Abstract
Background
Polycystic ovary syndrome (PCOS) is an endocrinopathy affecting women of reproductive age group at a global level. According to many community-based studies, the prevalence of PCOS in India ranges from 3.7 to 22.5% due to the country's enormous population. Upon ultrasound, it shows multiple cysts arranged in a bead of necklace-like appearance causing irregular menstrual cycles and infertility in most cases. It is manifested with abnormally raised testosterone and insulin levels and increased luteinizing hormone (LH)-to-follicle-stimulating hormone (FSH) ratio. Phenotypically, it is presented as obesity, hirsutism, acne and male pattern baldness, which impacts the self-esteem of young girls leading to depression and compromised quality of life.
Aim
Numerous potential genes have been shown to contribute to PCOS, and the genetic linkage of PCOS has been investigated in many studies. In this study we are looking into the candidate genes, the variants, and other responsible factors behind the genesis of PCOS. This will help in better understanding of its pathogenesis and, as a result, deciphering the mechanism by proper medication.
Method of the study
We comprehensively searched for publications including PCOS-relevant keywords in different areas in five different electronic databases: PubMed, Google Scholars, Elsevier, Springer Link and Science Direct up to March 2023 focusing on the new ones. We excluded non-English articles, conference papers and studies that were overlapping. Chosen articles were carefully read and further articles that were retrieved from their references were also reviewed so as to make the search complete with the inclusion criterion.
Result
This review summarizes PCOS as an polygenic and a multifactorial complex disease in which a vast array of genetic and environmental factors are involved. Genes that affect steroidogenesis (ovarian and adrenal), gonadotropin action and regulation, insulin action and secretion, body mass index and chronic inflammation are directly or indirectly associated with PCOS.
Conclusion
In this study, research of the genetic propensity to PCOS was made, though not in-depth. With the acquired knowledge of array of genes involved, targeted efforts can be made for the potential therapeutic management of the PCOS patients via the novel discovered routes. Moreover, understanding more about PCOS would be beneficial in prevention of the associated metabolic disorders, life-threatening morbidities, restoring fertility and raising the self-esteem of the young women.
Similar content being viewed by others
Background
Polycystic ovarian syndrome, also referred as Stein Leventhal Syndrome, is a multifactorial disease with a complex pathophysiology, affecting 5–15% of premenopausal women globally. It is characterized by metabolic disturbances, endocrine imbalances and life-threatening co-morbidities [1, 2]. Menstrual dysfunction affects an estimated one in five to six women owing to stress, obesity and hormonal fluctuations [3, 4]. The three most common factors associated with PCOS include irregular ovulation, elevated androgen levels and cystic ovaries on ultrasound. During the manifestation of PCOS, the observable ovarian phenotypic changes include thickened capsule layer and a drastic increase in the number of small antral follicles arrested at the stage of the cell cycle wherein the dominant follicles are undergoing the selection process. In comparison to normal ovulatory follicles, these cystic follicles demonstrate a decreased number of granulosa cell layers and elevated levels of the number of steroidogenic cells in theca interna, suggesting a strong correlation between abnormalities in proliferation and differentiation in PCOS’s theca interna and granulosa layers. It results in changed hormonal mileu in transformed ovaries of PCOS females leading to the development of cysts in the ovarian stroma [5].
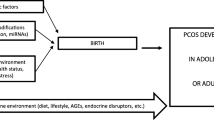
Earlier literature reported the association of genetic predisposition to PCOS development; however, no uniform consensus has been reached till now on established genetic marker for PCOS. As depicted in Fig. 1, PCOS progression is regulated by various environmental and genetic factors that affect ovaries directly or indirectly,. Several genes have a crucial role in the manifestation of this syndrome in females; these genes block and regulate the activity of various metabolic/hormonal pathways. The abnormal gene regulation at the genetic level leads to various post-translational modifications in the protein products, which causes the development of PCOS. The environmental or occupational factors such as sedentary lifestyle and dietary habits cause deterioration of the reproductive cycle by irregular menstruation, loss of physical activity, obesity, impairment of the menstrual cycle, etc., which contribute to the prevalence and modulation of PCOS. Throughout evolution, PCOS manifestation has occurred that can be demonstrated by the presence of 2–3 times increase in Anti-Mullerian hormone (AMH) in PCOS females, which serves as a diagnostic biomarker for monitoring the pathophysiology of PCOS [6,7,8].
This review presents a brief overview of pathophysiology of PCOS in light of vast array of genes involved in disruption of biochemical pathways—ovarian and adrenal steroidogenesis, gonadotropin action and regulation, insulin action and secretion, energy homeostasis and chronic inflammation.
Etiology of PCOS
The etiology of this syndrome is a combination of hereditary and environmental factors. Unhealthy eating habits, lifestyle choices and infectious agents further contribute to disease severity and progression [9, 10]. Hayes et al. in his study emphasized the association of PCOS with genetic components, candidate genes and single-nucleotide polymorphism (SNPs). As per the database, 241 gene variants have a direct implication in the etiology of PCOS manifestation [11,12,13]. The abnormal transcriptional activity of a gene due to polymorphisms or nucleotide mutations, results in PCOS [14]. Several polymorphisms have been identified as contributing to PCOS including steroidogenic acute regulatory (StAR) polymorphs, follicle-stimulating hormone receptor (FSHR) polymorphs, FTO alpha-ketoglutarate-dependent dioxygenase (FTO) polymorphs, vitamin D receptor (VDR) polymorphs, insulin resistance (IR) and insulin receptor substrate (IRS) polymorphs and gonadotropin-releasing hormone receptor (Gn-RHR) polymorphs. An ovary becomes dysfunctional due to a gene defect that disrupts the biochemical pathway. Mainly the genes encoding for hormone receptors such as androgen, LH, FSH, insulin and leptin are accountable [15]. With the increase in insulin secretion, ovarian theca cells respond by elevating the androgen level (androstenedione) that leads to anovulation. Unlike the normal ovary that metabolizes the androstenedione produced by the theca cells to estrone in peripheral tissues and estradiol in the granulosa cells, polycystic ovaries do not express the high aromatase activity thereby accumulating majority of androstenedione. The condition lowers the hepatic biosynthesis of sex hormone-binding globulin (SHBG) and insulin-like growth factor-binding protein-1 (IGFBP-1) produced in the liver. The stimulation of visceral adipose tissue (VAT) by increasing androgen levels leads to the production of free fatty acids (FFAs), which further contribute to insulin resistance [16]. An imbalanced secretion pattern of the gonadotropin-releasing hormone (GnRH) in PCOS contributes in the relative increase in LH-to-FSH ratio. As a result of this deranged ratio, ovulation does not occur in PCOS patients [17, 18].
Genetic predisposition and PCOS
There is a strong tie between the genetic predisposition and PCOS, which is demonstrated by the expression of different genes in different locations, illustrating other functions, as shown in Table 1. Expression of altered patterns of these genes is highly indicative of deranged signal transduction pathways involved in a cluster of a family of genes rather than a single gene. An overview of the genetic paradigm of PCOS is depicted in Table 1, and their roles in PCOS are discussed below in detail.
Genes involved in steroid hormone effect
Androgen receptor gene (AR)
This gene, which has 11 exons and is located on chromosome Xq12, codes for a protein-90 kb long and consists of 3 functional domains [19]. PCOS and androgen receptor AR are related. The androgen signaling pathway is disturbed and increased by X inactivation. A single copy of the X chromosome can affect the entire pathway of gene regulation of AR, an X-linked gene. Genome-wide association studies for PCOS can be used to find novel mutations and other genetic variations linked to the etiology of this condition [20].
Sex hormone-binding globulin gene (SHBG)
Sex steroid-binding globulin (SSBG), also known as sex hormone-binding globulin (SHBG), is a glycoprotein that binds to androgens and estrogens, located on chromosome 17p13-p12. It is also known as an androgen-binding protein when it is generated by Sertoli cells in the seminiferous tubules of the testis (ABP). Generally, SHBG gene polymorphism is associated with PCOS. Down-expression of this gene often serves as a potential biomarker of insulin resistance, abnormal glucose and lipid metabolism in PCOS patients.
