Abstract
Background
Chronic myeloid leukaemia is characterised by genetic instability which results in additional cytogenetic aberrations that have been linked to progression to advanced phase. Genomic study linked amplified genes in the form of c-MYC and/or the rare BCR::ABL1 genes amplification to chronic myeloid leukaemia. The effect of these genes’ amplification on patients’ characteristics and disease progression still needs further study.
This cross-sectional study aimed to investigate the frequency of additional chromosomal aberrations in addition to c-MYC and BCR::ABL1 genes amplification in chronic myeloid leukaemia patients and their impact on patient’s characteristics, disease progression, and level of remission. The study included cytogenetic analysis of 49 Philadelphia positive chronic myeloid leukaemia patients and investigation of c-MYC and BCR::ABL1 genes amplification by fluorescence in situ hybridization.
Results
Patients with additional chromosomal aberrations represented 36.7% and had significantly lower platelet count (P = 0.003) and higher blast count (P = 0.008). The acquisition of additional chromosomal aberrations was significantly higher in chronic myeloid leukaemia patients with advanced stages (P = 0.014). Follow-up of the patients for 6 months revealed significant higher frequency of additional chromosomal aberrations in patients with failure of remission (P < 0.0001). A highly significant association between cases with failure of molecular remission (P = 0.001) and co-existing additional chromosomal aberrations.
Amplification of the c-MYC gene was detected in 6 cases. The cases with c-MYC amplification showed significantly higher peripheral blood and bone marrow blasts (P = 0.029 and P = 0.008, respectively) and significantly lower platelet count (P = 0.044). Amplification of c-MYC was significantly associated with additional chromosomal aberrations (P = 0.011). Molecular remission was not achieved in any of the instances with c-MYC amplification. A highly significant association between c-MYC amplification and poor patient outcome was detected (P = 0.002). BCR::ABL1 amplification was detected in three cases, and ABL amplification was detected in four cases. Patients with BCR::ABL1 amplification showed significantly higher blast count. BCR::ABL1 amplification was significantly associated with disease progression and failure of molecular remission (P = 0.002).
Conclusion
Additional chromosomal aberrations, c-MYC amplification, and BCR:ABL1 amplification in chronic myeloid leukaemia stratify patients with disease progression, which may lead to better interventions and improved outcome in the future chronic myeloid leukaemia patients.
Similar content being viewed by others
Background
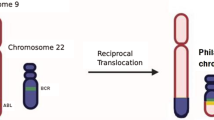
Chronic myeloid leukaemia (CML), a subtype of myeloproliferative neoplasm (MPN), is characterized by the translocation t(9;22)(q34;q11), the Philadelphia (Ph) chromosome [1]. The resulting breakpoint cluster region (BCR)::Abelson (ABL) chimeric protein which stimulates high levels of protein kinase activity is a hallmark of the leukemogenesis in CML patients [2, 3].
The course of CML is characterised by genetic instability which results in acquisition of additional cytogenetic aberrations (ACA) and clonal evolution [4]. A complex karyotype due to co-existing ACAs has been linked to the progression of CML to a more advanced phase [5].
According to genomic research, amplification of c-myelocytomatosis oncogene product (c-MYC) (located in 8q24) has been detected in myeloid malignancies [6]. Furthermore, a rare condition known as BCR::ABL1 intrachromosomal amplification has been linked to a poor prognosis and a poor response to imatinib therapy [7]. We believe further studies are still needed to identify the effect of ACAs and associated amplification of these genes on CML patients’ characteristics and outcome.
Objective
This study aimed to investigate frequency for ACA in addition to c-MYC and BCR::ABL1 genes amplification in CML patients and their impact on CML patient’s characteristics, disease progression, and level of remission.
Subjects and methods
The study protocol was approved by the Research Ethics Committee, Faculty of Medicine, Ain Shams University (FMASU R 195/2022), and written informed consent was obtained from all participants.
This is a cross-sectional study that had been conducted on a convenient sample of 49 patients diagnosed with CML. The study included 26 females and 23 males. Inclusion criteria of recruited patients were as follows: a) Patients more than 18 years, b) CML patients who were newly diagnosed with t(9;22)(q34;q11) detected by conventional cytogenetic analysis, confirmed by fluorescence in situ hybridization (FISH) and/or molecular testing for BCR::ABL1 fusion transcripts. Exclusion criteria were as follows: a) Patients less than 18 years old and b) CML patients who received treatment.
