Abstract
Background
Determining the initiation day of antagonist administration is an important and challenging issue and different results have been reported in the previous studies. The present study was designed to compare the controlled ovarian hyperstimulation (COH) cycles outcomes of early-onset gonadotropin-releasing hormone antagonist (GnRH-ant) protocol with conventional flexible GnRH-ant protocol in patients with poor ovarian response (POR) diagnosis. This randomized clinical trial was performed on infertile women who were diagnosed as poor responders in in vitro fertilization/intra-cytoplasmic sperm injection (IVF/ICSI) cycles at Arash Women’s Hospital affiliated to Tehran University of Medical Sciences. POR was defined according to the Bologna criteria and the eligible women were randomly allocated into an experimental (early-onset GnRH-ant) and control (conventional flexible GnRH-ant) groups. The women in the experimental group received recombinant gonadotropins (150–225 IU) and GnRH-ant (0.25 mg) simultaneously on the second day of the cycle. In the control group, the starting and the dose of gonadotropins were similar but daily administration of GnRH-ant was initiated when the leading follicle diameter was ≥ 13 mm. The COH outcomes were compared between groups (n=58 in each group).
Results
The analysis showed that the two groups did not have statistically significant differences in terms of the ovarian stimulation duration and the total dose of used gonadotropins. The total number of metaphase II (MII) oocytes in the experimental group was significantly higher than that of in control group (P = 0.04). Moreover, clinical and ongoing pregnancy rates per embryo transfer (ET) in the experimental group were significantly higher than those in the control group (P = 0.02 and P = 0.03, respectively); however, the implantation and miscarriage rates were similar between groups.
Conclusions
The early-onset GnRH-ant protocol can improve the number of retrieved and MII oocytes and probably the pregnancy outcomes after fresh embryo transfer in POR patients. However, larger randomized clinical trials are required to compare the pregnancy outcomes after this approach with other COH protocols with considering cost-effectiveness issue.
Trial registration
The name of the registry: Ladan Kashani.
The date of trial registration: 8.02.2020.
Similar content being viewed by others
Background
Despite the large number of studies on poor ovarian response (POR) in assisted reproduction (ART) cycles in the last 20 years, there is still debate in determining the best and most effective protocol for controlled ovarian stimulation (COS) in patients with POR. Among the various COS methods, the administration of the gonadotropin-releasing hormone antagonist (GnRH-ant) regimen in poor responders has had numerous benefits such as decreased stimulation duration, decreased the total amount of gonadotropin required, no symptoms of hormonal withdrawal, and no ovarian cyst formation [1,2,3]. In contrast to treatment by GnRH agonist, the main advantage of using GnRH-ant in the treatment of poor responders is that it prevents the premature surge of luteinizing hormone (LH) with preserving pituitary gland responsiveness [2, 4].
Based on the initiation day of administration, the GnRH-ant protocol could be divided into early onset (before day 6 of stimulation) and late onset (after day 6 of stimulation) [5, 6]. Early follicular administration of GnRH-ant has been reported to decrease exposure to LH and estradiol during controlled ovarian stimulation, negatively affecting the chance of pregnancy [4]. Moreover, the inhibition of the interphase peak of the follicle-stimulating hormone (FSH) by administering the antagonist at the beginning of the menstrual cycle leads to a better synchronization of the growth of the follicle cohort and consequently increases the yield of oocytes [4, 7, 8].
Determining the initiation day of antagonist administration is an important and challenging issue and different results have been reported in this regard in previous studies [5, 9,10,11]. In a flexible antagonist protocol, Inal et al. (2017) showed that the early initiation of the antagonist was more cost-effective regarding the number of the used gonadotropins and the number of the stimulation days [5].
Considering the limited number of clinical trial studies in this field, the present study was designed as a randomized clinical trial to compare the effects of the early onset of GnRH antagonist protocol with those of the flexible GnRH antagonist protocol on the outcomes of in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) cycles in patients with POR diagnosis.