Genes involved in gonadotropin action and regulation
Follicular-stimulating hormone receptor (FSHR)
The FSHR gene has 14 exons and is located on chromosome 2p16.3. This gene produces the G-coupled receptors protein, which is essential for the growth of the gonads [21]. Hormonal imbalances have an impact on the reproductive endocrine system. In addition to other hormone imbalances, FSH level is also accountable for the severity of PCOS. Follicular-stimulating hormone receptor is responsible for encoding FSH. Any aberration in the FSHR causes disturbances in follicular and ovarian function. Studies have reported a crystal-clear distinction between individuals of two cohorts—healthy and affected in the North of Iraq, by statistical analysis and RFLP using the Eam11051 restriction enzyme [22].
Anti-Müllerian hormone (AMH)
Polycystic ovaries produce many follicles—pre-antral and antral, which further up regulate the production of AMH, a member of the growth factor β family [23, 24]. The AMH gene is located on the long arm of chromosome 19 at cytogenetic location 13.3. Hence, elevated AMH levels are helpful for estimating ovarian volume in women with PCOS [25, 26]. The serum AMH levels are proposed as a diagnostic test for PCOS [27, 28]. The role of AMH in PCOS etiology has been reported, which mainly focuses on the AMH gene and its Type II Receptor (AMHR2). Kevenaar et al. studied the role of AMH SNPrs10407022 (Ile49Ser) and the AMHR2 polymorphism rs2002555 with the PCOS [29]. GATA4, FOXL2 and steroidogenic factor 1 are some transcription factors (TFs) that have been newly discovered genes that control AMH production in the ovary. There is an increase in the number of follicles, elevated expression of AMH levels per follicle as well as increased expression of AMH and AMHR2 in PCOS women, in contrast normal ovulatory females showed the absence of AMH gene expression after the follicle maturation [30,31,32,33].
Genes involved in insulin action and secretion
Insulin gene (INS)
Insulin is produced by the androgen receptors in the theca cells, which is also activated through the pathway (phosphoinositide 3-kinase/protein kinase B) in PCOS theca cells. Increased production of insulin corresponds to increased androgen synthesis, similarly to LH. The transcriptional activity of INS and IGF-II is often regulated by these VNTR polymorphisms which is associated with PCOS [34, 35].
Calcium-dependent cysteine protease (CAPN)
The calcium-dependent cysteine protease CAPN10 is often referred to as Caplain10. There are 12 exons in it, which are located on chromosome 2q37.3, a heterodimer protein associated with type 2 diabetes. It is located in a region where type 1 non-insulin-dependent diabetes mellitus is prevalent [36]. Calpain 10 is a cysteine protease encoded by the chromosomal region CAPN10. Calpain 10 has been identified to be involved in the action and secretion of insulin. Since insulin resistance and type 2 diabetes are linked to PCOS, genetic polymorphisms in CAPN10 result in PCOS, hence serving as an ideal candidate gene for PCOS [20].
Genes involved in insulin sensitivity and BMI
Fat mass obesity (FTO)
Alpha-ketoglutarate-dependent dioxygenase is another name for the FTO gene, which is located on chromosome 16q12.2 and contains 14 exons. Many investigations have demonstrated a link between FTO and type 2 diabetes, obesity and BMI [37]. A study carried out in Pakistan indicates the reported evidence of genetic polymorphism in the FTO gene among PCOS females. Patients with PCOS had the intronic variation of the rs9939609 SNP. The considerable BMI difference between affected patients and healthy persons has been found through genetic and statistical analyses. FTO genes (rs9939609 and rs1421085) in Saudi women suffering from PCOS shows increased obesity and provide likely a connection between FTO and PCOS susceptibility.
Tumor necrosis factor (TNF)
The TNF super family, which includes several transmembrane proteins with a homologous TNF domain, includes TNF-α as a member. The TNF-α is an adipokine and cytokine that causes insulin resistance and is linked to type 2 diabetes brought on by fat [38]. TNF-α is involved in chronic inflammation in PCOS females, but a direct association of polymorphism of TNF-α is not yet reported. But studies indicate that PCOS phenotypic traits are modified by TNF- gene polymorphism [39].
Genes involved in ovarian steroidogenesis and gametogenesis
Leptin
Leptin is acknowledged as a peripheral signal and a potential regulator of a variety of reproductive processes, including ovarian steroidogenesis and gametogenesis. Leptin is thought to be a link between diet and reproduction [40]. The hypothalamus–pituitary–ovarian (HPO) axis contains the leptin receptor and mRNA [41, 42] Leptin mRNA and protein production is also established in granulosa cells, oocytes and early cleavage-stage embryos. [43]. Leptin regulates ovarian folliculogenesis by leptin receptors in granulosa cells, and glucocorticoids regulate steroidogenesis [44]. It modulates the side chain cleavage enzyme and 17α-hydroxylase [45] and LH-stimulated estradiol production [46] in the ovary. Also, it serves as a potential marker in PCOS patients. Generally, PCOS individuals have elevated leptin levels than normal ovulatory females [47,48,49].
Aromatase gene
Aromatase, a member of the intricate Cytochrome P450 family, plays a crucial part in steroid conversion, and the conversion of testosterone into estrogen is one of the enzymes involved in steroidogenesis. A malfunction in the route caused by an aromatase deficit prevents its conversion [50]. Due to the lack of C19 to C18 conversion, this defect will disrupt ovarian function and boost androgen levels. Aromatase genes associated with PCOS are CYP11A1, CYP11B2, CYP17A1, CYP19A1, CYP1A1, CYP21A2 and CYP3A7 listed in Table 1. Cytochrome P450 abnormality is associated with an increased risk of PCOS manifestation and progression.
-
CYPA1A gene: The etiology of PCOS has been linked to the CYPA1A gene, which is also recognized as a candidate gene. Cytochrome P450 family 1, subfamily A, member 1 is the acronym for this gene, which is present on chromosome 15q24.1. There are seven exons in all. Polycyclic aromatic hydrocarbons (PAHs) have a significant role in inducing the expression of this gene, which encodes the Cytochrome P450 proteins found in the endoplasmic reticulum [51]. In a study of PCOS patients and healthy people, the ratio of isoleucine to valine in PCOS patients was higher than in healthy people. It was also determined through statistical analysis that PCOS patients have the genotype for valine and that isoleucine is replaced by valine in this condition. As a result, they concluded that the isoleucine/valine CYP1A1 genotype was 7.8 times more common than the valine genotype, which was 7.4 times more common [52]. Increased toxification and detoxification may result from polymorphism in phase 1 and 2 enzymes. Any change in such enzymes causes aberrant ovarian function and cyst development. Strong correlations exist between PCOS susceptibility and the genetic variant T6235C in the CYP1A1-encoded phase 1 enzyme. Because of the disruption to the enzymatic route caused by this mutant gene, PCOS propensity and advancement are at risk [53].
-
CYP11A1 gene: CYP11A1 is a member of the Cytochrome P450 family 11, subfamily A. The super family of cytochrome p450 is encoded. It is present in the inner membrane of mitochondria. Pregnenolone is produced primarily by the catalysis of cholesterol. In the pathway for steroid production, it is also essential. The ten exons that make up this gene are found on chromosome 15q24.1 [54]. The promotor pentanucleotide (TTTTA)n polymorphism is another genetic predisposition factor for PCOS. According to reports, CYP11A1 polymorphism is a molecular risk marker for PCOS. An interplay between hereditary and environmental variables raised the risk. Approximately 15 allele variations, with the most prevalent having eight repetitions, was found in a study of the South Indian population, generally ranging between 2–16 repeats. The occurrence of > 8 repeat alleles in PCOS-affected females was also examined in this study, which implies a threefold increased risk of PCOS predisposition compared to controls [55]. Case–control research conducted in China showed that polymorphism in CYP11A1 is thought to be the primary cause of PCOS. SNP rs4077582 in CYP11A1 is strongly linked to PCOS and increases androgen levels by regulating luteinizing hormones in different genotypes [56].