The study investigated the frequency of additional chromosomal aberrations (ACAs). In addition, we investigated c-MYC and BCR::ABL1 amplification by FISH analysis for studied CML patients. All patients were reassessed after 6 months for haematological, cytogenetic, and molecular levels of remission.
Patients either attended the haematology clinic or admitted to the University Hospital’s Haematology Department over 2022–2023. Patients were diagnosed with CML based on clinical features, complete blood count, peripheral blood smear examination, bone marrow smear findings, conventional cytogenetic analysis (CCA) by G-banding and FISH. After diagnosis, all patients were investigated for the presence of ACAs, as well as amplification of c-MYC and BCR::ABL1 genes. The stage of the disease was clearly defined as follows: a) Chronic phase (CP), b) CML with high-risk features associated with progression and resistance to targeted tyrosine kinase inhibitors (TKI), and c) Blast phase (BP). All studied patients were exposed to the CML protocol of therapy in the form of TKI. The studied patients were re-assessed at 6 months to detect the outcome of haematological, cytogenetic, and molecular levels of remission.
Sampling
Two millilitres of blood in sterile K2-EDTA vacutainers were used for complete blood count (CBC) and peripheral blood film preparation. Patients had CBC and blood film examination for counts of peripheral blood blasts. Bone marrow examination was carried for the disease phase. Three millilitres of heparinized bone marrow samples (2-3mL) were collected for karyotyping and FISH analysis.
According to Nardi et al. [8], conventional cytogenetic analysis was performed on at least 20 G-banding metaphases collected from unstimulated cultures from bone marrow aspirates. Interphase FISH analysis for the BCR::ABL1 fusion gene using LSI BCR::ABL1 double fusion probe was carried. Established procedures as described in Nardi et al. [8] were mounted using fixed cell suspensions acquired by direct or unstimulated overnight cultures of bone marrow [9]. The BCR::ABL1 translocation probe was an Abbott Molecular-Vysis LSI dual-colour, dual-fusion model. The LSI c-MYC (8q24) was an Abbott Molecular-Vysis LSI dual colour break apart rearrangement probe. Two independent observers examined each sample. Examination included a minimum of 200 interphase cells for each sample in addition to any accessible metaphases. Microscope imaging software of Leica microsystems with combined microscope, digital camera, and accessories was integrated in one system that was used.
Data were collected and analysed using the Statistical Package for Social Science (IBM SPSS) version 23. Quantitative data were shown as median and interquartile range. The comparison between Philadelphia positive (Ph +) CML patients with ACAs and other with no ACAs was studied using Chi-square test. The same was applied on comparing CML patients with c-MYC amplification, BCR::ABL1 amplification and ABL amplification and the other who do not have amplification of genes. Values less than 0.05 were appraised as significant. Qualitative data were displayed as number and percentages, and the comparison between groups was carried by using Chi-square test and/or Fisher exact test. The comparison between two independent groups with quantitative data and parametric distribution was settled using independent t test while with non-parametric distribution was settled by using Mann–Whitney test.
Results
The study included 49 Philadelphia positive CML patients (26 females and 23 males) with a mean age of 46.8. Out of 49 cases, 31 (63.3%) were diagnosed with CP, eight (16.3%) had CML with high-risk features associated with progression and resistance to targeted tyrosine kinase inhibitors (TKI) according to WHO 2022, and ten (20.4%) were in BP [9]. Out of the ten cases diagnosed with BP, nine cases revealed an immunophenotyping (IPT) of myeloblastic pattern, while only one case had lymphoblastic pattern.
After 6 months of follow-up, 12 of the 49 patients in the study achieved complete haematological, cytogenetic, and molecular remission (CHR-CCR-CMR) (Table 1), and all were diagnosed with CP. Six patients out of 49 failed to achieve any remission (NHR-NCR-NMR) (Table 1), three of whom had BP and the other three were in progression and resistance to TKI.
Karyotyping was successful in 43 patients; 32 patients had translocation t(9;22)(q34;q11) as a sole abnormality and 11 cases had ACAs in addition to t(9;22)(q34;q11). FISH analysis revealed t(9;22)(q34;q11) in all studied CML patients and confirmed co-existing ACAs in 18 patients only.
