Abstract
Background
WAGR syndrome is a rare genetic disorder characterized by a de novo deletion of 11p13 and is usually clinically associated with Wilms’ tumor, aniridia, genitourinary anomalies, and mental retardation (W-A-G-R). Although the genotypic defects in WAGR syndrome have been well established. The congenital aniridia is caused, in nearly 90% of cases by mutations in the gene PAX6. In the face of congenital aniridia, it is imperative to specify whether it falls within the scope of a WAGR syndrome or if it is an isolated congenital aniridia or inherited by performing karyotype, FISH (Fluorescence In Situ Hybridization) or a CGH array for genetic counseling.
Case presentation
We report here a case of genetic testing for newborn with aniridia, to detect 11p13 rearrangements, using karyotyping and CGH array to complete picture of the chromosomal deletions and breakpoints in aniridia. Results show either a loss of 3811.196 kb on chromosome 11 delimited by the bands p14.1 and p13 with formula or a loss of a 1867.287 kb on chromosome 18 fragment delimited by q21.33 and q22.1 bands, that has not been detected by karyotype analysis.
Conclusions
Cytogenetics screening is a good strategy for the genetic diagnosis of aniridia and associated syndromes, allowing for a better identification of breakpoints. Our results underline the clinical importance of performing exhaustive and accurate analysis of chromosomal rearrangements for patients with aniridia, especially newborns to improve survival and quality of life for affected individuals.
Similar content being viewed by others
Introduction
WAGR syndrome is a syndrome grouping systemic and ocular abnormalities. It is the acronym for a set of malformations and clinical characteristics with W standing for Wilms tumor (or nephroblastoma), A for congenital aniridia, G for urogenital abnormalities, and R for mental retardation/intellectual disability and retardation of psychomotor development [1, 2]. A large variety of phenotypic manifestations of the syndrome has been reported [3, 4]. Clinical diagnosis is usually made at the infancy stage by the presence of congenital aniridia (revealed by the total absence of a black pupil type iris or partial absence of iris) or by the existence of nystagmus; and/or urogenital abnormalities. The association of congenital aniridia and genital or urogenital abnormalities (more discrete in girls) should alert the clinician to the possibility of WAGR syndrome. Children with WAGR syndrome can undergo delayed development (difficulty processing, learning, speaking or understanding language…) or psychiatric or behavioral problems (attention-deficit/hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), or autism spectrum disorder). When WAGR syndrome includes childhood-onset obesity, it is often referred to as WAGRO syndrome [5].
WAGR syndrome is sporadic in the vast majority of cases regardless of family history, resulting from the de novo occurrence of a chromosomal accident microdeletion type. In rare cases, WAGR syndrome is “inherited.” In these cases, a parent carrying a balanced translocation rearrangement with no signs and symptoms can transmit during meiosis a reciprocal unbalanced translocations to the offspring during tetravalent chromosome segregation showing a partial trisomy or partial monosomy [6].
The annual incidence of congenital aniridia varies from 1/40,000 to 1/100,000 births. This ratio is perhaps underestimated, regardless of ethnicity or sex prevalence, it can reach one in 500,000 to 1 million, and thus, it is a rare genetic pathology [7, 8]. It is, therefore, considered a rare disease with a prevalence of less than 1/2000 people, according to the European estimations.
The WAGR syndrome genotypic and phenotypic abnormalities have been well established and detected by cytogenetic analysis [3, 9]. This syndrome is the consequence of a heterozygous interstitial microdeletion on the short arm of chromosome 11 which causes at least the loss of several genes contiguous at 11p13 [10] such as the WT1 Wilms tumor 1 gene (kidney tumor suppressor gene), responsible for urogenital abnormalities congenital and in case of a secondary event on the other allele, the occurrence of Wilms tumor (nephroblastoma) [9], and the PAX6 gene, coding for transcription factors involved in the development of the eye, sensory organs and brain. Neurodevelopmental disorders may be related to the additional loss of still ill-defined genes in the 11p13 region. Microdeletions wider, involving the 11p14 band which encompasses the BDNF gene (Brain-Derived Neurotrophic Factor) and other genes such as LMO2, EXT2, ALX4 [11], PRRG4, and TRIM44, have been causally connected to obesity and potentially intellectual disability associated with the disease [12]. The size of the deletion varies between individuals from 1 million to 26.5 million pairs of bases (or Mb), the average being 11 Mb. The variability in the size of the genetic microdeletion explains the variable phenotype.
Congenital aniridia is caused, in nearly 90% of cases, by mutations in the gene PAX6 located on the short arm of a chromosome 11 at 11p13, which codes for a factor of transcription linked to the process of development of the eyeball [13, 14]. Mutations in the PAX6 gene cause corneal cytokeratin expression problems, as well as abnormalities cell adhesion and migration and the glycoconjugate process.