-
CYP11b2 gene: Cytochrome P450, family 11, subfamily B and member 2 is its short form. Nine exons make up this gene, which is found on chromosome 8q24.3. Its job is to transmit instructions for the adrenal gland aldosterone synthetases to create new molecules [57]. According to reports, this additional gene is in charge of the developing of PCOS. According to the results of a case–control study, the etiology of PCOS is due to polymorphism in the aldosterone synthetase promoter area. When compared to people without PCOS, the frequency of polymorphism was significantly high. Since PCOS-affected individuals also had significantly higher levels of aldosterone and testosterone, the likelihood of developing PCOS was enhanced [58].
-
CYP17A1 gene: Another monooxygenase involved in steroidogenesis is Cytochrome P450, Family 17, Subfamily A and Member 1. It has eight exons and is located on chromosome 10q24.32 [59]. According to reports, the pathophysiology of PCOS involves the gene CYP17. According to a study done on the population of Chile, the CYP17 polymorphism C>T causes PCOS to progress. Through hormonal and clinical evidence, the comparison of polymorphism with body weight and insulin resistance was also made. Furthermore, an increase in body weight, insulin resistance and excessive lipid are caused by the polymorphism in CYP17 and the gene defect discovered by RFLP PCR. As a result, it is linked to PCOS and metabolic pathways [60]. Another study found that the Chinese population has T/C polymorphism in the CYP17A1 gene. The genotypes of TC, TT and CC, which were 43.71%, 49.69% and 6.6%, were shown by the clinical and genetic criteria. In comparison to those who have the TT and TC genotypes, affected females with the CC genotype showed higher testosterone levels. Furthermore, T/C polymorphism might not be directly related to PCOS [61]. When there is increased insulin resistance and testosterone levels, the association may be based on polymorphism. Among Indian women with PCOS, Pusalkar et al. reported that the frequency of the C allele rises, which could have an influence on their hyperandrogenic phenotype [62]. However, this correlation was not found by Xing et al., suggesting the role of CYP17A1 rs7435721 polymorphisms as protective factors for PCOS [63].
-
CYP21A2 gene: Another gene implicated in the development and progression of PCOS is Cytochrome P450, Family 21, Subfamily A, Member 2. It has ten exons and is located on chromosome 6p21.33 [64]. A heterozygous mutation in CYP21A2 may be involved in the etiology of PCOS. For the advancement of PCOS, about 14 molecular abnormalities in CYP21A2 have been documented. 5.9% and 7.6% of afflicted and control individuals had mutations, respectively. In the case of CYP21A2, however, it still isn't an acceptable response [65].
-
CYP3A7 gene: Cytochrome P450, Family 3, Subfamily A, Member 7 is another name for CYP3A7. It predominantly manifests in the liver. It has 13 exons located at chromosome 7q22.1 [66]. According to reports, females with abnormal levels of androgen have inherited them. DHEAS metabolism is helped by CYP3A7. The CYP3A7 promoter variant allele lowers the activity of the metabolic pathway. In a study, the variant's overall frequency was 2.7%. It was 2.2% in the affected person and 3.6% in control. As a result, it is proven that females with PCOS have decreased DHEAS due to a mutant mutation in CYP3A7. The androgen metabolic pathway is affected by polymorphism, which can also lessen the severity of increased androgen levels and the PCOS phenotype [67].
-
CYP19A1 gene: It is also mentioned that the SNP rs2414096 discovered in the CYP19 gene in the Chinese population contributes to hyperandrogenism. In PCOS patients compared to controls, the genotype for rs2414096 was expressible different (AG, AA, GG), suggesting that the CYP19 SNP may also be linked to PCOS risk [68]. PCOS development is also caused by a gene called CYP19A1 that encodes aromatase. Cytochrome P450, Family 19, Subfamily A, Polypeptide 1 is called CYP19A1. The manufacture of lipids, steroids and cholesterol is carried out by monooxygenase. It is found in the endoplasmic reticulum and is essential for the estrogen production. The CYP19A1 gene, located on chromosome 15q21.2 and contains a total of 18 exons and 17 introns [69], can exhibit abnormalities that disrupt the estrogen pathway and aromatase activity. The exon part of this gene contains the SNP rs700519(C/T), and the intronic part contains rs710059(C/T) [70]. The regulatory part of CYP19A1 is 93 kb long, whereas the coding region is 30 kb long [71]. Due to CYP19A1 gene polymorphism, there is additional evidence that PCOS advancement increases the risk of endometrial cancer, breast cancer and prostate cancer [72]. The identification of SNPs, which are determined to be crucial in the disruption of the estrogen pathway, is also seen in the Korean population. Nineteen variants, found in 10 introns, four exons, 1 SNP in the 30 UTR and 6 SNP in the 50 untranslated part, have been identified [73]. The SNPs rs700519 at the exon region and rs2414096 and rs60271534 at the intronic region were also discovered in the South Indian population. These variations are what give rise to PCOS. The exon region Arg264Cys shows a substantial correlation with variance, according to statistical research. While the in-silico study revealed that the structure of the aromatase substrate recognition site 3 was destabilized, resulting in decreased enzymatic activity.
Genes involved in chronic inflammation
Plasminogen activator inhibitor-1 (PAI-1)
Cardiovascular disease is known to be associated with abnormalities in the fibrinolytic and coagulation pathways among patients with PCOS, with the possibility of involvement of associated candidate genes for PCOS [74]. Plasminogen activator inhibitor-1 (PAI-1) is one of the gene involved in chronic inflammation. PCOS women develop increasing amounts of PAI-1, and the 4G allele in the PAI-1 promoter is expressed, which enhances its expression [75].
Interleukin 6 (IL-6)
Several studies have demonstrated a correlation between PCOS women's chronic low-grade inflammation and hyperandrogenism. With conflicting existing literature, the involvement of IL-6 in insulin sensitivity is less established. It has been hypothesized that the influence of IL-6 on insulin metabolism fluctuates according to the type of tissue, the physiological status and the duration of IL-6 elevation (transient or chronic) [76]. During exercise, a transient surge of IL-6 has both an anti-inflammatory response and stimulated absorption of glucose in the skeletal muscle [77]. IL-6 also induces insulin sensitivity in fatty tissue and the liver by over-expressing the protein suppressor of cytokine signaling 3 (SOCS3), which binds to the insulin receptor and inhibits it. It also suppresses the transcription and phosphorylation of IRS-1 [78,79,80]. IL-1β has a major role in various stages of implantation that induced the secretion of urokinase plasminogen activator (uPA), PAL-1 and PAL-2. IL-1β-511C/T polymorphism has a positive correlation with PCOS pathogenesis along with recurrent spontaneous abortion (RSA) in Chinese and Saudi populations whereas no such effect was observed in Korean, Indian, Caucasian and Iranian Azeri individuals [81,82,83,84,85]. Similarly, IL-6-174G/C and TNF-α-1031 T/C polymorphisms showed significant disease pathogenesis and also reflected chemical characteristics of RSA with PCOS women in Saudi women [86, 87].
Transforming growth factor-beta (TGF)
TGF-β1 is a multifunctional cytokine which is associated with the maintenance of ovarian functions, from granulosa cells differentiation, regulating progesterone production, maintaining corpus luteum to inducing follicular atresia [88]. It is also associated with tissue fibrosis, wound healing and embryonic development. TGF-1 levels in PCOS patients' serum and ovaries were found to be elevated than in non-PCOS women, making it a major therapeutic target for PCOS pathophysiology [89]. TGF-1 gene SNPs and haplotypes were linked to PCOS in Chinese women [90]. The TGF-1 gene polymorphism (rs1800469C/T) is linked to the emergence of PCOS and metabolic problems, which are more common among Koreans [91].