When we compared CML patients with ACAs (n = 18) to CML patients who have Philadelphia as a sole cytogenetic abnormality (n = 31), we found that CML patients with ACAs had significantly lower platelet count (P = 0.003) and higher blast count in the bone marrow (P = 0.008).
The acquisition of ACAs was significantly higher in CML patients with advanced stages (P = 0.014). ACAs were significantly associated with poor outcome in the form of failure of remission (NHR-NCR-NMR) with a highly significant association between cases with failure of molecular remission (NMR) and acquisition of ACAs (P < 0.0001 and P = 0.001, respectively).
c-MYC gene amplification represented as three composite signals or more (Fig. 1). The amplification of the c-MYC gene was found in six cases. Out of those six cases, five cases had associated + 8. BCR::ABL1 amplification was detected in three cases (Fig. 1), while ABL amplification was observed in four cases. None of the cases with BCR::ABL1 amplification or ABL amplification had associated double Ph chromosome.
The cases with c-MYC amplification showed significantly higher peripheral blood and bone marrow blasts (P = 0.029 and P = 0.008, respectively) and significantly lower platelet count (P = 0.044) (Table 2). CML cases with c-MYC amplification had a statistically significant higher frequency of ACAs (P = 0.011), with + 8 being the most common (p = 0.0001) (Table 3). Three out of the six cases with c-MYC amplification did not achieve any level of remission (NHR-NCR-NMR). Although haematological and cytogenetic remission was achieved in the other three cases, molecular remission was not achieved in any of the instances with c-MYC amplification. Follow-up for 6 months revealed a highly significant association between c-MYC amplification and poor patient outcome (P = 0.002).
The cases with BCR::ABL1 and ABL amplification revealed significantly higher peripheral blood blasts (p = 0.03) (Table 4). This study reported no significant association between BCR::ABL1 or ABL amplification and presence of ACAs or patient outcome.
Discussion
The studied patients in the current study had a mean age of 46.8 which is consistent with the age of CML reported at middle eastern centres in 2019 [10]. It has been noted that the age of CML has dropped to the fifth decade. The reasons behind this early incidence of CML, however, are unclear [11, 12]. This appears to be at odds with studies conducted in the West that described CML in older age groups [13]. This may be attributed to the growth of population in developing countries and the increase of ageing in developed countries [14]. In developed countries, ageing is a risk factor related to a decrease in haematopoietic stem cell function and leukemogenesis and negatively affects therapy [15, 16]
In CML, ACAs are continuously acquired and have been described to be associated with progression to more advanced phases and/or CML BP [17]. In our study, 18 of 49 CML patients (36.7%) had ACAs by FISH analysis. Azzazi et al., in 2018, reported ACAs in CML patients at a lower rate [17]. In 2011, Hsiao et al. reported a lower percentage in newly diagnosed CML subjects had ACAs [18]. This may be explained by our lower sample size.
In the current study, patients with ACAs had a significantly higher blast count and more advanced stages. These findings are completely consistent with previous research [19, 20]. In 2019, a study by Chandran and his colleagues came to a similar conclusion when they reported a considerably greater incidence of ACAs in patients diagnosed with BP and accelerated phase (AP) of CML [4].
According to the findings of the present study, patients with failure of remission (NHR-NCR-NMR) have a considerably greater incidence of ACAs with a highly significant association between cases with failure of molecular remission (NMR) and frequency of ACAs. Our results tie well with previous studies [21, 22]. Bozkurt et al., study reported progression to advanced phases and/or BP in 58% of CML patients with ACAs and most did not have a cytogenetic remission (CR) [21].
Cellular effects of the proto-oncogene c-MYC on cell cycle dynamics, apoptosis, DNA damage response, and haematopoiesis are extensive. Its expression is controlled on several different levels due to its multifunctionality [23]. Although the expression of the BCR::ABL1 kinase is the molecular hallmark of CML [24], earlier research reported additional processes at various levels, such as JAK2, which is discovered to boost c-MYC expression and is driven by BCR::ABL1 [25] and collaborates with c-MYC for transformation [26].
In this study, we investigated the amplification of c-MYC in CML subjects. We detected c-MYC amplification in 6 of our 49 studied subjects. A significant association between the presence of c-MYC amplification and ACAs was also detected. Patients with amplification of c-MYC had a significant lower platelet count and a significantly higher peripheral blood and bone marrow blasts. These findings are consistent with earlier research showing high expression of c-MYC in CML patients in BP [27, 28].