To handle a congenital aniridia, it is compulsory to specify whether it falls within the scope of a WAGR syndrome or if it is an isolated congenital aniridia by performing karyotype, Fluorescence In Situ Hybridization (FISH) or an array-based comparative genomic hybridization (array CGH) or if possible searching for a PAX6 and WT1 microdeletion [8, 15]. If the gene locus PAX6 is intact, the study by FISH must also target the regulatory region of the gene located in introns 7–9 of the neighboring gene, ELP4. Besides the classic features for which the syndrome is named, it can have other clinical abnormalities and complications (craniofacial dysmorphism and deficits sensory) for which the disorder is less well known.
Conventional cytogenetic analysis is considered as important tool for routine laboratory testing providing a significant diagnostic and prognostic results for many human diseases. We report here an application of conventional and molecular cytogenetics for genetic diagnosis of WAGR syndrom in Morocco.
Patients and methods
Case presentation
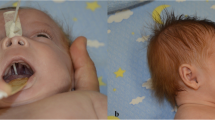
We present a case of a child received for a medical genetic consultation in the National Laboratory of Reference, Cheikh Khalifa Hospital, Casablanca. Our patient is a 14-month-old male, the first and unique child of a non-consanguineous young Moroccan couple (mother and father aged 24 and 33 years old, respectively). The child was born at term without any pregnancy-related complications. He has received his vaccines according to the Moroccan National Vaccination Program. The child presents photophobia and whitish lesion in eyes. Clinical and fundus eye examination revealed abnormal eyes (bilateral aniridia) and hypoplasia. He has genitourinary anomalies (hypospadias and undescended testes) associated with psychomotor developmental delay. These symptoms imply that the WAGR syndrome or a congenital aniridia to be confirmed by the presence or absence of the interstitial deletion in the short arm of one chromosome 11. There was no family history of aniridia or renal tumors.
Methods
Conventional cytogenetic analysis
The patient’s parents gave informed consent for this study, which was performed in accordance with the Declaration of Helsinki protocols and approved by the ethics committee of Cheikh Khalifa Hospital. Three–5 ml of blood put in a heparinized tube was taken from the patient for cytogenetic analysis. Chromosomal analysis was performed by R-banding at the resolution level of 400 bands according to standard procedures using a Metaphase Chromosome Harvester (HANABI PII, ADS Biotec company). The spreading was done by the metaphase Auto Spreader Mini (HANABI PV, ADS Biotec company). Chromosome classification and analysis was performed using an automatic system “GenASIS, Version 7.2.7.34276 (https://spectral-imaging.com).” Karyotype was described according to the International System for Human Cytogenetic Nomenclature ISCN (2020) [16] by analyzing about 20 mitoses.
Molecular cytogenetic: array-based comparative genomic hybridization (array CGH)
To specify with precision the boundaries of deletions, we performed an array CGH using the Cytoscan HD platform from Affymetrix Cytoscan HD array containing roughly around 1 million probes (Applied Biosystems: ex Affymetrix). It provides a genome-wide approach for the detection of CNVs (gains or losses), LOH, and UPD on a single test. Blood was collected on EDTA tube from the proband, and DNA was extracted using Maxi-QIAamp DNA Extraction Kit (Qiagen, Hidden, Germany) according to the manufacturer's instructions. The quality of DNA evaluated by electrophoresis on agarose gel and DNA was quantified using Qubit (Thermo Fisher Scientific). The protocol was conducted following the manufacture recommendations with some modifications (data not shown) in the presence of a positive and negative controls. After labeling PCR fragmented product and hybridization process on the GeneChip® hybridization oven 645, the array was washed on the GeneChip® Fluidic station 450 and scanned using GeneChip® 3000 Scanner. The scanner generates images of the array and converts them to a «.cel» file. This output file was analyzed using the software «Affymetrix Chromosomal Analysis Suites: ChAs» version 4.3 (Thermo).
Results
Cytogenetic investigation revealed a male karyotype with an interstitial deletion at the short arm of chromosome 11 (partial monosomy 11p, monosomy 11p13-p14.2) (Fig. 1).
To precisely specify the boundaries of deletions, we performed array CGH on the blood sample of the proband. Results show either a loss of 3811.196 kb on chromosome 11 delimited by the bands p14.1 and p13 with formula; arr[GRCh38] 11p14.1p13(28,763,339_32,574,534) × 1 (Fig. 2) or a loss of a 1867.287 kb on chromosome 18 fragment delimited by q21.33 and q22.1 bands (Fig. 3), that has not been detected by karyotype analysis before, with formula: arr[GRCh38] 18q21.33q22.1(62,053,563_63,920,849) × 1.