Role of oxidative stress in PCOS
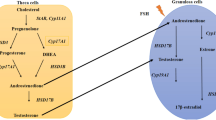
Inflammation and oxidative stress (OS) are an essential parameter in PCOS pathogenesis. Studies have reported elevated levels of markers in the oxidative circulation, demonstrated by Fig. 2 [92, 93]. Reactive oxygen species (ROS) acts as secondary redox messenger and helps in the development and progression of cancer, diabetes and cardiovascular diseases. ROS modulates gene expression by regulating cell growth, differentiation and apoptosis. It has been found that different phenotypes impact oxidative stress, but the mechanism remains open to investigation in the future. ROS are often associated with mitochondrial dysfunction since mitochondria are the energy currency of the cell, and also the primary source of ROS generation, which is a by-product of nutrient translation [94]. An increase in ROS may induce mitochondrial DNA damage (mt-DNA), proteins and lipids and can cause apoptosis [95]. Two factors demonstrate the correlation of ROS with PCOS: first studies have shown a decrease in mt-DNA copy number in PCOS females, which is essential for ROS generation [96]. Secondly, mitochondrial gene mutations such as single-point mutations of genes encoding mt-tRNA cause PCOS complications such as diabetes and hypertension [97, 98]. Mutations in mt-DNA displacement loop (D-loop) potentially disrupt mt-DNA replication and transcription, altering ETC and enhancing cellular ROS production and oxidative stress (OS). Many human disorders, including PCOS, have been linked to sequence variations in the mt-DNA D-loop. Hence, the development of PCOS is closely related to mitochondrial oxidative metabolism. Variations in the genes for antioxidant enzymes may make patients more susceptible to oxidative stress and hence aid in the etiology of PCOS [99,100,101].
Also, the effect of oxidative stress is impaired on glucose uptake, mainly in the muscle and adipose tissues. Also, insulin secretion by pancreatic cells is reduced. Hyperinsulinemia prevents nitric oxide (NO) secretion from the vascular endothelium, which leads to a series of events, thereby resulting in endothelial dysfunction, caused mainly by the decrease in endothelial fluid and increase in intracellular calcium levels. Thus, endothelial dysfunction may occur in PCOS patients which is also an early symptom of atherosclerosis. Oxidative stress is further maintained and regulated by other features of PCOS, such as abdominal adiposity, insulin resistance, obesity and androgen excess [102]. A cumulative increase in oxidative stress may also facilitate the development of Atherosclerotic heart disease (AHD) [103].
Diet-induced ROS-related oxidative stress (OS) causes an inflammatory response that activates an over-expression of Nuclear Factor Kappa B (NFKB) [104, 105]. It triggers the action of TNF and IL-6 from monocytes which are altered in PCOS. The ROS generation also causes increased expression of the p47 (phok) protein which is translocated from cytosol to membrane-bound nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and causes superoxide generation [106]. Hence, diet-induced response contributes to OS.
Hormonal-induced ROS have also been studied; hand is often related to PCOS. The administration of oral androgens causes mononuclear cell (MNC) activation, which further generates ROS, activating the cascade of activation of nuclear factor kappa B (NFKB) and TNF-mRNA. Tsai-Turton studied similar results with androgen-resistant mice models, which were not treated with testosterone or dihydrotestosterone (DHT). Oxidative Stress also leads to follicular apoptosis in PCOS individuals which blocks the maturation of follicles and is inhibited by glutathione [94, 107,108,109]. PCOS-obese women also suffer from frequent pregnancy and miscarriages mediated by OS [110]. Increased production of ROS causes antioxidant depletion and sometimes causes the abnormal distribution of mitochondria in oocytes [111]. Therefore, increasing oxidative stress with low antioxidants and insulin resistance, as reported in PCOS patients, support the concept of the role of oxidative stress in the pathophysiology of PCOS.
Epigenetics and PCOS
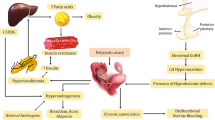
Genome-wide association studies (GWAS) have identified that the susceptibility of hereditary loci in PCOS females are less than ~ 10%. In contrast, studies have reported that monozygotic twins have hereditary PCOS susceptibility loci of approximately ~ 70%. Hence, there is a strong association between epigenetic changes and PCOS. Both environmental and epigenetic mechanisms have an important role in etiology and pathophysiology of PCOS. Tissue-specific epigenetic regulations such as promoter activity, histone modifications, DNA methylation, enhancer-binding activity and transcription factor binding profiles in PCOS females have been reported. Over-expression of DENND1A (DENN/ MADD domain containing 1A) in theca cells of PCOS females has a significant role in elevated androgen and progestin synthesis [112]. Hox A family genes identified in cumulus cells and luteinizing hormone/chorionic gonadotropic receptors (LHCGR) identified in theca and mature granulosa cells associated polymorphisms drive excess androgen production which also play a crucial role in etiology of PCOS [113]. Over-expression of androgen receptors during prenatal life leads to DNA hypomethylation in androgenized rats at specific CpG sites from the promoter region of GATA6 (-520) and STAR (-822) genes. These genes play an essential role in steroidogenesis, which alters the epigenetic landscape and thus confers PCOS [114], as shown in Fig. 3. GWAS studies have reported many gene that differ in DNA methylation status in normal and PCOS females. For instance, the LHCGR gene encodes for LH receptors, FST gene for follistatin, LMNA for Lamin A/C, PRARGC1A for the proliferation the peroxisomes and EPHX1 encoding epoxide hydrolase in PCOS individuals showed modified gene expression. It is to be noted that any individual gene methylation alterations do not cause the disease, instead they encode for the fundamental physiological process such as steroidogenesis, glucose metabolism, inflammation, follicular development and insulin regulation [115]. It is the modification in their methylation status that leads to syndromic conditions.
Histone epigenetic modifications such as acetylation, methylation, phosphorylation and ubiquitination are versatile alterations closely linked to disease pathophysiology and development such as PCOS. Hosseini et al. reported the CYP19A1 in ovarian cumulus cells when compared to control showed a direct pathophysiological link between histone acetylation and PCOS. Low cytochrome p450 aromatase activity was caused due to upregulation of serum levels of histone H3 acetylation and methylation of H3K9 in PCOS individuals which downregulated the CYP19A1 expression [116, 117].
Another aspect of epigenetic modifications that influence gene expression involves the microRNA (miRNA); these are non-coding single-stranded RNA that modulated the activity of two enzymes—DNA methyltransferases and histone deacetylases [118]. Numerous miRNAs have been reported that influence PCOS pathogenesis [119] and serve as potential biomarkers. For instance, the expression of miR-182 and miR-15a was downregulated in PCOS rat models which is essential for the maintenance of granulosa cell (GC) in the ovaries [120]; hence, similar studies were conducted on PCOS women and by targeting a particular pathway helps decipher the exact role of a certain miRNA that aids in the understanding of metabolic consequences and diagnosis of PCOS [121]. There are diverse functions of miRNAs such as in steroidogenesis by regulating development and maturation of oocytes, in glucose metabolism via GLUT-4, insulin signaling system, cholesterol homeostasis, lipid metabolism, BMI, adipogenesis, etc. The miR-222 and miR-93 are known to regulate insulin metabolism [122]. These miRNAs are the major contributors of PCOS pathophysiology since miR-93 over-expression causes decreased expression of GLUT4, the major insulin mediated glucose transporter into adiposities and miR-222 over-expression is linked with elevated serum levels [123]. Various other miRNAs reported in adipose generation and inflammation are miR-132, miR-103, miR-27b, miR-9, miR-18b, miR-21, miR-135a and miR-320 and in androgen regulation are miR-155, miR-146a and miR-222 [124, 125]. Deswal et al. demonstrated various expression levels of miRNAs in PCOS as shown in Fig. 4, corresponding upregulation levels of miR-518-3p, miR-27b, miR-301a-3p, miR-93, miR-21, miR-19b, miR-24, miR-93, miR-222, miR-223, miR-93, miR-27a-3p, miR-27a-5p, miR-23a, miR-24-2, miR-25, miR-106, miR-509-3p, let-7i-3p, miR-5706, miR-4463, miR-3665, miR-638, miR-502-3p, miR-200c, miR-1, hsa-let-7g, miR-32, miR-513-3p, miR-508-3p, miR-513b, miR-144, miR-146a, miR-30c, miR-16, miR-200a-3p, miR-10b-3p, miR-200b-3p, miR-29c-3p, miR-99a-3p, miR-125a-5p and miR-424, whereas downregulation levels of miR-24-3p, miR-29a -5p, miR-151-3p, miR-574-3p, miR-155, miR-4522, miR-324-3p, miR-9, miR-18b, miR-32, miR-34c, miR-26a-5p, miR-135a, miR-23a, miR-23b, miR-105-3p, miR-19a, miR-186, miR-320, miR-128 and let-7c in PCOS women. Among these, miR-29a-5p and miR-320 can be potentially used for PCOS diagnosis as biomarkers [126].