Methods
This randomized controlled trial was performed on infertile women with POR diagnosis who underwent IVF/ICSI cycles at Arash Women’s Hospital affiliated to Tehran University of Medical Sciences between March 2020 and December 2020. The trial protocol was approved by the Review Boards and Ethics Committee of Tehran University of Medical Sciences (ethics reference number: IR.TUMS.MEDICINE.REC.1398.544) and was registered in Iranian Registry of Clinical Trials Website (www.irct.ir, IRCT20110731007165N9). The women with POR diagnosis undergoing IVF/ICSI cycles were determined on the basis of the Bologna criteria [12] and the existence of at least two of the following criteria: (1) the previous history of POR (retrieved oocytes ≤ 3) in a conventional stimulation protocol, (2) advanced maternal age (≥40 years) or any other risk factors for POR (e.g., a history of ovarian surgery), and( 3) abnormal ovarian reserve test (i.e. antral follicle count (AFC) < 5 follicles or anti-Mȕllerian hormone (AMH) < 1.1 ng/ml). The patients with age over 44 years, uterine factor and/or severe male factor infertility, hypothalamic amenorrhea, chronic diseases, history of recurrent miscarriage, and repeated implantation failure were excluded from the study. The eligible patients were randomly allocated into two groups: an experimental (early-onset GnRH-ant) and control (flexible GnRH-ant) group. The permuted block randomization was conducted by the statistician advisor with a computer-generated list. The type of treatment was placed in sealed envelopes and the assignment to intervention and control groups was performed by the out-of-study nurse. In addition, signed informed consents were obtained from all patients before the intervention.
Controlled ovarian hyperstimulation (COH) was performed using the recombinant follicle-stimulating hormone FSH (rFSH) (Gonal-F, Merck-Serono) and human menopausal gonadotropin (hMG; Menopur; Ferring). In both study groups serial two-dimensional follicle monitoring by transvaginal ultrasonography (Philips Affiniti 70 machine with a C10-3v Pure-Wave endovaginal probe) and hormonal assay (as needed) were performed.
The women in the experimental group received rFSH (150–225 IU) and GnRH-ant (0.25 mg) (Cetrotide: Merck-Serono) simultaneously on the second day of the cycle. The patients in the control group received daily injections of recombinant FSH (150–225 IU) from day 2 of the cycle and the GnRH-ant (Cetrotide: 0.25 mg daily) was initiated when the leading follicle diameter was ≥ 13 mm. In both groups, when at least two dominant follicles with 17 mm or greater in diameter were observed in ultrasound monitoring, the final oocyte maturation was triggered by human chorionic gonadotropin (hCG) (10,000 IU, Choriomon, IBSA). The serum estradiol, progesterone, and LH levels were measured at two points: baseline assessment (day 1 or 2 of menstrual cycle) and hCG administration day.
The ovarian puncture was carried out 34–36 h after hCG injection and IVF/ICSI process was then applied in accordance with our standards clinical procedures. The embryos were graded as proposed by Cummins et al. [13]. This classification considers different features such as the number of blastomeres, the degree of fragmentation, multinucleation, and the symmetry of the blastomeres on the third day after oocyte retrieval. On the basis of the women’s age and embryos quality, up to three embryos were transferred at the cleavage stage (day 3 after ovum pickup). Luteal phase was supported by using vaginal progesterone suppositories 400 mg BID (Cyclogest®, Actoverco, Iran) daily for 14 days until to pregnancy test day. In the case of the positive pregnancy, vaginal progesterone was administrated until 10 weeks of gestation.
The main outcomes were the total number of the retrieved and metaphase II (MII) oocytes, the implantation rate (the number of observed gestational sacs divided by the number of embryos transferred for each patient), clinical pregnancy (the presence of a gestational sac with fetal heart beat on vaginal ultrasound), early miscarriage (spontaneous loss of a clinical pregnancy ≤ 12 weeks of gestation), and ongoing pregnancy (pregnancies continued more than 12 weeks after ET).
Statistical analysis was done using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) version 23.0. The comparisons of continuous variables between groups were provided by Student’s t test for parametric data and by the Mann–Whitney U test for non-parametric data. and presented as mean ± standard deviation (SD). The chi-square test was used for comparing the categorical variables between groups. The statistical significance level was considered at p value < 0.05.