The deletion at chromosome 11 includes 12 OMIM genes: KCNA4 (176266), FSHB (136530), ARL14EP (612295), MPPED2 (600911), DCDC1 (608062), DNAJC24 (611072), IMMP1L (612323), ELP4 (606985), RCN1 (602735), PAX6 (607108), WT1 (607102), and WT1-AS (607899). The deletion of the last three genes confirms the suspected phenotype and confirms the WAGR syndrome without ongoing Fluorescent In Situ Hybridization (FISH) [17, 18].
The deletion at chromosome 18 includes 17 OMIM genes: PIGN (606097), RELCH (618001), TNFRSF11A (603499), PHLPP1 (609396), BCL2 (151430), KDSR (136440), VPS4B (609983), and long intergenic noncoding RNA genes: SERPINB5 (154790), SERPINB12 (615662), SERPINB13 (604445), SERPINB4 (600518), SERPINB3 (600517), SERPINB11 (615682), SERPINB7 (603357), SERPINB2 (173390), and SERPINB10 (602058). These genes have no relation with the phenotype. Thus, this deletion is not linked to the phenotype but, rather, to the deletion on chromosome 11.
Discussion
Our study has shown that the patient showed typical signs (Wilms tumor, aniridia, genitourinary anomalies, and psychomotor retardation) of WAGR. The karyotype analysis showed monosomy 11p13-p14.2 confirmed by CGH array analysis showing a loss of 3811.196 kb on chromosome 11 delimited by the bands p14.1 and p13, including WT1 and PAX 6 genes. Nevertheless, the scope of these contributions remains undefined in terms of developmental delay observed in our patient, which could mostly result from the KCNA gene deletion (KCNA4: potassium voltage-gated channel, shaker-related subfamily, member 4). This gene is linked to microcephaly, cataracts, impaired intellectual development, and dystonia with abnormal striatum phenotype segregating in autosomic recessive inheritance originally reported by Al-Owain et al. (2013), Kaya et al. (2016) in a consanguineous Saudi family, presenting homozygosity for a missense mutation leading to the loss of KCNA4 gene function [19, 20].
This finding is very important in managing and evaluating WAGR syndrome because it helps to deliver the genetic anomalies information to confirm the diagnosis of the associated syndromes. The use of classical and molecular cytogenetic techniques is important to confirm the diagnosis of WAGR syndrome. However, while karyotyping has been considered the gold standard technique for detecting chromosomal alterations for many years, it has limitations regarding the severity of the phenotype which depends not only on the size of the deletion, but also on the genes mapped to the deleted segments responsible for the associated syndromes, well characterized by chromosomal microarray [21]. The use of CGH array highlighted the main cause of obesity associated with WAGR due to the deletion of BDNF gene [22] and a high risk of developing either multiple exostoses (a disorder characterized by cartilaginous excrescences near the ends of the diaphysis of the long bones) or Potocki–Shaffer syndrome by deletion including ALX4 or ETX2 genes [23].
In conclusion, we report the first molecular cytogenetic description using Cytoscan HD-CGH array tool in Morocco of a patient with WAGR syndrome. The absence of WT1 and PAX 6 genes makes obligatory the health supervision for the child by recommending an appropriate medical intervention by regular monitoring of visual function, of renal ultrasound examinations, and of renal function to prevent secondary expected complications due to solid molecular diagnostics to improve his survival and quality of life. A correct detection of these genetic defects has important consequences for prognostic and genetic counseling of patients and families with aniridia, particularly for syndromic patients and sporadic cases.