Furthermore, the circular RNAs (circRNAs) are non-coding RNAs that form a covalently closed-loop due to back-splicing of exons, hence excluding 5′-end cap and 3′-end poly (A) tails. CircRNAs function in protein sequestration, elevated parental gene expression, translation leading to polypeptides, and miRNA sponges. These circular molecules are more abundant and specialized than other types of RNA. They are therefore referred to as possible biomarkers for various disorders [127]. Interactions between miRNAs and circRNAs could influence the miRNAs' downstream targets, resulting to the hormonal imbalance as reported by the interactions between miR-139-5p and five circRNAs have sponging effects, mainly hsa_circ_0063309, hsa_circ_0054275, hsa_circ_0056196, hsa_circ_0018108 and hsa_circ_007098 [128]. Zhang et al. identified miR-217-RUNX2 upregulation in the cumulus cells of PCOS women, whereas when silenced, it causes estradiol biosynthesis by over-expression of CYP11A1 and CYP19A1 genes [129].
Potential miRNA-based therapeutics are being developed that can either restore or inhibit the function of miRNA by using miRNA mimics and inhibitors (anti-miRNAs) such as small interfering RNA (siRNA), anti-miRNA oligonucleotides and miRNA mimics. This may explain the reason behind heterogeneity in PCOS women. Therefore, further studies need to be conducted for understanding the involvement of non-coding RNAs in the etiology of PCOS that might lead to new insights and treatments.
Discussion
Polycystic ovary syndrome (PCOS) has a significant genetic, epigenetic and deranged lifestyle basis involving interplay of individual genes, genetic polymorphism and altered genes’ environmental conditions. Numerous genes, including the sex hormone-binding globulin gene (SHBG), androgen receptor gene (AR), follicular-stimulating hormone receptor (FSHR), fat mass obesity (FTO), calcium-dependent cysteine protease (CAPN), leptin and anti-Müllerian hormone (AMH), have been proposed as playing a role in the etiopathogenesis of PCOS. Affected women have hyper-synthesis of androgens owing to altered expression of critical enzymes involved in the steroid hormone biosynthesis pathway chiefly Cytochrome P450 enzymes: CYP17, CYP21, CYP19 and CYP11A. Hypothalamic–pituitary–adrenal axis impairment, ovarian dysfunctions and ovarian expression of certain genes (mainly of inflammatory origin) contribute in the pathophysiology of PCOS. Due to complexity and heterogeneity of PCOS, there is a difference in genetic basis of PCOS between families and within families. An estimate of 20–40% of the first-degree female family members of PCOS patients are also diagnosed with the syndrome, and the heritability is estimated to be around 65% indicating that genetic factors chiefly determine PCOS susceptibility. Thus, we can say that PCOS is a complicated multisystem disorder, where numerous genetic, epigenetic and environmental factors contribute to its pathophysiology. Small genetic effects taken together increase disease risk and modulate the phenotype of any specific patient.
Conclusion
PCOS is a multifactorial, polygenic and complex infertility disorder with overlapping symptoms. The current review has summarized the influence of several factors that contribute to PCOS progression; including altered metabolic pathways caused by a genetic abnormality that result in ovarian dysfunction. Genes involved in steroidogenesis, obesity, inflammation, gonadotropin action, insulin production and resistance along with the epigenetic factors are central to manifestation of PCOS. The proper preventative measures, such as losing weight, eating a nutritious diet and taking prescribed medications, can assist in alleviating the critical symptoms of PCOS and minimize the psycho-social trauma and severity associated with it. With better understanding of the pathogenesis of PCOS and by shedding light on the genetics of PCOS, we can do earlier intervention in controlling the co-morbidities that would allow treatment to be tailored with more personalized care.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PCOS:
-
Polycystic ovary syndrome
- HPO:
-
Hypothalamic–pituitary–ovarian axis
- HA:
-
Hyperandrogenemia
- STRP:
-
Short tandem repeat polymorphisms
- FTO:
-
Fat mass obesity
- SHBG:
-
Sex hormone-binding globulin gene
- AMH:
-
Anti-Mullerian hormone
- CAPN:
-
Calcium-dependent cysteine protease
- AR:
-
Androgen receptor gene
- FSHR:
-
Follicular-stimulating hormone receptor
- TNF:
-
Tumor necrosis factor
- PAI:
-
Plasminogen activator inhibitor-1
- OS:
-
Oxidative stress
- NO:
-
Nitric oxide
- GWAS:
-
Genome-wide association studies
References
Zhu JL, Chen Z, Feng WJ, Long SL, Mo ZC (2019) Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta Int J Clin Chem 499:142–148
Singh S, Pal N, Shubham S, Sarma DK, Verma V, Marotta F, Kumar M (2023) Polycystic ovary syndrome: etiology, current management, and future therapeutics. J Clin Med 12(4):1454
Siddiqui S, Mateen S, Ahmad R, Moin S (2022) A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J Assist Reprod Genet 39(11):2439–2473
Turan V, Sezer ED, Zeybek B, Sendag F (2015) Infertility and the presence of insulin resistance are associated with increased oxidative stress in young, non-obese Turkish women with polycystic ovary syndrome. J Pediatr Adolesc Gynecol 28(2):119–123
Priyadarshini A (2022) Effects of opium alkaloid, noscapine in RU486 induced experimental model of polycystic ovary syndrome. Indian J Biochem Biophys 59:468–478
Vural F, Vural B, Kardaş E, Ertürk Coşkun AD, Yildirim İ (2023) The diagnostic performance of antimullerian hormone for polycystic ovarian syndrome and polycystic ovarian morphology. Arch Gynecol Obstet 307(4):1083–1090
Peigné M, Pigny P, Pankhurst MW, Drumez E, Loyens A, Dewailly D et al (2020) The proportion of cleaved anti-Müllerian hormone is higher in serum but not follicular fluid of obese women independently of polycystic ovary syndrome. Reprod Biomed Online 41(6):1112–1121
Mukherjee S (2018) Pathomechanisms of polycystic ovary syndrome multidimensional approaches. Front Biosci 10(3):384–422
Sadeghi HM, Adeli I, Calina D, Docea AO, Mousavi T, Daniali M, Nikfar S, Tsatsakis A, Abdollahi M (2022) Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci 23(2):583
Amini L, Tehranian N, Movahedin M, Ramezani Tehrani F, Ziaee S (2015) Antioxidants and management of polycystic ovary syndrome in Iran: a systematic review of clinical trials. Iran J Reprod Med 13(1):1–8
Ding H, Zhang J, Zhang F, Zhang S, Chen X, Liang W, Xie Q (2021) Resistance to the insulin and elevated level of androgen: a major cause of polycystic ovary syndrome. Front Endocrinol 12:741764
Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH, Alvero R, Barnhart HX, Baker V, Barnhart KT, Bates GW, Brzyski RG, Carr BR, Carson SA, Casson P, Cataldo NA, Christman G, Coutifaris C, Diamond MP, Eisenberg E, Gosman GG, Giudice LC, Haisenleder DJ, Huang H, Krawetz SA, Lucidi S, McGovern PG, Myers ER, Nestler JE, Ohl D, Santoro N, Schlaff WD, Snyder P, Steinkampf MP, Trussell JC, Usadi R, Yan Q, Zhang H, Stener-Victorin E, Legro RS, Dunaif A (2015) Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun 6:7502
Ajmal N, Khan SZ, Shaikh R (2019) Polycystic ovary syndrome (PCOS) and genetic predisposition: a review article. Eur J Obstet Gynecol Reprod Biol X 8(3):100060
