Abstract
Background
Abnormal folate metabolism is a risk factor for DNA hypomethylation and chromosomal nondisjunction. MTHFR is a candidate gene for folliculogenesis and ovarian development. In the present study, we aimed to investigate the distribution of the MTHFR C677T polymorphism in individuals with primary amenorrhea and it’s association with the cytogenetic and clinical profile. The MTHFR polymorphism (C677T) was checked in 45 females with PA and 45 control females (age-matched) with regular menstrual cycles using polymerase chain reaction-restriction fragment length polymorphism.
Results
We observed the CC genotype in 84.4% (n = 38) of the control group females and 48.9% (n = 22) of the case group females, CT genotype in 13.3% (n = 6) in the control group females and 24.4% (n = 11) in the case group females (p = 0.039; χ2 value 4.253; odd ratio 0.316, 95%CI 0.103–0.973) and TT genotype in 2.2% (n = 1) in the control group females and 26.7% (n = 12) in the case group females (p = 0.000266; χ2 value 13.294; odd ratio 0.048, 95%CI 0.006–0.397). Out of 45 case group females, 26 females showed 46,XX karyotype, 4 females showed male karyotype, 3 females showed pure Turner karyotype, 2 females were mosaic Turner and the rest of the females showed structural abnormalities like deletion, isochromosome and normal variants. The serum values revealed significantly lower levels (p = 0.032) of progesterone in the individuals with the TT genotype as compared to the CC genotype and the radiology profile showed a significant role of the MTHFR gene in ovarian development (p = 0.024).
Conclusion
We suggest that the MTHFR polymorphism (C677T) might be responsible for the chromosomal nondisjunction in monosomy X females. It also influences the progesterone level and ovarian development, thus affecting folliculogenesis and the ovarian reserve responsible for primary amenorrhea.
Similar content being viewed by others
Introduction
Primary amenorrhea (PA) is characterized by the absence of the menarche in a female by the age of 14, regardless of whether secondary sexual characteristics (SSCs) are present or not [1]. The incidence of PA is less than 0.1%, and the World Health Organization (WHO) has estimated amenorrhea as the sixth major cause of female infertility [2]. PA results due to several different causes viz. anatomic, endocrinologic, or genetic [3]. Among them, chromosomal abnormalities like Turner syndrome or its variants, male karyotype (presence of Y chromosome), and X abnormalities contribute from 15.9 to 63.3% in the etiology of PA [2]. Candidate genes responsible for normal ovarian development and function have been identified, and any defect in these genes will cause PA. It has been suggested that female carrying MTHFR polymorphism (C677T) has reduced ovarian follicular activity and/or may experience early menopause [4, 5]. Studies have confirmed a potential role of MTHFR gene polymorphism associated with abnormal folate metabolism, and DNA hypomethylation reaction might increase the risk of chromosomal nondisjunction [6]. Moreover, in ovarian follicular growth or oocyte maturity, the MTHFR polymorphisms may interfere with granulosa cells (GC) activity in growing follicles by causing apoptosis in the GC [5]. The literature has suggested that MTHFR polymorphism increases the risk of chromosomal nondisjunction [6] and also interferes with female’s ovarian cycles [5], suggesting that this polymorphism plays a major role in female reproduction. Based on the above-mentioned role of the MTHFR gene, we aimed to check the distribution of the MTHFR C677T polymorphism in the individuals with primary amenorrhea and its association with the endocrinology and radiology profiles of the case group females.
Materials and method
The present study was conducted at the Department of Zoology, BioMedical Technology and Human Genetics, Ahmedabad, where 90 females were recruited, of which 45 females had a chief complaint of primary amenorrhea (PA) and were categorized as “case group” and the rest 45 were “control group” females who had a regular menstrual cycle with no complaints regarding menstruation. Ethical approval was obtained from Institutional Ethics Committee of Gujarat University (No.: GU/IEC/02/2018). Females over 14 years and below 30 years were considered for the study. Informed consent was obtained from each female, and in the case of minor females, permission was obtained from one of the family members.
