (2021) Prevalence of common gynecological conditions in the Middle East: systematic review and meta-analysis. Front Reprod Health Frontiers 3:7. https://doi.org/10.3389/frph.2021.661360
Burney RO, Giudice LC (2012) Pathogenesis and pathophysiology of endometriosis. Fertil Steril 98:511–519. https://doi.org/10.1016/j.fertnstert.2012.06.029
Moradi, Y., Shams-Beyranvand, M., Khateri, S., Gharahjeh, S., Tehrani, S., Varse, F., Tiyuri, A. and Najmi, Z. (2021) A systematic review on the prevalence of endometriosis in women. Indian Journal of Medical Research, Wolters Kluwer -- Medknow Publications, 154, 446. https://doi.org/10.4103/ijmr.IJMR_817_18
Nnoaham KE, Hummelshoj L, Webster P, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT, Carmona F, Kennedy S (2011) Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries Europe PMC Funders Group. Fertil Steril 96:366–373. https://doi.org/10.1016/j.fertnstert.2011.05.090
Sulaiman M, Al-Farsi Y, Al-Khaduri M, Saleh J, Waly M (2018) Polycystic ovarian syndrome is linked to increased oxidative stress in Omani women. Int J Womens Health Dove Medical Press Ltd 10:763–771. https://doi.org/10.2147/IJWH.S166461
Darwish, A., Hassanin, M.S. and Sekkin, A. (2006) Epidemiology and risk factors associated with laparoscopically diagnosed typical and atypical endometriosis among Egyptian women. Middle East Fertility Society Journal, 11, 196–201. http://www.bioline.org.br/pdf?mf06033
Zegers-Hochschild, F., Adamson, G.D., Dyer, S., Racowsky, C., de Mouzon, J., Sokol, R., Rienzi, L., Sunde, A., Schmidt, L., Cooke, I.D., Simpson, J.L. and van der Poel, S. (2017) The international glossary on infertility and fertility care, 2017. Fertility and Sterility, Elsevier Inc., 108, 393–406. https://doi.org/10.1016/J.FERTNSTERT.2017.06.005
Borght, M. Vander and Wyns, C. (2018) Fertility and infertility: definition and epidemiology. https://doi.org/10.1016/j.clinbiochem.2018.03.012
Okafor, N.I., Joe-Ikechebelu, N.N. and Ikechebelu, J.I. (2017) Perceptions of infertility and in vitro fertilization treatment among married couples in Anambra State, Nigeria. African Journal of Reproductive Health, 21, 55–66. https://doi.org/10.29063/ajrh2017/v21i4.6
Al Shukri, M., Al Riyami, A.S., Al Ghafri, W. and Gowri, V. (2022) Based on clinical profile: are there predictors of earlier diagnosis of endometriosis? A retrospective study in Oman. Oman Medical Journal. https://doi.org/10.5001/omj.2023.35
Vatsa R, Sethi A (2021) Impact of endometriosis on female fertility and the management options for endometriosis-related infertility in reproductive age women: a scoping review with recent evidences. Fertil Soc J 26:36. https://doi.org/10.1186/s43043-021-00082-3
Coccia ME, Nardone L, Rizzello F (2022) Endometriosis and infertility: a long-life approach to preserve reproductive integrity. Int J Environ Res Public Health 19:6162. https://doi.org/10.3390/IJERPH19106162. (MDPI AG)
ESHRE Endometriosis Guideline Development Group. (2022) Endometriosis: Guideline of European Society of Human Reproduction and Embryology. https://www.eshre.eu/guidelines
Becker, C.M., Bokor, A., Heikinheimo, O., Horne, A., Jansen, F., Kiesel, L., King, K., Kvaskoff, M., Nap, A., Petersen, K., Saridogan, E., Tomassetti, C., van Hanegem, N., Vulliemoz, N., Vermeulen, N. and Group, E.E.G. (2022) Endometriosis: European Society of Human Reproduction and Emberyology. Human Reproduction Open, hoac009. https://doi.org/10.1093/hropen/hoac009
Vidal, F., Guerby, P., Simon, C., Lesourd, F., Cartron, G., Parinaud, J., Tanguy le Gac, Y. and Dupuis, N. (2021) Spontaneous pregnancy rate following surgery for deep infiltrating endometriosis in infertile women: the impact of the learning curve. Journal of Gynecology Obstetrics and Human Reproduction, Elsevier Masson, 50, 101942. https://doi.org/10.1016/J.JOGOH.2020.101942
Bafort, C., Beebeejaun, Y., Tomassetti, C., Bosteels, J. and Duffy, J.M.N. (2020) Laparoscopic Surgery for Endometriosis. The Cochrane database of systematic reviews, Cochrane Database Syst Rev, 10. https://doi.org/10.1002/14651858.CD011031.PUB3
Bulletti C, Coccia ME, Battistoni S, Borini A (2010) Endometriosis and Infertility. J Assist Reprod Genet 27:441–447. https://doi.org/10.1007/s10815-010-9436-1