Dong XC, Liu C, Zhuo GC, Ding Y (2023) Potential Roles of mtDNA mutations in PCOS-IR: a review. Diabetes Metab Syndr Obes 25(16):139–149
Ramadhan RS, Algafari RN, Jarallah AL (2022) Investigating pathogenic SNPs in androgen receptor with direct influence on polycystic ovary syndrome (PCOS) in women. Egypt J Med Hum Genet 23:77
Chen Y, Fang SY (2018) Potential genetic polymorphisms predicting polycystic ovary syndrome. Endocr Connect 7(5):R187–R195
Shaikh N, Dadachanji R, Mukherjee S (2014) Genetic markers of polycystic ovary syndrome: emphasis on insulin resistance. Int J Med Genet 2014:478972. https://doi.org/10.1155/2014/478972
Magoffin DA (2006) Ovarian enzyme activities in women with polycystic ovary syndrome. Fertil Steril 86(Suppl 1):S9–S11
2023. AR androgen receptor [Homo sapiens (human)] [database on the Internet]. https://www.ncbi.nlm.nih.gov/gene/367
Urbanek M (2007) The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 3(2):103–111
FSHR follicle stimulating hormone receptor [Homo sapiens (human)] [database on the Internet]. https://www.ncbi.nlm.nih.gov/gene/2492
Baban ASS, Korsheed SH, AlHayawi AY (2018) The FSHR polymorphisms association with polycystic ovary syndrome in women of Erbil, Kurdistan in North of Iraq. Ibn AL-Haitham J Pure Appl Sci 257–272
Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP, Fisher RA, Bertonis JM, Torres G, Wallner BP, Ramachandran KL, Ragin RC, Manganaro TF, MacLaughlin DT, Donahoe PK (1986) Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell 45(5):685–698
Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BCJM, Themmen AP (2004) Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. MHR Basic Sci Reprod Med 10(2):77–83
Dewailly D, Barbotin AL, Dumont A, Catteau-Jonard S, Robin G (2020) Role of anti-Müllerian hormone in the pathogenesis of polycystic ovary syndrome. Front Endocrinol (Lausanne) 11:641
Silva MSB, Giacobini P (2021) New insights into anti-Müllerian hormone role in the hypothalamic–pituitary–gonadal axis and neuroendocrine development. Cell Mol Life Sci 78(1):1–16
Dewailly D (2016) Diagnostic criteria for PCOS: is there a need for a rethink? Best Pract Res Clin Obstet Gynaecol 37:5–11
Lie Fong S, Laven JSE, Duhamel A, Dewailly D (2017) Polycystic ovarian morphology and the diagnosis of polycystic ovary syndrome: redefining threshold levels for follicle count and serum anti-Müllerian hormone using cluster analysis. Hum Reprod (Oxf, Engl) 32(8):1723–1731
Kevenaar ME, Laven JS, Fong SL, Uitterlinden AG, De Jong FH, Themmen AP, Visser JA (2008) A functional anti-mullerian hormone gene polymorphism is associated with follicle number and androgen levels in polycystic ovary syndrome patients. J Clin Endocrinol Metab 93(4):1310–1316
Catteau-Jonard S, Jamin SP, Leclerc A, Gonzalès J, Dewailly D, Di Clemente N (2008) Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab 93(11):4456–4461
Pierre A, Taieb J, Giton F, Grynberg M, Touleimat S, El Hachem H, Fanchin R, Monniaux D, Cohen-Tannoudji J, di Clemente N, Racine C (2017) Dysregulation of the anti-Müllerian hormone system by steroids in women with polycystic ovary syndrome. J Clin Endocrinol Metabol 102(11):3970–3978
Bhattacharya K, Saha I, Sen D et al (2022) Role of anti-Mullerian hormone in polycystic ovary syndrome. Middle East Fertil Soc J 27:32
Kristensen SG, Kumar A, Kalra B, Pors SE, Bøtkjær JA, Mamsen LS, Colmorn LB, Fedder J, Ernst E, Owens LA, Hardy K, Franks S, Andersen CY (2019) Quantitative differences in TGF-β family members measured in small antral follicle fluids from women with or without PCO. J Clin Endocrinol Metab 104(12):6371–6384
2023. INS insulin [Homo sapiens (human)]. https://www.ncbi.nlm.nih.gov/gene?Cmd=DetailsSearch&Term=3630
Shaaban Z, Khoradmehr A, Amiri-Yekta A, Nowzari F, Jafarzadeh Shirazi MR, Tamadon A (2021) Pathophysiologic mechanisms of insulin secretion and signaling-related genes in etiology of polycystic ovary syndrome. Genet Res 2021:7781823
2023. CAPN10 calpain 10 [Homo sapiens (human)] [database on the Internet]. https://www.ncbi.nlm.nih.gov/gene/11132
FTO (2023) Alpha-ketoglutarate dependent dioxygenase [Homo sapiens (human)] [database on the Internet]. https://www.ncbi.nlm.nih.gov/gene/79068
Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM (1996) IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science (New York, NY) 271(5249):665–668
Escobar-Morreale HF, Calvo RM, Sancho J, San Millan JL (2001) TNF-α and hyperandrogenism: a clinical, biochemical, and molecular genetic study. J Clin Endocrinol Metab 86:3761–3767
Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, Norman RJ (1995) Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod (Oxf, Engl) 10(10):2705–2712
Brannian JD, Hansen KA (2002) Leptin and ovarian folliculogenesis: implications for ovulation induction and ART outcomes. Semin Reprod Med 20(2):103–112
Cioffi JA, van Blerkom J, Antczak M, Shafer A, Wittmer ST, Snodgrass HR (1997) The expression of leptin and its receptors in pre-ovulatory human follicles. Mol Hum Reprod 3(6):467–472
Finn PD, Cunningham MJ, Pau KYF, Spies HG, Clifton DK, Steiner RA (1998) The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology 139(11):4652–4662
Chakrabarti J (2013) Serum leptin level in women with polycystic ovary syndrome: correlation with adiposity, insulin, and circulating testosterone. Ann Med Health Sci Res 3(2):191–196
Donato J Jr, Cravo RM, Frazão R, Elias CF (2011) Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendocrinology 93(1):9–18
Karlsson C, Lindell K, Svensson E, Bergh C, Lind P, Billig H, Carlsson LM, Carlsson B (1997) Expression of functional leptin receptors in the human ovary. J Clin Endocrinol Metab 82(12):4144–4148
Pandey G, Shihabudeen MS, David HP, Thirumurugan E, Thirumurugan K (2015) Association between hyperleptinemia and oxidative stress in obese diabetic subjects. J Diabetes Metab Disord 14:24
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372(6505):425–432
MacDougald OA, Hwang CS, Fan H, Lane MD (1995) Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Natl Acad Sci USA 92(20):9034–9037
Ashraf S, Nabi M, Rasool, S.u.A. et al (2019) Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: a review. Egypt J Med Hum Genet 20:25
2023. CYP1A1 cytochrome P450 family 1 subfamily A member 1 [Homo sapiens (human)] [database on the Internet]. https://www.ncbi.nlm.nih.gov/gene/1543
Esinler I, Aktas D, Otegen U, Alikasifoglu M, Yarali H, Tuncbilek E (2008) CYP1A1 gene polymorphism and polycystic ovary syndrome. Reprod Biomed Online 16(3):356–360
Babu KA, Rao KL, Kanakavalli MK, Suryanarayana VV, Deenadayal M, Singh L (2004) CYP1A1, GSTM1 and GSTT1 genetic polymorphism is associated with susceptibility to polycystic ovaries in South Indian women. Reprod Biomed Online 9(2):194–200
2023. CYP11A1 cytochrome P450 family 11 subfamily A member 1 [Homo sapiens (human)] [database on the Internet]. https://www.ncbi.nlm.nih.gov/gene/1583
Reddy KR, Deepika ML, Supriya K, Latha KP, Rao SS, Rani VU, Jahan P (2014) CYP11A1 microsatellite (tttta)n polymorphism in PCOS women from South India. J Assist Reprod Genet 31(7):857–863