In our study, all the studied cases with c-MYC amplification failed to achieve molecular remission. A highly significant association between the presence of c-MYC amplification and failure of remission (NHR- NCR-NMR) was reported. These findings are consistent with most of previous studies, which found that amplified c-MYC was associated with disease progression and poor prognosis in patients with myeloid malignancies [29, 30]. Our results support the role of c-MYC in genomic instability and differentiation arrest [31].
c-MYC amplification was significantly associated with + 8. Previous studies disclosed low-level amplification of c-MYC in number of patients with + 8 acute myeloid leukaemia (AML), + 8 myelodysplastic syndrome and CML in BP [32, 33]. Some authors elaborated that the expression of other gene(s) included in 8q24 amplicon is a pathogenetically important consequence [34].
This interrelationship between trisomy 8 and c-MYC amplification in myeloid leukemogenesis has been of concern, where the biological mechanism of the + 8 cell clones' onco-proliferative activity was explained by a gene dosage effect [35].
Furthermore, a previous comparative genomic hybridization ratio measurement revealed that a gain of 8q24 is associated with mutation of the p53 tumour suppressor gene. The association between the gain at 8q24 and the p53 mutation might be responsible for the transactivation of the c-MYC gene by the p53 promoter [36]. Our results together with the previously reported results call attention to amplification of c-MYC and trisomy 8 as evolution events in CML advanced phases with ACAs.
Three CML patients in the study group had BCR::ABL1 amplification, all were in BP, and all three cases failed to achieve molecular remission. BCR::ABL1 fusion gene amplification was associated with advanced stages of CML. Similarly, a previous study published in 2019 by Chandran and his colleagues reported multiple copies of the BCR::ABL1 fusion gene in advanced stages of CML and IM resistant CP patients. In accordance with our results, Chandran et al. study recommended using FISH to detect BCR::ABL1 fusion gene amplification in the future CML patients in order to improve their outcomes [37]. Other studies have found that BCR::ABL1 fusion gene amplification associated BP is highly resistant to chemotherapy, with a response rate < 30% and a 5-year survival rate of only 6% [38, 39].
Interestingly, our studied patients with ABL amplification had higher bone marrow and peripheral blood blast and failed to achieve molecular remission. Though amplification of ABL alone is rare, sixfold and 15-fold amplification of the gene have been described in the CML-derived cell line, K562 [40] and in a patient with CML in lymphoid blast crisis, respectively [41]. The only other FISH report of amplification involving ABL was in three cases of secondary AML [42] which still needs further study.
Limitation of the study
One limitation of this study is the small sample size. This study examined the frequency of ACAs in CML patients and its relation to clinical features, haematological parameters, and patient outcome. In addition, the study revealed preliminary results of the infrequent c-MYC and BCR::ABL1 genes amplification detected in the studied CML patients. We recommend further studies on larger scale investigating c-MYC and BCR::ABL1 amplification in CML patients for clinically relevant and conclusive results.
Conclusion
The study reported the frequency of ACAs in CML patients and described the considerably greater frequency of ACAs in patients diagnosed with disease progression. This study reported higher frequency of ACAs in CML patients with poor outcome and failure of remission.