The females with complete absence of menstruation were recruited under the case group, who were referred from government hospitals of Ahmedabad and nearby districts to our department for karyotyping; and age-matched females with normal menstrual cycles were recruited under the control group. Exclusion criteria included infection, history of surgery before referral or drug-induced menstruation. For the control group, the females with a family history of amenorrhea or infertility were excluded.
A detailed proforma with informed consent was filled for each individual, which included a detailed pedigree minimum of up to 3 generations, a clinical profile (proband’s clinical information such as serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), prolactin, progesterone, estradiol and testosterone). A detailed ultrasonography (USG) profile was obtained from the clinician at the time of the recruitment.
Cytogenetic analysis
For cytogenetic analysis, the peripheral blood lymphocyte culture (PBLC) was done according to the standard protocol with slight modifications [7]. Venous blood was drawn under an aseptic condition, and 0.5 ml was added to RPMI 1640 culture media pre-supplemented with the fetal bovine serum (FBS). Phytohemagglutinin (PHA) was added to each culture for stimulation, and cultures were kept at 37 °C for 72 hours. Colchicine (1mg/5 ml) was added to arrest the cells at metaphase, followed by treatment with a pre-warmed hypotonic solution (KCl: 0.75M). The cells were fixed in Carnoy’s solution (acetomethanol 3:1), and slides were prepared. Slides were subjected to GTG banding [8], 100 metaphase plates were counted to check for mosaicism, and 20 plates were karyotyped according to ISCN 2016. The images were captured by Zeiss Microscope, and karyotyping was carried out with the help of Ikaros Imaging System karyotype (Metasystems®, North Royalton, OH).
Molecular analysis
Genomic DNA extraction was carried out by John et al. [9] method with slight modifications like centrifuge timing and resuspension of genomic DNA in TE buffer. The quantity and quality of DNA were checked by nano spectrometer and agarose-gel electrophoresis, respectively. The extracted DNA was subjected to a polymerase chain reaction (PCR) according to the method described by Cyril et al. [10] with slight modifications in the initial denaturation and annealing temperature. The PCR reaction was carried out in a final volume of 25 µl containing 2X PCR master mix (EmeraldAmp® GT PCR Master Mix, Takara Bio Inc., Shiga, Japan), 20 pM of each primer (forward primer: 5'TGAAGGAGAAGGTGTCTGCGGGA3' and reverse primer: 5'AGGACGGTGCGGTGAGAGTG3' synthesized from Sigma-Aldrich Chemical Pvt Limited, (Milwaukee, Wis., USA), 100 ng of the DNA template and nuclease-free water. The PCR conditions were set as follows: initial denaturation at 95 °C for 5 minutes followed by 30 cycles of denaturation at 94 °C for 30 seconds, annealing at 64 °C for 1 minute, extension at 72 °C for 30 seconds and final extension at 72 °C at 5 minutes. For restriction fragment length polymorphism (RFLP) analysis, 198 bp amplicon was digested with the HinfI enzyme (ThermoFisher Scientific, Massachusetts, USA). Digestion was performed in 30 µl of reaction volume containing 1X reaction buffer, 1 unit of restriction enzyme and 10 µl of PCR product. The mixture was incubated at 37 °C overnight. After digestion, the product was run on 3% agarose gel stained with ethidium bromide (EtBr) at 110 volts for 40 minutes. Bands were visualized under a UV transilluminator. The gel electrophoresis bands for all individuals were photographed (Fig. 1). The band at 198 bp shows homozygous CC genotype, the bands at 198/175 bp show heterozygous CT genotype and the band at 175 bp shows homozygous polymorphic TT genotype against 100 bp ladder.
Statistical analysis
The statistical analysis was conducted on SPSS version 23 software (IBM Corp., Armonk, NY, USA). The Chi-square (χ2) test, odds ratio (OR) and 95% confidence interval (95%CI) were calculated to check the association between the control and case group. One-way ANOVA was performed to analyze the differences in the serum level of hormones as well as in the Mullerian duct development within the groups. The serum level of hormones was represented as mean and standard deviation (SD). The Pearson’s p-value<0.05 was considered statistically significant.