Zhang CW, Zhang XL, Xia YJ, Cao YX, Wang WJ, Xu P, Che YN, Wu XK, Yi L, Gao Q, Wang Y (2012) Association between polymorphisms of the CYP11A1 gene and polycystic ovary syndrome in Chinese women. Mol Biol Rep 39(8):8379–8385
2023. CYP11B2 cytochrome P450 family 11 subfamily B member 2 [Homo sapiens (human)] [database on the Internet]. https://www.ncbi.nlm.nih.gov/gene/1585
Zhao SP, Tang XM, Shao DH, Dai HY, Dai SZ (2003) Zhonghua fu chan ke za zhi 38(2):94–97
2023. CYP17A1 cytochrome P450 family 17 subfamily A member 1 [Homo sapiens (human)] [database on the Internet]. https://www.ncbi.nlm.nih.gov/gene/1586
Banerjee U, Dasgupta A, Khan A, Ghosh MK, Roy P, Rout JK, Roy P, Dhara S (2016) A cross-sectional study to assess any possible linkage of C/T polymorphism in CYP17A1 gene with insulin resistance in non-obese women with polycystic ovarian syndrome. Indian J Med Res 143(6):739–747
Li L, Gu ZP, Bo QM, Wang D, Yang XS, Cai GH (2015) Association of CYP17A1 gene -34T/C polymorphism with polycystic ovary syndrome in Han Chinese population. Gynecol Endocrinol 31(1):40–44
Pusalkar M, Meherji P, Gokral J, Chinnaraj S, Maitra A (2009) CYP11A1 and CYP17 promoter polymorphisms associate with hyperandrogenemia in polycystic ovary syndrome. Fertil Steril 92:653–659
Xing C, Zhao H, Zhang J, He B (2022) The association of CYP17A1, CYP19A1, and SHBG gene polymorphisms in polycystic ovary syndrome susceptibility: a systematic review and meta-analysis. Front Physiol 13:741285
2023. NCBI. CYP21A2 cytochrome P450 family 21 subfamily A member 2 [Homo sapiens (human)]. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=1589
Settas N, Dracopoulou-Vabouli M, Dastamani A, Katsikis I, Chrousos G, Panidis D, Dacou-Voutetakis C (2013) CYP21A2 mutations in women with polycystic ovary syndrome (PCOS). Horm Metab Res Hormon- und Stoffwechselforschung Hormones et metabolisme 45(5):383–386
2023. CYP3A7 cytochrome P450 family 3 subfamily A member 7 [Homo sapiens (human)] [database on the Internet]. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=1551
Goodarzi MO, Xu N, Azziz R (2008) Association of CYP3A7*1C and serum dehydroepiandrosterone sulfate levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab 93(7):2909–2912
Jin JL, Sun J, Ge HJ, Cao YX, Wu XK, Liang FJ, Sun HX, Ke L, Yi L, Wu ZW, Wang Y (2009) Association between CYP19 gene SNP rs2414096 polymorphism and polycystic ovary syndrome in Chinese women. BMC Med Genet 10:139
2023. CYP19A1 cytochrome P450 family 19 subfamily A member 1 [Homo sapiens (human)] [database on the Internet]. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=1588
Joseph S, Barai RS, Bhujbalrao R, Idicula-Thomas S (2016) PCOSKB: a KnowledgeBase on genes, diseases, ontology terms and biochemical pathways associated with polycystic ovary syndrome. Nucleic Acids Res 44(D1):D1032–D1035
Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M (2003) The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol 86(3–5):219–224
Kanda S, Tsuchiya N, Narita S, Inoue T, Huang M, Chiba S, Akihama S, Saito M, Numakura K, Tsuruta H, Satoh S, Saito S, Ohyama C, Arai Y, Ogawa O, Habuchi T (2015) Effects of functional genetic polymorphisms in the CYP19A1 gene on prostate cancer risk and survival. Int J Cancer 136(1):74–82
Lee SJ, Kim WY, Choi JY, Lee SS, Shin JG (2010) Identification of CYP19A1 single-nucleotide polymorphisms and their haplotype distributions in a Korean population. J Hum Genet 55(3):189–193
Yildiz BO, Haznedaroğlu IC, Kirazli S, Bayraktar M (2002) Global fibrinolytic capacity is decreased in polycystic ovary syndrome, suggesting a prothrombotic state. J Clin Endocrinol Metab 87(8):3871–3875
Diamanti-Kandarakis E, Palioniko G, Alexandraki K, Bergiele A, Koutsouba T, Bartzis M (2004) The prevalence of 4G5G polymorphism of plasminogen activator inhibitor-1 (PAI-1) gene in polycystic ovarian syndrome and its association with plasma PAI-1 levels. Eur J Endocrinol 150(6):793–798
Makki K, Froguel P, Wolowczuk I (2013) Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 2013:139239
Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK (2003) Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J 17(8):884–886
Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA (2003) Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 278(16):13740–13746
Galic S, Oakhill JS, Steinberg GR (2010) Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316:129–139
Torres-Leal FL, Fonseca-Alaniz MH, Rogero MM, Tirapegui J (2010) The role of inflamed adipose tissue in the insulin resistance. Cell Biochem Funct 28(8):623–631
Mu Y, Liu J, Wang B et al (2010) Interleukin 1 beta (IL-1β) promoter C [-511] T polymorphism but not C [+3953] T polymorphism is associated with polycystic ovary syndrome. Endocrine 37(1):71–75
Kim JO, Lee WS, Lee BE, Jeon YJ, Kim YR, Jung SH, Chang SW, Kim NK (2014) Interleukin-1beta -511T>C genetic variant contributes to recurrent pregnancy loss risk and peripheral natural killer cell proportion. Fertil Steril 102(1):206-212.e5
Rashid N, Nigam A, Saxena P, Jain SK, Wajid S (2017) Association of IL-1β, IL-1Ra and FABP1 gene polymorphisms with the metabolic features of polycystic ovary syndrome. Inflamm Res 66(7):621–636
Kolbus A, Walch K, Nagele F, Wenzl R, Unfried G, Huber JC (2017) Interleukin-1 alpha but not interleukin-1 beta gene polymorphism is associated with polycystic ovary syndrome. J Reprod Immunol 73(2):188–193
Ali Rahmani S, Paknejad Z, Mohammadkhanlou M, Daneshparvar M (2018) Association of of IL-1 receptor antagonist (IL-1RN) and interleukin-1β genes (IL-1β) polymorphisms with recurrent pregnancy loss in Iranian Azeri women. Horm Mol Biol Clin Investig 33(3):20170044
Alkhuriji AF, Al Omar SY, Babay ZA, El-Khadragy MF, Mansour LA, Alharbi WG, Khalil MI (2020) Association of IL-1β, IL-6, TNF-α, and TGFβ1 gene polymorphisms with recurrent spontaneous abortion in polycystic ovary syndrome. Dis Markers 2020:6076274
Vural P, Değirmencioğlu S, Saral NY, Akgül C (2010) Tumor necrosis factor α (−308), interleukin-6 (−174) and interleukin-10 (−1082) gene polymorphisms in polycystic ovary syndrome”. Eur J Obstet Gynecol Reprod Biol 150(1):61–65
Rotello RJ, Lieberman RC, Purchio AF, Gerschenson LE (1991) Coordinated regulation of apoptosis and cell proliferation by transforming growth factor beta 1 in cultured uterine epithelial cells. Proc Natl Acad Sci USA 88(8):3412–3415
Tal R, Seifer DB, Shohat-Tal A, Grazi RV, Malter HE (2013) Transforming growth factor-β1 and its receptor soluble endoglin are altered in polycystic ovary syndrome during controlled ovarian stimulation. Fertil Steril 100(2):538–543
Yang J, Zhong T, Xiao G et al (2015) Polymorphisms and haplotypes of the TGF-β1 gene are associated with risk of polycystic ovary syndrome in Chinese Han women. Eur J Obstet Gynecol Reprod Biol 186:1–7
Roh EY, Yoon JH, Song EY, Kim JJ, Hwang KR, Seo SH, Shin S (2017) Single nucleotide polymorphisms in the TGF-β1 gene are associated with polycystic ovary syndrome susceptibility and characteristics: a study in Korean women. J Assist Reprod Genet 34(1):139–147
Khashchenko EP, Tsvirkun DV (2016) Chronic systemic inflammation and mitochondrial dysfunction in the origin of polycystic ovary syndrome. Obstet Gynecol 12:41–46
Uçkan K, Demir H, Turan K, Sarıkaya E, Demir C (2022) Role of oxidative stress in obese and nonobese PCOS patients. Int J Clin Pract 2022:4579831