References
Xu S, Han JC, Morales A, Menzie CM, Williams K, Fan Y-S (2008) Characterization of 11p14-p12 deletion in WAGR syndrome by array CGH for identifying genes contributing to mental retardation and autism. Cytogenet Genome Res 122:181–187
Miller RW, Fraumeni JF Jr, Manning MD (1964) Association of Wilms’s tumor with aniridia, hemihypertrophy and other congenital malformations. N Engl J Med 270:922–927
Fischbach BV, Trout KL, Lewis J, Luis CA, SikaM. (2005) WAGR syndrome: a clinical review of 54 cases. Pediatrics 116:984–988
Scott RH, Stiller CA, Walker L, Rahman N (2006) Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet 43:705–715
Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA (2008) Brain-derived neurotrophic factor and obesity in the WAGR Syndrome. N Engl J Med 359:918–927
Crolla JA, Heyningen V (2002) Frequent chromosome aberrations revealed by molecular cytogenetic studies in patients with aniridia. Am J Hum Genet 71(5):1138–1149
Hingorani M, Hanson I, Van Heyningen V (2012) Aniridia. Eur J Hum Genet 20(10):1011–1017
Tezcan B, Rich P, Bhide A (2015) Prenatal Diagnosis of WAGR Syndrome. Case Rep Obstet Gynecol. 2015:928585
Lange J, Peterson SM, Takashima JR, Grigoriev Y, Ritchey ML, Shamberger RC, Beckwith JB, Perlman E, Green DM, Breslow NE (2011) Risk factors for end stage renal disease in non-WT1-syndromic Wilms tumor. J Urol 186(2):378–386
Schmickel RD (1986) Chromosomal deletions and enzyme deficiencies. J Pediatr 108(2):244–246
Bremond-Gignac D, Crolla JA, Copin H, Guichet A, Bonneau D, Taine L, Lacombe D, Baumann C, Benzacken B, Verloes A (2005) Combination of WAGR and Potocki-Shaffer contiguous deletion syndromes in a patient with an 11p11.2–p14 deletion. Eur J Hum Genet 13(4):409–413
Hall HN, Williamson KA, FitzPatrick DR (2019) The genetic architecture of aniridia and Gillespie syndrome. Hum Genet 138(8–9):881–898
Bobilev AM, McDougal ME, Taylor WL, Geisert EE, Netland PA, Lauderdale JD (2016) Assessment of PAX6 alleles in 66 families with aniridia. Clin Genet 89(6):669–677
Vasilyeva TA, Marakhonov AV, Voskresenskaya AA, Kadyshev VV, Käsmann-Kellner B, Sukhanova NV, Katargina LA, Kutsev SI, Zinchenko RA (2021) Analysis of genotype-phenotype correlations in PAX6-associated aniridia. J Med Genet 58(4):270–274
Blanco-Kelly F, Palomares M, Vallespın E, Villaverde C, Martın-Arenas R, Velez-Monsalve C, Lorda-Sánchez I, Nevado J, Trujillo-Tiebas MJ, Lapunzina P, Ayuso C, Corton M (2017) Improving molecular diagnosis of aniridia and WAGR syndrome using customized targeted array-based CGH. PLoS ONE 12(2):e0172363
ISCN 2020: An International System for Human Cytogenomic Nomenclature (2020). Reprint of: Cytogenetic and Genome Research 2020, Vol. 160, No. 7–8. Karger, S; 2020
Muto R, Yamamori S, Ohashi H, Osawa M (2002) Prediction by FISH analysis of the occurrence of Wilms’ tumor in Aniridia patients. Am J Med Genet 108:285–289
Natiq A, Amasdl S, Liehr T, Kreskowski K, Meyer B, Ratbi I, Amzazi S, Sefiani A (2014) 11p13 deletion syndrome: first case in Morocco detected by FISH. J Pediatrics Neonatal Care 1(7):00048
Owain M, Al-Zahrani J, Al-Bakheet A, Abudheim N, Al-Younes B, Aldhalaan H, Al-Zaidan H, Colak D, Almohaileb F, Abouzied ME, Al-Fadhli F, Meyer B, Kaya N (2013) A novel syndrome of abnormal striatum and congenital cataract: evidence for linkage to chromosomes (sic) 11. Clin Genet 84:258–264
Kaya N, Alsagob M, D’Adamo MC, Al-Bakheet A, Hasan S, Muccioli M, Almutairi FB, Almass R, Aldosary M, Monies D, Mustafa OM, Alyounes B et al (2016) KCNA4 deficiency leads to a syndrome of abnormal striatum, congenital cataract and intellectual disability. J Med Genet 53:786–792
De Souzaa VS, Rodrigues da Cunhaa GC, Versianic BR, De Oliveirac CP, Alves Silva Rosad MT, De Oliveiraa SF, Morettie PN, Mazzeub JF, Pic-Taylor A (2022) Characterization of associated nonclassical phenotypes in patients with deletion in the WAGR region identified by chromosomal microarray: new insights and literature review. Mol Syndromol 13:290–304
Jung R, Rauch A, Salomons GS, Verhoeven NM, Jakobs C, Gibson KM, Lachmann E, Sass JO, Trautmann U, Zweier C, Staatz G, Knerr I (2006) Clinical, cytogenetic and molecular characterization of a patient with combined succinic semialdehyde dehydrogenase deficiency and incomplete WAGR syndrome with obesity. Mol Genet Metab 88:256–260
Meng Y, Yang J, Tian C, Qiao J (2020) Identification of a 6-month-old baby with a combination of WAGR and Potocki-Shaffer contiguous deletion syndromes by SNP array testing. Hereditas 157(1):23
Author information
Authors and Affiliations
Contributions
FC: conceptualized the study, CGH array analysis analyzed the data, and was responsible for redaction and submission. HH: performed cytogenetic analysis. HM: genetic conselling. HB: analyzed the data and revised the manuscript. KO: contributed to clinical investigation and conception.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chbel, F., Hamdaoui, H., Mossafa, H. et al. Conventional and molecular cytogenetic characterization of a Moroccan patient with WAGR syndrome. Egypt J Med Hum Genet 25, 37 (2024). https://doi.org/10.1186/s43042-024-00514-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-024-00514-5