Results
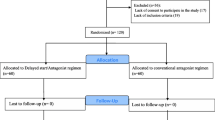
The study subjects’ sampling flow chart according to the Consolidated Standards of Reporting Trials (CONSORT) guideline was presented in Fig. 1. During the study period, 192 infertile patients were evaluated for participation in the study. Of these, 34 women were excluded due to non-eligibility for entering the study and a total of 158 women were randomly assigned to the experimental group (n = 84) and control group (n = 74). After follow-up, the cycle outcomes of 58 patients in the experimental group and 58 patients in the control group were compared. The baseline characteristics of the patients were shown in Table 1. No statistically significant differences were found between groups regarding the age of women, BMI, cause and duration of infertility, total antral follicle count, and the baseline hormone levels.
The comparison of the COH cycle and pregnancy outcomes between groups was presented in Table 2. The analysis showed that the two groups did not have statistically significant differences in terms of the ovarian stimulation duration and the total dose of used gonadotropins. Moreover, the total number of MII oocytes in the experimental group was significantly higher than that of in control group (P=0.04). In addition, there were no significant differences in the number of transferred embryos and endometrial thickness on ET day between groups. In the following, clinical and ongoing pregnancy rates per ET in the experimental group were significantly higher than those in the control group (P = 0.02 and P = 0.03, respectively). However, the implantation and miscarriage rates were not significant between groups (Table 2).
Discussion
In the present study, the outcomes of COH cycle and pregnancy after early-onset GnRH-ant protocol were compared with conventional GnRH-ant protocol in patients with POR diagnosis. Our results revealed a higher number of retrieved and MII oocytes and also a higher rate of clinical pregnancy following fresh embryo transfer in early start
GnRH-ant protocol than those of the control group, however, the rates of implantation and miscarriage were not significantly different between groups. It is worth noting that this strategy did not affect the total dose of gonadotropins, duration of stimulation, and cycle cancelation rate in patients with POR.
In this regard, Kolibianakis et al. in a randomized clinical study concluded that high exposure of the genital tract to LH and E2 in the early follicular phase is associated with a reduced chance of pregnancy in cycles stimulated with recombinant FSH and GnRH-ant for IVF/ICSI, therefore, it is assumed that the endocrine environment of the early follicular phase in antagonist cycles might be related to the reproductive outcomes [9]. In agreement with them, in our study higher MII oocytes and clinical pregnancy rate after fresh embryo transfer were observed in early-onset GnRH-ant protocol.
The beneficial effects of early follicular phase GnRH-ant on improving the number of retrieved and MII oocytes in normal responder patients were shown in previous studies; however, they have reported no significant positive effect on pregnancy rate in these patients [14, 15]. Park et al. in a retrospective study of normal responder women, concluded that the modified early-onset antagonist protocol may improve the mature oocyte yield, possibly via enhanced follicular synchronization, while resulting in superior CPR as compared to the conventional antagonist protocol, which requires to be studied further in prospective randomized controlled trials. Regarding to patients with POR diagnosis, the most of previous studies with early-onset GnRH-ant protocol, estradiol pre-treatment (E2 priming) was used in the late luteal phase prior to the start of the antagonist protocol [7, 8, 16]; hence, the results of the present study were not comparable to them. In whatever way regardless of how most of the mentioned studies have shown evidence of improved ovarian stimulation outcomes [7, 8, 16, 17] and pregnancy rates [8] after early-onset GnRH-ant protocol with E2 priming in POR patients.
In the flexible type, the early onset of the antagonist appears to have beneficial effects compared to the late onset of the antagonist. On the other hand, due to the possibility of LH surge occurring earlier than the sixth day, stimulation and release of oocytes before puncture, especially in POR individuals with fewer eggs, early onset of antagonist will prevent the loss of these limited growing follicles [18]. Furthermore, early onset of GnRH antagonist may lead to better follicular synchronization, which increases follicle maturation. It is difficult to determine the exact time of increasing FSH level at interphase due to intrinsic changes in FSH because it can occur before or at the beginning of the follicular phase. Therefore, it is believed that the interphase peak of FSH can be suppressed by early GnRH antagonist onset at the beginning of the menstrual cycle and primary FSH suppression may be helpful in achieving follicles coordination [19].