Results
Polymorphism screening
The polymorphism screening was carried out to determine its distribution in females with PA. The homozygous wild CC genotype was found in 84.4% (n = 38) of control group females compared to 48.9% (n = 22) of case group females. The heterozygous CT genotype was observed in 13.3% (n = 6) of the control group females and 24.4% (n = 11) in the case group females (p = 0.039; χ2 value 4.253; odd ratio 0.316, 95%CI 0.103–0.973). Similarly, the homozygous polymorphic TT genotype was observed in 2.2% (n = 1) of the control group females and 26.7% (n = 12) in case group females (p = 0.000266; χ2 value 13.294; odd ratio 0.048, 95%CI 0.006–0.397). The frequency of the “C” allele distribution was found to be higher in the control group (91.1%) than the case group (61.1%). In contrast, the frequency of the “T” allele distribution was found to be higher in the case group (38.9%) than the control group (8.9%) (p = 0.000002; χ2 value 22.275; odd ratio 0.153, 95%CI 0.066–0.355) (Table 1).
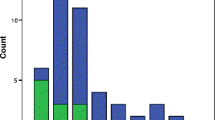
The cytogenetic profile of PA females revealed 57.7% (n = 26) females with 46,XX karyotype, out of which 12 females reported CC genotype, 8 with CT genotype and 6 with TT genotype. Out of 42.3% (n = 19) females with abnormal karyotypes, all three females with 45,X reported TT genotype, females with 46,XY karyotype reported one with CC, two with CT and one with TT genotype. In females with mosaicism, the CC and the TT genotypes were observed in 1 individual each. All the females with Turner variants reported CC genotype, and out of 8 females with normal variants, six females reported CC genotype, one female with CT and one female with TT genotype (Table 2) (Additional file 1 shows the different karyotypes observed in case group (PA) individuals).
Hormonal profile
The individuals with PA were screened for FSH, LH, TSH, prolactin, estrogen, progesterone and testosterone. The serum level of these hormones was not statistically significant in individuals with the CT genotype when compared with the CC genotype. In individuals with the TT genotype, the serum level of progesterone was significantly lower (1.3±2.4 ng/mL, p = 0.032) than the females with the CC genotype (3.7±2.7 ng/mL) (Table 3).
Radiology profile
The individuals with PA were assessed for the role of the MTHFR polymorphism in Mullerian structure development. Out of 45 case group females, the frequency of the uterine (n = 38 individuals of 45) and ovarian (n = 23 individuals of 45) abnormalities were higher in all three genotypes, whereas the frequency of individuals reported with normal mullerian duct structures (other than uterus and ovaries) was higher (Table 4). The result of one-way ANOVA revealed a statistically significant role (p = 0.024) of the MTHFR gene in the development of the structure of ovaries, whereas a non-significant role of the MTHFR gene was observed with the development of other Mullerian structures (p>0.05) (Table 4).
Discussion
MTHFR catalyzes the conversion of 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which donates methyl group for the remethylation of homocysteine to methionine. The reaction is significant for synthesising S-adenosylmethionine (SAM), a significant methyl group donor for DNA, protein and lipid methylation reactions. The C to T transition at position 677 in the MTHFR gene (C677T) causes alanine to valine substitution in the MTHFR protein, thus reducing enzyme activity. Reduced enzyme activity requires increased dietary folate intake to maintain normal homocysteine to methionine remethylation reaction. The low levels of folic acid cause homocysteine accumulation, reducing the SAM to S-adenosylhomocysteine (SAH) ratio resulting in the hypomethylation of DNA [6, 11]. Furthermore, the MTHFR gene is also involved in folliculogenesis [12], indicating its association with the risk of premature ovarian failure (POF). Based on the observations of the influence of MTHFR polymorphism on ovarian function, we have attempted to check the role of MTHFR (C677T) polymorphism in females with PA as to the best of our knowledge no similar study has been conducted till date. Nevertheless, various studies have been conducted to check MTHFR polymorphism's association with Turner syndrome and females with polycystic ovarian syndrome (PCOs) [4, 5, 13,14,15,16,17]. The frequency of the TT genotype in females aligns with previous reports [13]. This suggests that the C677T polymorphism could result in DNA hypomethylation and potentially impact the occurrence of the condition by directly affecting folliculogenesis and depletion of the ovarian reserve. Further exploration of the mechanisms involved is recommended.