Gandhi AR, Carvalho LF, Nutter B, Falcone T (2014) Determining the fertility benefit of controlled ovarian hyperstimulation with intrauterine insemination after operative laparoscopy in patients with endometriosis. J Minim Invasive Gynecol 21:101–108. https://doi.org/10.1016/J.JMIG.2013.07.009. (Elsevier)
Mon Khine, Y., Fuminori Taniguchi, • and Harada, • Tasuku. Clinical Management of Endometriosis-Associated Infertility. https://doi.org/10.1007/s12522-016-0237-9
Kobayashi H, Sumimoto K, Moniwa N, Imai M, Takakura K, Kuromaki T, Morioka E, Arisawa K, Terao T (2007) Risk of developing Ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan. Int J Gynecol Cancer 17:37–43. https://doi.org/10.1111/j.1525-1438.2006.00754.x. (England)
Tummon, I.S., Asher, L.J., Martin, J.S.B. and Tulandi, T. (1997) Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertility and Sterility, Elsevier Inc., 68, 8–12. https://doi.org/10.1016/S0015-0282(97)81467-7
Nulsen JC, Walsh S, Dumez S, Metzger DA (1993) A randomized and longitudinal study of human menopausal gonadotropin with intrauterine insemination in the treatment of infertility. Obstet Gynecol 82:780–786 (United States)
Van Der Houwen LEE, Schreurs AMF, Schats R, Heymans MW, Lambalk CB, Hompes PGA, Mijatovic V (2014) Efficacy and safety of intrauterine insemination in patients with moderate-to-severe endometriosis. Reprod Biomed Online 28:590–598. https://doi.org/10.1016/J.RBMO.2014.01.005. (Elsevier)
Holoch KJ, Lessey BA (2010) Endometriosis and infertility. Clin Obstet Gynecol 53:429–438. https://doi.org/10.1097/GRF.0b013e3181db7d71. (United States)
Roeder C, Lukyanov V, de Agustin Calvo E, Schwarze J (2022) POSC104 determining cost data for fertility treatment in a Spanish setting. Value Health 25:S107. https://doi.org/10.1016/J.JVAL.2021.11.509. (Elsevier BV)
Shahin, A. and Shahin, A.Y. (2007) The problem of IVF cost in developing countries: has natural cycle IVF a place? 15, 51–56. https://doi.org/10.1016/S1472-6483(10)60691-8
Sharma, R.S., Saxena, R. and Singh, R. (2018) Infertility & assisted reproduction: a historical & modern scientific perspective. The Indian Journal of Medical Research, Wolters Kluwer -- Medknow Publications, 148, S10. https://doi.org/10.4103/IJMR.IJMR_636_18
Patel, A., Sharma, P.S.V.N. and Kumar, P. (2018) Role of mental health practitioner in infertility clinics: a review on past, present and future directions. https://doi.org/10.4103/jhrs.JHRS_41_18
Burns LH (2007) Psychiatric aspects of infertility and infertility treatments. Psychiatr Clin North Am 30:689–716. https://doi.org/10.1016/j.psc.2007.08.001
Verit FF, Yucel O (2013) Endometriosis, leiomyoma and adenomyosis: the risk of gynecologic malignancy. Asian Pac J Cancer Prev 14:5589–5597. https://doi.org/10.7314/APJCP.2013.14.10.5589
Petraglia F, Musacchio C, Luisi S, De Leo V (2008) Hormone-dependent gynaecological disorders: a pathophysiological perspective for appropriate treatment. Best Pract Res Clin Obstet Gynaecol 22:235–249. https://doi.org/10.1016/J.BPOBGYN.2007.07.005
Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, Petraglia F (2016) Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update 22:104–115. https://doi.org/10.1093/HUMUPD/DMV044
Khan, K.N., Kitajima, M., Hiraki, K., Fujishita, A., Nakashima, M., Ishimaru, T. and Masuzaki, H. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. https://doi.org/10.1093/humrep/deq240
Lin, K.Y.H., Yang, C.Y., Lam, A., Chang, C.Y.Y. and Lin, W.C. (2021) Uterine leiomyoma is associated with the risk of developing endometriosis: a nationwide cohort study involving 156,195 women. PLoS ONE, Public Library of Science, 16. https://doi.org/10.1371/JOURNAL.PONE.0256772
Maclaran K, Agarwal N, Odejinmi F (2014) Co-existence of uterine myomas and endometriosis in women undergoing laparoscopic myomectomy: risk factors and surgical implications. J Minim Invasive Gynecol 21:1086–1090. https://doi.org/10.1016/J.JMIG.2014.05.013. (Elsevier)
Capezzuoli T, Vannuccini S, Fantappiè G, Orlandi G, Rizzello F, Coccia ME, Petraglia F (2020) Ultrasound findings in infertile women with endometriosis: evidence of concomitant uterine disorders. Gynecol Endocrinol 36:808–812. https://doi.org/10.1080/09513590.2020.1736027. (Taylor and Francis Ltd)