The study described c-MYC and BCR::ABL1 genes amplification in CML patients. The CML studied patients with genes amplification showed co-acquisition of ACAs, disease progression and failure of remission. These results call the attention to the impact of c-MYC and BCR::ABL1 amplification on CML and recommend further studies on larger scale investigating c-MYC and BCR::ABL1 amplification in CML patients
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- c-MYC :
-
C-myelocytomatosis oncogene product
- ABL :
-
Abelson
- BCR :
-
Breakpoint cluster region
- CML:
-
Chronic myeloid leukaemia
- MPN:
-
Myeloproliferative neoplasm
- ACA:
-
Additional chromosomal aberrations
- FISH:
-
Fluorescence in situ hybridization
- CP:
-
Chronic phase
- BP:
-
Blast phase
- Ph+ :
-
Philadelphia chromosome positive
- IPT:
-
Immunophenotyping
- TKI:
-
Targeted tyrosine kinase inhibitors
- CHR:
-
Complete haematological remission
- CCR:
-
Complete cytogenetic remission
- CMR:
-
Complete molecular remission
- NHR:
-
No haematological remission
- NCR:
-
No cytogenetic remission
- NMR:
-
No molecular remission
- MMR:
-
Major molecular remission
- AP:
-
Accelerated phase
- CR:
-
Cytogenetic remission
- IM:
-
Imatinib
- AML:
-
Acute myeloid leukaemia
References
Minciacchi VR, Kumar R, Krause DS (2021) Chronic myeloid leukaemia: a model disease of the past, present and future. Cells 10(1):117
Barnes EJ, Eide CA, Kaempf A, Bottomly D, Romine KA, Wilmot B et al (2023) Secondary fusion proteins as a mechanism of BCR::ABL1 kinase-independent resistance in chronic myeloid leukaemia. Br J Haematol 200:323–328. https://doi.org/10.1111/bjh.18515
Zhao H, Chen Y, Shen C et al (2021) Breakpoint mapping of a t(9;22; 12) chronic myeloid leukaemia patient with e14a3 BCR-ABL1 transcript using Nanopore sequencing. J Gene Med 23:e3276. https://doi.org/10.1002/jgm.3276
Krishna Chandran R, Geetha N, Sakthivel KM, Suresh Kumar R, Jagathnath Krishna KMN, Sreedharan H (2019) Impact of additional chromosomal aberrations on the disease progression of chronic myelogenous leukemia. Front Oncol 9:88
Bacher U, Haferlach T, Hiddemann W, Schnittger S, Kern W, Schoch C (2005) Additional clonal abnormalities in Philadelphia-positive ALL and CML demonstrate a different cytogenetic pattern at diagnosis and follow different pathways at progression. Cancer Genet Cytogen 157:53–61. https://doi.org/10.1016/j.cancergencyto
Vincelette ND, Moon J, Kuykendall AT, Zhang L, Komrokji RS, Murphy D, Cleveland JL, Yun S (2021) C-MYC augments the proliferation and survival of hematopoietic stem cells and multipotent progenitors to drive myeloproliferative neoplasms. Blood 138(Supplement 1):28. https://doi.org/10.1182/blood-2021-146399
Virgili A, Nacheva EP (2010) Genomic amplification of BCR/ABL1 and a region downstream of ABL1 in chronic myeloid leukaemia: a FISH mapping study of CML patients and cell lines. Mol Cytogenet 1(3):15. https://doi.org/10.1186/1755-8166-3-15
Nardi V, Pulluqi O, Abramson JS, Dal Cin P, Hasserjian RP (2015) Routine conventional karyotyping of lymphoma staging bone marrow samples does not contribute clinically relevant information. Am J Hematol 90(6):529–533. https://doi.org/10.1002/ajh.24008
Khoury JD, Solary E, Abla O et al (2022) The 5th edition of the World health organization classification of haematolymphoid tumours myeloid and histiocytic/dendritic neoplasms. Leukemia 36:1703–1719. https://doi.org/10.1038/s41375-022-01613-1
Khaled SA, Nabih O, Abdel Aziz NM, Mahran DG (2019) Myeloid leukemias: a glance at middle Eastern centers. J Blood Med 16(10):425–433. https://doi.org/10.2147/JBM.S221317
Babu GK, Thanky A, Jacob LA et al (2015) Outcome of young adults with chronic myeloid leukaemia treated with upfront imatinib: a single institutional experience. J Appl Hematol 6:157–161. https://doi.org/10.4103/1658-5127.171987
Phekoo KJ, Richards MA, Møller H, Schey SA (2006) South Thames haematology specialist Committee the incidence and outcome of myeloid malignancies in 2112 adult patients in southeast England. Haematologica 91(10):1400–1404