Previous studies have proved the importance of DNA methylation for chromosomal segregation and stabilization and showed that the C677T polymorphism significantly increases the risk for somatic chromosomal nondisjunction because of abnormal folate metabolism and DNA hypomethylation [18]. The direct effect of DNA methylation in oocytes due to impaired reactions mediated by the MTHFR gene polymorphism is responsible for aneuploidies preferentially during oocyte maturation or conception [14], consequently responsible for Turner phenotype in females. In the present study also, case group females showing chromosomal complement of Turner syndrome, i.e., 45,X (n = 3) and mosaic Turner, i.e., mos 45,X[84]/47,XXX[16] (n = 1) reported TT genotype indicating that presence of the T allele decreases the MTHFR activity and contributes toward the somatic chromosomal nondisjunction resulting in monosomy X in females. The presented suggestion supports the previous findings and that of Santos et al. [13], who reported the significant presence of the TT genotype in TS patients.
The “T” allele distribution frequency in the Indian population was reported to be 10.1% [19]. However, the present study has unequivocally observed a significantly higher frequency. Globally various studies have been carried out in diverse population groups to know the frequency of MTHFR C677T polymorphism and 677T allele distribution [20,21,22] and found that distribution of 677T was highest in Europe (24.1–64.3%), subsequently in North America (6–64.3%), East Asia (2–55%) and South America (2–48.7%). In India, out of 23 population groups studied from different states, the 677T allele distribution is highest among north Indians compared to other regions, thus indicating the heterogeneous distribution of the MTHFR 677T allele in the Indian population group [19]. Unlike other studies [15, 16, 21], the present study observes a significantly higher distribution of T allele in case group individuals, suggesting that the presence of T allele may affect the mechanism of chromosomal segregation, consequently causing the Turner syndrome in females featuring PA as a clinical symptom. Another suggestion could be that the T allele may interfere with the other genes responsible for the normal development of the female’s Mullerian duct. Also, studies suggest that variations in results are due to linkage disequilibrium and different dietary habits of the various populations [16].
Hormonal profile
We observed significantly lower progesterone levels in the case group females with the TT genotype. Progesterone is involved in the feedback mechanism of the menstrual cycle, thus exerting its direct effect on folliculogenesis, oocyte release and maturation, and endometrial implantation [23]. The exact mechanism of MTHFR polymorphism on progesterone level is unknown but studies have tried to check and found no significant association [24]. To our knowledge, this is the first study that reported a significant association between progesterone level and MTHFR gene polymorphism in PA females. Previous studies reported that the TT genotype significantly impaired estrogen production from granulosa cells and partial inhibition of FSH activity, indicating its role in folliculogenesis [25]. However, no correlation was observed with progesterone levels. We understand that progesterone and MTHFR are involved in the mechanism of folliculogenesis and control of oocyte regulation. Thus, progesterone levels are compromised in the polymorphic (TT) genotype, consequently affecting the feedback mechanism involved in the menstrual cycle suggesting MTHFR polymorphism is one of the reasons for the occurrence of PA in females.
Radiology profile
Our attempt to check the role of MTHFR polymorphism on the development of Mullerian structures showed a significant association between abnormal ovarian structure and TT genotype. Earlier, it was shown that MTHFR polymorphism increases the plasma Hcy concentration, which significantly interferes with ovarian activity and is associated with lower estrogen levels in females by influencing the ovarian responsiveness to FSH for follicular maturation and is also responsible for decreasing ovarian reserve [5]. Later, contradicting results were reported about the C677T genotype's association with the depletion of ovarian reserve [4]. The discrepancies in the studies indicate the requirement for further well-designed research, for which the present study provides the foundation for assessing the involvement of the C677T genotype in ovarian development and ovarian function. We observed that 9 out of 12 females with the TT genotype showed either absent or streak ovaries, suggesting the vital role of MTHFR in the development of ovaries during embryogenesis.