Zhang R, Liu H, Bai H, Zhang Y, Liu Q, Guan L, Fan P (2017) Oxidative stress status in Chinese women with different clinical phenotypes of polycystic ovary syndrome. Clin Endocrinol 86(1):88–96
Zhang J, Bao Y, Zhou X, Zheng L (2019) Polycystic ovary syndrome and mitochondrial dysfunction. Reprod Biol Endocrinol RB&E 17(1):67
Wallace DC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39:359–407
Lee SH, Chung DJ, Lee HS, Kim TJ, Kim MH, Jeong HJ, Im JA, Lee DC, Lee JW (2011) Mitochondrial DNA copy number in peripheral blood in polycystic ovary syndrome. Metab Clin Exp 60(12):1677–1682
Lee HK, Song JH, Shin CS, Park DJ, Park KS, Lee KU, Koh CS (1998) Decreased mitochondrial DNA content in peripheral blood precedes the development of non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 42(3):161–167
Finsterer J, Zarrouk-Mahjoub S (2018) Polycystic ovary syndrome in mitochondrial disorders due mtDNA or nDNA variants. Am J Transl Res 10(1):13–15
Reddy TV, Govatati S, Deenadayal M, Sisinthy S, Bhanoori M (2019) Impact of mitochondrial DNA copy number and displacement loop alterations on polycystic ovary syndrome risk in south Indian women. Mitochondrion 44:35–40
Francis A, Pooja S, Rajender S, Govindaraj P, Tipirisetti NR, Surekha D, Rao DR, Rao L, Ramachandra L, Vishnupriya S, Ramalingam K, Satyamoorthy K, Thangaraj K (2013) A mitochondrial DNA variant 10398G>A in breast cancer among South Indians: an original study with meta-analysis. Mitochondrion 13(6):559–565
Amini L, Tehranian N, Movahedin M, Tehrani FR, Ziaee S (2015) Antioxidants and management of polycystic ovary syndrome in Iran: a systematic review of clinical trials. Iran J Reprod Med 13(1):1
Jung HH, Choi DH, Lee SH (2004) Serum malondialdehyde and coronary artery disease in hemodialysis patients. Am J Nephrol 24(5):537–542
González F, Rote NS, Minium J, Kirwan JP (2006) Increased activation of nuclear factor κB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab 91(4):1508–1512
González F, Rote NS, Minium J, Kirwan JP (2006) Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab 91(1):336–340
Ladrón de Guevara A, Fux-Otta C, Crisosto N, Szafryk de Mereshian P, Echiburú B, Iraci G, Perez-Bravo F, Sir-Petermann T (2014) Metabolic profile of the different phenotypes of polycystic ovary syndrome in two Latin American populations. Fertil Steril 101(6):1732–9.e92
Liu S, Navarro G, Mauvais-Jarvis F (2010) Androgen excess produces systemic oxidative stress and predisposes to beta-cell failure in female mice. PLoS ONE 5(6):e11302
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I (2017) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Investig 114(12):1752–1761
Tsai-Turton M, Luong BT, Tan Y, Luderer U (2007) Cyclophosphamide-induced apoptosis in COV434 human granulosa cells involves oxidative stress and glutathione depletion. Toxicol Sci 98(1):216–230
Özer A, Bakacak M, Kıran H, Ercan Ö, Köstü B, Kanat-Pektaş M, Kılınç M, Aslan F (2016) Increased oxidative stress is associated with insulin resistance and infertility in polycystic ovary syndrome. Ginekol Pol 87(11):733–738
Zhang J, Bao Y, Zhou X, Zheng L (2019) Polycystic ovary syndrome and mitochondrial dysfunction. Reprod Biol Endocrinol 17(1):1–15
McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF 3rd (2014) Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci USA 111(15):E1519–E1527
Narayan P (2015) Genetic models for the study of luteinizing hormone receptor function. Front Endocrinol 6:152
Cao P, Yang W, Wang P, Li X, Nashun B (2021) Characterization of DNA methylation and screening of epigenetic markers in polycystic ovary syndrome. Front Cell Dev Biol
Vázquez-Martínez ER, Gómez-Viais YI, García-Gómez E, Reyes-Mayoral C, Reyes-Muñoz E, Camacho-Arroyo I, Cerbón M (2019) DNA methylation in the pathogenesis of polycystic ovary syndrome. Reproduction (Cambridge, England) 158(1):R27–R40
Hosseini E, Shahhoseini M, Afsharian P, Karimian L, Ashrafi M, Mehraein F, Afatoonian R (2019) Role of epigenetic modifications in the aberrant CYP19A1 gene expression in polycystic ovary syndrome. Arch Med Sci AMS 15(4):887–895
Ambros V (2001) microRNAs: tiny regulators with great potential. Cell 107(7):823–826
Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, Chen BS, Chazenbalk G, Azziz R (2013) miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 62(7):2278–2286
Sirotkin AV, Kisová G, Brenaut P, Ovcharenko D, Grossmann R, Mlyncek M (2014) Involvement of microRNA Mir15a in control of human ovarian granulosa cell proliferation, apoptosis, steroidogenesis, and response to FSH. MicroRNA (Shariqah, United Arab Emirates) 3(1):29–36
Vitale SG, Fulghesu AM, Mikuš M, Watrowski R, D’Alterio MN, Lin LT, Shah M, Reyes-Muñoz E, Sathyapalan T, Angioni S (2022) The translational role of miRNA in polycystic ovary syndrome: from bench to bedside-a systematic literature review. Biomedicines 10(8):1816
Sørensen AE, Udesen PB, Wissing ML, Englund ALM, Dalgaard LT (2016) MicroRNAs related to androgen metabolism and polycystic ovary syndrome. Chem Biol Interact 259(Pt A):8–16
Sørensen AE, Wissing ML, Salö S, Englund AL, Dalgaard LT (2014) MicroRNAs related to polycystic ovary syndrome (PCOS). Genes 5(3):684–708
Butler AE, Ramachandran V, Cunningham TK, David R, Gooderham NJ, Benurwar M, Dargham SR, Hayat S, Sathyapalan T, Najafi-Shoushtari SH, Atkin SL (2020) Increased microRNA levels in women with polycystic ovarian syndrome but without insulin resistance: a pilot prospective study. Front Endocrinol 11:571357
Sathyapalan T, David R, Gooderham NJ, Atkin SL (2015) Increased expression of circulating miRNA-93 in women with polycystic ovary syndrome may represent a novel, non-invasive biomarker for diagnosis. Sci Rep 5:16890
Schroen B, Heymans S (2012) Small but smart–microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome, and ageing. Cardiovasc Res 93(4):605–613
Deswal R, Dang AS (2020) Dissecting the role of micro-RNAs as a diagnostic marker for polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril 113(3):661–669
Combs JC, Hill MJ, Decherney AH (2021) Polycystic ovarian syndrome genetics and epigenetics. Clin Obstet Gynecol 64(1):20–25
Mazloomi S, Mousavi V, Aghadavod E, Mafi A (2023) Circular RNAs: emerging modulators in the pathophysiology of polycystic ovary syndrome and their clinical implications. Curr Mol Med. https://doi.org/10.2174/1566524023666230110151155
Zhang CL, Wang H, Yan CY, Gao XF, Ling XJ (2017) Deregulation of RUNX2 by miR-320a deficiency impairs steroidogenesis in cumulus granulosa cells from polycystic ovary syndrome (PCOS) patients. Biochem Biophys Res Commun 482(4):1469–1476
Acknowledgements
We would like to thank Principal, Kirori Mal College, University of Delhi, Prof. Dinesh Khattar for allowing us to conduct this research.
Funding
No funding received.
Author information
Authors and Affiliations
Contributions
AP conceived and designed the review. Puneet, TB and AP cowrote, edited and revised the draft critically and approved for the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhimwal, T., Puneet & Priyadarshani, A. Understanding polycystic ovary syndrome in light of associated key genes. Egypt J Med Hum Genet 24, 38 (2023). https://doi.org/10.1186/s43042-023-00418-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-023-00418-w