Nevertheless, the present study has some limitations and some strength points that should be mentioned. The strengths of the current study were the randomized clinical trial methodology and the selection of homogenous population of POR patients. The rate of ET cancelation was a little high which can reduce the power of the study (Fig. 1). However, further studies with a larger sample size are needed to validate our findings.
Conclusions
Early-onset GnRH-ant protocol can improve the number of retrieved and MII oocytes and clinical pregnancy rate after fresh embryo transfer; however, larger randomized studies are required to compare the pregnancy outcomes after this protocol versus other COH protocols with considering cost-effectiveness issue.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Abbreviations
- POR:
-
Poor ovarian response
- ART:
-
Assisted reproduction technology
- AFC:
-
Antral follicle count
- AMH:
-
Anti-Mȕllerian hormone
- COH:
-
Controlled ovarian hyperstimulation
- E2:
-
Estradiol
- GnRH-ant:
-
Gonadotropin-releasing hormone antagonist
- HMG:
-
Human menopausal gonadotrophins
- hCG:
-
Human chorionic gonadotropin
- SD:
-
Standard deviation
- LH:
-
Luteinizing hormone
- IVF/ICSI:
-
In vitro fertilization/intra-cytoplasmic sperm injection
- MII:
-
Metaphase II
References
Albano C, Felberbaum R, Smitz J, Riethmuller-Winzen H, Engel J, Diedrich K, Devroey P (2000) Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist cetrorelix and the LHRH-agonist buserelin. Hum Reprod 15(3):526–531. https://doi.org/10.1093/humrep/15.3.526
Abdul-Hameed KM, Al-Kawaz UM, Al-Hilli NM (2019) Early and Short Follicular GnRH Antagonist (Sandwich) Protocol Versus Conventional GnRH Antagonist Protocol in Normal Responders. Iraqi J Embryos Infertil Res 9(1):68–88. https://doi.org/10.28969/IJEIR.v9.i1.r5
Shapiro DB, Mitchell-Leef D, Carter M, Nagy ZP (2005) Ganirelix acetate use in normal-and poor-prognosis patients and the impact of estradiol patterns. Fertil Steril 83(3):666–670. https://doi.org/10.1016/j.fertnstert.2004.11.001
Al-Jeborry MM (2019) Comparison of sandwich, conventional antagonist and microdose protocols in poor responders
Inal ZO, Yilmaz N, Inal HA, Hancerliogullari N, Coskun B (2017) Are there any differences between antagonist administration on days< 6 and 6 of COH on assisted reproductive technique outcomes? J Chin Med Assoc 20:1e5
Kolibianakis EM, Albano C, Camus M, Tournaye H, Van Steirteghem AC, Devroey P (2003) Initiation of gonadotropin-releasing hormone antagonist on day 1 as compared to day 6 of stimulation: effect on hormonal levels and follicular development in in vitro fertilization cycles. J Clin Endocrinol Metabol 88(12):5632–5637. https://doi.org/10.1210/jc.2003-030805
Cakmak H, Tran ND, Zamah AM, Cedars MI, Rosen MP (2014) A novel “delayed start” protocol with gonadotropin-releasing hormone antagonist improves outcomes in poor responders. Fertil Steril 101(5):1308–1314. https://doi.org/10.1016/j.fertnstert.2014.01.050
Maged AM, Nada AM, Abohamila F, Hashem AT, Mostafa WA, Elzayat AR (2015) Delayed start versus conventional GnRH antagonist protocol in poor responders pretreated with estradiol in luteal phase: a randomized controlled trial. Reprod Sci 22(12):1627–1631. https://doi.org/10.1177/1933719115590666
Kolibianakis EM, Albano C, Kahn J, Camus M, Tournaye H, Van Steirteghem AC, Devroey P (2003) Exposure to high levels of luteinizing hormone and estradiol in the early follicular phase of gonadotropin-releasing hormone antagonist cycles is associated with a reduced chance of pregnancy. Fertil Steril 79(4):873–880. https://doi.org/10.1016/S0015-0282(02)04920-8
Tannus S, Weissman A, Boaz M, Horowitz E, Ravhon A, Golan A, Levran D (2013) The effect of delayed initiation of gonadotropin-releasing hormone antagonist in a flexible protocol on in vitro fertilization outcome. Fertil Steril 99(3):725–730. https://doi.org/10.1016/j.fertnstert.2012.11.020