In conclusion, our result suggests a strong association between MTHFR polymorphism (C677T) and PA in our population of West India. Since the studies reporting monosomy X due to chromosomal nondisjunction caused by the MTHFR polymorphism are limited, this report provides the foundation for studying the role of the C677T polymorphism in the females with monosomy X and PA because of the significant presence of the “T” allele. The strong association between MTHFR polymorphism and progesterone level and ovarian development provides mechanistic information on MTHFR’s impact on these factors. Due to the small sample size, the other considered factors were found to be non-significant. However, a study with a larger cohort might provide a clear idea about the role of MTHFR in the occurrence of PA and its influence on progesterone levels and ovarian development.
Limitations and Recommendations: The limitation of the present study is the sample size due to the low prevalence of the condition. We further recommend the examination of other polymorphic variants of the MTHFR gene for linkage disequilibrium study and also its association with the mechanism of chromosomal segregation as well as female ovulatory cycles.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PA:
-
Primary amenorrhea
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- TSH:
-
Thyroid-stimulating hormone
- MTHFR:
-
Methylenetetrahydrofolate reductase
- MD:
-
Mullerian duct
- PCR:
-
Polymerase chain reaction
- POF:
-
Premature ovarian failure
- RFLP:
-
Restriction-fragment length polymorphism
- DNA:
-
Deoxyribonucleic acid
- SSC:
-
Secondary sexual characters
- RPMI 1640:
-
Roswell Park Memorial Institute 1640 culture medium
- ISCN:
-
International System for Human Cytogenetic Nomenclature
- SAM:
-
S-adenosylmethionine
- SAH:
-
S-adenosylhomocysteine
- PCOs:
-
Polycystic ovarian syndrome
- USG:
-
Ultrasonography
References
Rajangam S, Nanjappa L (2007) Cytogenetic studies in amenorrhea. Saudi Med J 28(2):187
Dutta UR, Ponnala R, Pidugu VK, Dalal AB (2013) Chromosomal abnormalities in amenorrhea: a retrospective study and review of 637 patients in South India. Arch Iran Med 16(5): 0–0
Klein DA, Poth MA (2013) Amenorrhea: an approach to diagnosis and management. Am Family Phys 87(11):781–8
Rosen MP, Shen S, McCulloch CE, Rinaudo PF, Cedars MI, Dobson AT (2007) Methylenetetrahydrofolate reductase (MTHFR) is associated with ovarian follicular activity. Fertil Steril 88(3):632–8
Thaler CJ, Budiman H, Ruebsamen H, Nagel D, Lohse P (2006) Effects of the common 677C> T mutation of the 5, 10-methylenetetrahydrofolate reductase (MTHFR) gene on ovarian responsiveness to recombinant follicle-stimulating hormone. Am J Reprod Immunol 55(4):251–8
Hobbs CA, Sherman SL, Yi P, Hopkins SE, Torfs CP, Hine RJ et al (2000) Polymorphisms in genes involved in folate metabolism as maternal risk factors for Down syndrome. Am J Hum Genet 67(3):623–30
Hungerford DA (1965) Leukocytes cultured from small inocula of whole blood and the preparation of metaphase chromosomes by treatment with hypotonic KCl. Stain Technol 40(6):333–8
Seabright M (1971) A rapid banding technique for human chromosomes. Lancet 2:971–2
John SW, Weitzner G, Rozen R, Scriver CR (1991) A rapid procedure for extracting genomic DNA from leukocytes. Nucleic Acids Res 19(2):408
Cyril C, Rai P, Chandra N, Gopinath PM, Satyamoorthy K (2009) MTHFR gene variants C677T, A1298C and association with down syndrome: a case-control study from South India. Indian J Hum Genet 15(2):60
James SJ, Pogribna M, Pogribny IP, Melnyk S, Hine RJ, Gibson JB et al (1999) Abnormal folate metabolism and mutation in the methylenetetrahydrofolate reductase gene may be maternal risk factors for Down syndrome. Am J Clin Nutr 70(4):495–501
Pouresmaeili F, Fazeli Z (2014) Premature ovarian failure: a critical condition in the reproductive potential with various genetic causes. Int J Fertil Steril. 8(1):1–12