Just PA, Moret S, Borghese B, Chapron C (2021) Endométriose et Adénomyose. Ann Pathol 41:521–534. https://doi.org/10.1016/J.ANNPAT.2021.03.012. (Elsevier Masson)
Maruyama S, Imanaka S, Nagayasu M, Kimura M, Kobayashi H (2020) Relationship between adenomyosis and endometriosis; different phenotypes of a single disease? Eur J Obstet Gynecol Reprod Biol 253:191–197. https://doi.org/10.1016/J.EJOGRB.2020.08.019. (Elsevier)
Bourdon M, Santulli P, Oliveira J, Marcellin L, Maignien C, Melka L, Bordonne C, Millisher AE, Plu-Bureau G, Cormier J, Chapron C (2020) Focal adenomyosis is associated with primary infertility. Fertil Steril 114:1271–1277. https://doi.org/10.1016/J.FERTNSTERT.2020.06.018
Pinzauti S, Lazzeri L, Tosti C, Centini G, Orlandini C, Luisi S, Zupi E, Exacoustos C, Petraglia F (2015) Transvaginal sonographic features of diffuse adenomyosis in 18–30-year-old nulligravid women without endometriosis: association with symptoms. Ultrasound Obstet Gynecol 46:730–736. https://doi.org/10.1002/UOG.14834. (John Wiley and Sons Ltd)
Leyendecker G, Bilgicyildirim A, Inacker M, Stalf T, Huppert P, Mall G, Böttcher B, Wildt L (2015) Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI Study. Arch Gynecol Obstet 291:917–932. https://doi.org/10.1007/S00404-014-3437-8/FIGURES/12. (Springer Verlag)
Loring M, Chen TY, Isaacson KB (2021) A systematic review of adenomyosis: it is time to reassess what we thought we knew about the disease. J Minim Invasive Gynecol 28:644–655. https://doi.org/10.1016/J.JMIG.2020.10.012
Chapron C, Tosti C, Marcellin L, Bourdon M, Lafay-Pillet MC, Millischer AE, Streuli I, Borghese B, Petraglia F, Santulli P (2017) Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod 32:1393–1401. https://doi.org/10.1093/HUMREP/DEX088. (Oxford University Press)
Olive DL, Henderson DY (1987) Endometriosis and Mullerian anomalies. Obstet Gynecol 69:412–415 (United States)
Pirtea P, Cicinelli E, De Nola R, de Ziegler D, Ayoubi JM (2021) Endometrial causes of recurrent pregnancy losses: endometriosis, adenomyosis, and chronic endometritis. Fertil Steril 115:546–560. https://doi.org/10.1016/J.FERTNSTERT.2020.12.010. (Elsevier)
de Ziegler D, Pirtea P, Ayoubi JM (2019) Inflammation and uterine fibrosis: the possible role of chronic endometritis. Fertil Steril 111:890–891. https://doi.org/10.1016/J.FERTNSTERT.2019.02.005
Takebayashi, A., Kimura, F., Kishi, Y., Ishida, M., Takahashi, A., Yamanaka, A., Takahashi, K., Suginami, H. and Murakami, T. (2014) The association between endometriosis and chronic endometritis. PloS one, PLoS One, 9. https://doi.org/10.1371/JOURNAL.PONE.0088354
Gao, Y., Qu, P., Zhou, Y. and Ding, W. (2020) Risk factors for the development of tubo-ovarian abscesses in women with ovarian endometriosis: a retrospective matched case-control study.https://doi.org/10.1186/s12905-021-01188-6
Singh KB, Patel YC, Wortsman J (1989) Coexistence of polycystic ovary syndrome and pelvic endometriosis. Obstet Gynecol 74:650–652. https://doi.org/10.1111/j.1447
Kichukova, D. (1996) Polycystic ovaries in association with pelvic endometriosis in infertile women diagnosed by laparoscopy. Folia Med (Plovdiv), 38, 71–3. https://pubmed.ncbi.nlm.nih.gov/9145594/
Schwartz CL, Christiansen S, Vinggaard AM, Axelstad M, Hass U, Svingen T (2019) Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch Toxikol 93:253–272. https://doi.org/10.1007/S00204-018-2350-5. (Springer Verlag)
Funding
This is a non-funded project.
Author information
Authors and Affiliations
Contributions
MA: data collection, data analysis, manuscript writing. SA: data collection. WA: originated the idea, manuscript editing. VG: brainstorming the manuscript ideas and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study is approved by the research and ethics committee of the college of medicine and biomedical sciences at Sultan Qaboos University. MREC # 1696.
Consent for publication
Consent from individual participants was not required.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al Shukri, M.N., Al Riyami, A.S.A., Al Ghafri, W.M. et al. Reproductive outcome and gynecologic comorbidities in women with endometriosis in a non-IVF setting: a retrospective study. Middle East Fertil Soc J 28, 17 (2023). https://doi.org/10.1186/s43043-023-00141-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-023-00141-x