Hamdine O, Macklon N, Eijkemans M, Laven J, Cohlen B, Verhoeff A, van Dop P, Bernardus R, Lambalk C, Oosterhuis G (2013) Comparison of early versus late initiation of GnRH antagonist co-treatment for controlled ovarian stimulation in IVF: a randomized controlled trial. Hum Reprod 28(12):3227–3235. https://doi.org/10.1093/humrep/det374
Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L (2011) ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 26(7):1616–1624. https://doi.org/10.1093/humrep/der092
Cummins J, Breen T, Harrison K, Shaw J, Wilson L, Hennessey J (1986) A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fertil Embryo Transfer 3(5):284–295. https://doi.org/10.1007/BF01133388
Younis JS, Soltsman S, Izhaki I, Radin O, Bar-Ami S, Ben-Ami M (2010) Early and short follicular gonadotropin-releasing hormone antagonist supplementation improves the meiotic status and competence of retrieved oocytes in in vitro fertilization–embryo transfer cycles. Fertil Steril 94(4):1350–1355. https://doi.org/10.1016/j.fertnstert.2009.08.033
Blockeel C, Riva A, De Vos M, Haentjens P, Devroey P (2011) Administration of a gonadotropin-releasing hormone antagonist during the 3 days before the initiation of the in vitro fertilization/intracytoplasmic sperm injection treatment cycle: impact on ovarian stimulation. A pilot study. Fertil Steril 95(5):1714–1719. e1712
Davar R, Neghab N, Naghshineh E (2018) Pregnancy outcome in delayed start antagonist versus microdose flare GnRH agonist protocol in poor responders undergoing IVF/ICSI: An RCT. Int J Reprod BioMed 16(4):255–260
Ashrafi M, Arabipoor A, Yahyaei A, Zolfaghari Z, Ghaffari F (2018) Does the “delayed start” protocol with gonadotropin-releasing hormone antagonist improve the pregnancy outcome in Bologna poor responders? a randomized clinical trial. Reprod Biol Endocrinol 94(4):1350–1355
Hamdine O, Broekmans FJ, Eijkemans MJC, Lambalk CB, Fauser BM, Laven JSE, Macklon NS, CETRO trial study group (2013) Early initiation of gonadotropin-releasing hormone antagonist treatment results in a more stable endocrine milieu during the mid-and late-follicular phases: a randomized controlled trial comparing gonadotropin-releasing hormone antagonist initiation on cycle day 2 or 6. Fertil Steril 100(3):867–874. https://doi.org/10.1016/j.fertnstert.2013.05.031
Park CW, Hwang YI, Koo HS, Kang IS, Yang KM, Song IO (2014) Early gonadotropin-releasing hormone antagonist start improves follicular synchronization and pregnancy outcome as compared to the conventional antagonist protocol. Clin Exp Reprod Med 41(4):158–164. https://doi.org/10.5653/cerm.2014.41.4.158
Acknowledgements
We would like to thank all the colleagues in Arash Women’s Hospital and all of the participants for their assistance in this study.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
TE, LK, and AM designed the research. TE, AM, LK, MFM, and SHM contributed in patient selection, data collection, interpretation of data, and manuscript writing/editing. TE, ES, and LK wrote the manuscript. AA helped in the analysis of the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Boards and the Ethics Committees of Tehran University of Medical Sciences approved this study (ethics code: IR.TUMS.MEDICINE.REC.1398.834). All procedures conducted in the present study involving human participants were in accordance with the ethical standards of Tehran University of Medical Sciences and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The eligible patients signed written informed consent prior to participation in the clinical trial.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esfidani, T., Moini, A., Arabipoor, A. et al. Early administration of gonadotropin-releasing hormone antagonist versus flexible antagonist ovarian stimulation protocol in poor responders: a randomized clinical trial. Middle East Fertil Soc J 26, 35 (2021). https://doi.org/10.1186/s43043-021-00079-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-021-00079-y