Cai S, Zhong Z, Li X, Wang L, Wang H, You Y et al (2019) ACA rearrangement is associated with the prognosis of CML in the Era of TKI. Ann Hematol Oncol 6(6):1252
Ning L, Hu C, Lu P, Que Y, Zhu X, Li D (2020) Trends in disease burden of chronic myeloid leukemia at the global, regional, and national levels: a population-based epidemiologic study. Exp Hematol Oncol 9(1):29. https://doi.org/10.1186/s40164-020-00185-z.PMID:33292593;PMCID:PMC7607878
de Haan G, Lazare SS (2018) Aging of hematopoietic stem cells. Blood 131:479–487
Verovskaya EV, Dellorusso PV, Passegué E (2019) Losing sense of self and surroundings: hematopoietic stem cell aging and Leukemic transformation. Trends Mol Med 25:494–515
Azzazi M, Moussa M, Amro El-Ghammaz A, Eissa A, Hamza M (2018) Study of additional chromosomal abnormalities in young adult Egyptian chronic myeloid leukaemia patients. Egyptian J Haematol Bone marrow Transplantation 5(5):8–14
Hsiao HH, Liu YC, Tsai HJ, Hsu JF, Yang WC, Chang CS, Lin SF (2011) Additional chromosome abnormalities in chronic myeloid leukemia. Kaohsiung J Med Sci 27(2):49–54. https://doi.org/10.1016/j.kjms.2010.09.001
Wang W, Tang G, Cortes JE, Liu H, Ai D, Yin CC, Li S, Khoury JD, Bueso-Ramos C, Medeiros LJ, Hu S (2015) Chromosomal rearrangement involving 11q23 locus in chronic myelogenous leukemia: a rare phenomenon frequently associated with disease progression and poor prognosis. J Hematol Oncol 8(8):32. https://doi.org/10.1186/s13045-015-0128-2
Wang W, Cortes JE, Tang G, Khoury JD, Wang S, Bueso-Ramos CE, DiGiuseppe JA, Chen Z, Kantarjian HM, Medeiros LJ, Hu S (2016) Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood 127(22):2742–2750. https://doi.org/10.1182/blood-2016-01-690230
Bozkurt S, Uz B, Buyukasik Y, Bektas O, Inanc A, Goker H, Kansu E (2013) Prognostic importance of additional cytogenetic anomalies in chronic myeloid leukemia. Med Oncol 30(1):443. https://doi.org/10.1007/s12032-012-0443-1
Cortes JE, Kantarjian HM, Goldberg SL, Powell BL, Giles FJ, Wetzler M, Akard L, Burke JM, Kerr R, Saleh M, Salvado A, McDougall K, Albitar M, Radich J, Rationale and Insight for Gleevec High-Dose Therapy (RIGHT) Trial Study Group (2009) High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J Clin Oncol 27(28):4754–4759. https://doi.org/10.1200/JCO.2008.20.3869
Chandran RK, Geetha N, Sakthivel KM, Aswathy CG, Gopinath P, Nair JKKM, Sreedharan H (2018) Prognostic implications of derivative chromosome 9 deletions in patients with advanced-stage chronic myelogenous leukemia. J Environ Pathol Toxicol Oncol 37(2):117–126. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2018026023
Ahmadi SE, Rahimi S, Zarandi B et al (2021) MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol 202(14):121
Melo JV, Barnes DJ (2007) Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer 7(6):441–453. https://doi.org/10.1038/nrc2147
Xie S, Lin H, Sun T, Arlinghaus RB (2002) Jak2 is involved in c-MYC induction by Bcr-Abl. Oncogene 21(47):7137–7146. https://doi.org/10.1038/sj.onc.1205942
Afar DE, Goga A, McLaughlin J, Witte ON, Sawyers CL (1994) Differential complementation of Bcr-Abl point mutants with c-MYC. Science 264(5157):424–426. https://doi.org/10.1126/science.8153630
Handa H, Hegde UP, Kotelnikov VM, Mundle SD, Dong LM, Burke P, Rose S, Gaskin F, Raza A, Preisler HD (1997) Bcl-2 and c-MYC expression, cell cycle kinetics and apoptosis during the progression of chronic myelogenous leukemia from diagnosis to blastic phase. Leuk Res 21(6):479–489. https://doi.org/10.1016/s0145-2126(97)00006-4
Wolman SR, Gundacker H, Appelbaum FR, Slovak ML, Southwest Oncology Group (2002) Impact of trisomy 8 (+8) on clinical presentation, treatment response, and survival in acute myeloid leukemia: a Southwest oncology group study. Blood 100(1):29–35. https://doi.org/10.1182/blood.v100.1.29
Schoch C, Kohlmann A, Dugas M, Kern W, Hiddemann W, Schnittger S, Haferlach T (2005) Genomic gains and losses influence expression levels of genes located within the affected regions: a study on acute myeloid leukemias with trisomy 8, 11, or 13, monosomy 7, or deletion 5q. Leukemia 19(7):1224–1228. https://doi.org/10.1038/sj.leu.2403810