Santos K, Lemos-Marini SH, Baptista M, Bonadia LC, Pinto Júnior W, Bertuzzo CS (2006) Frequency of 677C-> T and 1298A-> C polymorphisms in the 5, 10-methylenetetrahydrofolate reductase (MTHFR) gene in Turner syndrome individuals. Genet Mol Biol 29:41–4
de Oliveira KC, Bianco B, Verreschi ITN, Guedes AD, Galera BB, Galera MF et al (2008) Prevalence of the polymorphism MTHFR A1298C and not MTHFR C677T is related to chromosomal aneuploidy in Brazilian turner syndrome patients. Arq Bras Endocrinol Metab 52(8):1374–81
Ismail MF, Zarouk WA, Ruby MO, Mahmoud WM, Gad RS (2015) Methylenetetrahydrofolate reductase gene polymorphisms in Egyptian turner syndrome patients. Acta Biochim Pol. 62(3):529–32
Oliveira KC, Verreschi IT, Sugawara EK, Silva VC, Galera BB, Galera MF et al (2012) C677T and A1298C polymorphisms of MTHFR gene and their relation to homocysteine levels in Turner syndrome. Genet Test Mol Biomark 16(5):396–400
Jain M, Pandey P, Tiwary N, Jain S (2012) MTHFR C677T polymorphism is associated with hyperlipidemia in women with polycystic ovary syndrome. J Hum Reprod Sci 5(1):52
Kedar R, Chandel D (2019) MTHFR gene polymorphism and associated nutritional deficiency in the etiology and pathogenesis of Down syndrome. Egypt J Med Hum Genet 20(1):1–10
Saraswathy KN, Asghar M, Samtani R, Murry B, Mondal PR, Ghosh PK et al (2012) Spectrum of MTHFR gene SNPs C677T and A1298C: a study among 23 population groups of India. Mol Biol Rep 39(4):5025–31
Reyes-Engel A, Munoz E, Gaitan MJ, Fabre E, Gallo M, Dieguez JL et al (2002) Implications on human fertility of the 677C→ T and 1298A→ C polymorphisms of the MTHFR gene: consequences of a possible genetic selection. Mol Hum Reprod 8(10):952–7
Rosenberg N, Murata M, Ikeda Y, Opare-Sem O, Zivelin A, Geffen E et al (2002) The frequent 5, 10-methylenetetrahydrofolate reductase C677T polymorphism is associated with a common haplotype in whites, Japanese, and Africans. The Am J Hum Genet 70(3):758–62
Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Redlund M et al (2003) Geographical and ethnic variation of the 677C> T allele of 5, 10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. J Med Genet 40(8):619–25
Casarini L, Simoni M (2014) Gene polymorphisms in female reproduction. In: Rosenwaks Z, Wassarman PM (eds) Human fertility. Springer, New York, New York. https://doi.org/10.1007/978-1-4939-0659-8_4
Zeng S, Wang X, Wang Y, Xu Z, Zhang J, Liu W et al (2019) MTHFR C677T polymorphism is associated with follicle-stimulating hormone levels and controlled ovarian hyperstimulation response: a retrospective study from the clinical database. Fertil Steril 111(5):982–90
Rah H, Jeon YJ, Choi Y, Shim SH, Yoon TK, Choi DH et al (2012) Association of methylenetetrahydrofolate reductase (MTHFR 677C>T) and thymidylate synthase (TSER and TS 1494del6) polymorphisms with premature ovarian failure in Korean women. Menopause 19(11):1260–6
Acknowledgements
Not applicable
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
DC conceptualized the study and performed karyotyping. PS performed the assays and recorded the results. Both the authors contributed equally to the result interpretation, manuscript preparation, and revision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants were aware of and consented to enter this study (Ethical number: GU/IEC/02/2018).
Consent for publication
Informed written consent was obtained from all participants regarding use of sample for research purpose and to publish; provided the confidentiality of identity is maintained (in case of non-adult female, the consent was obtained from one of the parents). (Ethical number: GU/IEC/02/2018).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Karyotype Images.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanghavi, P.M., Chandel, D. MTHFR (C677T) polymorphism and its association with cytogenetic and clinical profile in individuals with primary amenorrhea. Egypt J Med Hum Genet 25, 5 (2024). https://doi.org/10.1186/s43042-023-00471-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-023-00471-5