Meyer N, Penn LZ (2008) Reflecting on 25 years with MYC. Nat Rev Cancer 8(12):976–990
Quintás-Cardama A, Cortes J (2009) Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood 113(8):1619–1630. https://doi.org/10.1182/blood-2008-03-144790
Mathew S, Lorsbach RB, Shearer P, Sandlund JT, Raimondi SC (2000) Double minute chromosomes and c-MYC amplification in a child with secondary myelodysplastic syndrome after treatment for acute lymphoblastic leukemia. Leukemia 14(7):1314–1315. https://doi.org/10.1038/sj.leu.2401782
Slovak ML, Ho JP, Pettenati MJ, Khan A, Douer D, Lal S, Traweek ST (1994) Localization of amplified MYC gene sequences to double minute chromosomes in acute myelogenous leukemia. Genes Chromosomes Cancer 9(1):62–67. https://doi.org/10.1002/gcc.2870090111
Angelova S, Jordanova M, Spassov B, Shivarov V, Simeonova M, Christov I, Angelova P, Alexandrova K, Stoimenov A, Nikolova V, Dimova I, Ganeva P, Tzvetkov N, Hadjiev E, Toshkov S (2011) Amplification of c-MYC and MLL genes as a marker of clonal cell progression in patients with myeloid malignancy and trisomy of chromosomes 8 or 11. Balkan J Med Genet 14(2):17–24. https://doi.org/10.2478/v10034-011-0043-y
Jain AN, Chin K, Børresen-Dale AL, Erikstein BK, EynsteinLonning P, Kaaresen R, Gray JW (2001) Quantitative analysis of chromosomal CGH in human breast tumors associates copy number abnormalities with p53 status and patient survival. Proc Natl Acad Sci U S A 98(14):7952–7957. https://doi.org/10.1073/pnas.151241198
Chandran RK, Geetha N, Sakthivel KM, Aswathy CG, Gopinath P, Raj TVA, Priya G, Nair JKKM, Sreedharan H (2019) Genomic amplification of BCR-ABL1 fusion gene and its impact on the disease progression mechanism in patients with chronic myelogenous leukemia. Gene 20(686):85–91. https://doi.org/10.1016/j.gene.2018.11.005
Kantarjian H, Susan O’Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, Rios MB, Ravandi F, Fader S, Kadia T, Borthakur G, Huang X, Champlin R, Talpaz M, Cortes J (2012) Improved survival in chronic myeloid leukaemia since the introduction of imatinib therapy: a single-institution historical experience. Blood 119(9):1981–1987
Jabbour EJ, Hughes TP, Cortés JE, Kantarjian HM, Hochhaus A (2014) Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukaemia. Leuk Lymphoma 55(7):1451–1462. https://doi.org/10.3109/10428194.2013.845883
Collins SJ, Groudine MT (1983) Rearrangement and amplification of c-abl sequences in the human chronic myelogenous leukemia cell line K-562. Proc Natl Acad Sci U S A 80(15):4813–4817. https://doi.org/10.1073/pnas.80.15.4813
Kenner L, Beham-Schmid C, Kerbl R, Schmidt HH, Sill H, Hoefler G (1999) ABL amplification in a patient with lymphoid blast crisis of chronic myelogenous leukaemia. Virchows Arch 434(3):255–257. https://doi.org/10.1007/s004280050337
Tanaka K, Arif M, Eguchi M, Kyo T, Dohy H, Kamada N (1997) Frequent jumping translocations of chromosomal segments involving the ABL oncogene alone or in combination with CD3-MLL genes in secondary leukemias. Blood 89(2):596–600
Acknowledgements
NIL
Funding
Self-funded (NIL).
Author information
Authors and Affiliations
Contributions
Conception and design were performed by Dr. Fouad. Data collection was done by Dr. Fouad. Data analysis and interpretation were performed by Dr. Attia and Dr. Samy. Manuscript preparation was done by Dr. Attia. Critical revision of the manuscript was done by Dr. Fouad. Final approval of the manuscript was done by Dr. Fouad, Dr. Attia and Dr. Samy. Supervision was done by Dr. Fouad.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Research Ethics Committee, Faculty of Medicine, Ain Shams University (FMASU R 195/2022), and written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Attia, H., Fouad, D.A. & Samy, H. The study of the impact of additional chromosomal aberrations and c-MYC and BCR::ABL1 genes amplification on CML patient’s characteristics: relation to haematological parameters and patient outcome. Egypt J Med Hum Genet 24, 78 (2023). https://doi.org/10.1186/s43042-023-00460-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-023-00460-8