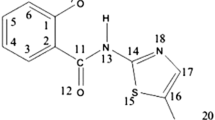
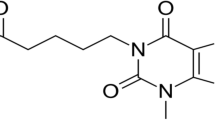
Abstract
Background
Coronavirus disease 2019 (Covid-19) is an infectious worldwide pandemic triggered by severe acute respiratory coronavirus 2 (SARS-CoV-2). This pandemic disease can lead to pro-inflammatory activation with associated acute lung injury and acute respiratory distress syndrome.
Main body of the abstract
SARS-CoV-2 infection is linked with inhibition of adenosine and activation of phosphodiesterase. Dipyridamole (DIP) is a nucleoside transport and phosphodiesterase inhibitor so that it may potentially affect SARS-CoV-2 infection and its accompanying inflammations. Therefore, the primary objective of this mini-review study was to elucidate the potential beneficial impacts of DIP on the adenosinergic pathway in Covid-19. A systemic search was done using online databases with relevant keywords. The findings of the present study illustrated that DIP directly or indirectly, through augmentation of adenosine and inhibition of phosphodiesterase, mitigates Covid-19 outcomes.
Conclusion
Our study concluded that DIP has a potential therapeutic effect in the management and treatment of Covid-19. This could be attained either directly, through anti-SARS-CoV-2, anti-inflammatory, and anti-platelets properties, or indirectly, through augmentation of extracellular adenosine, which has anti-inflammatory and immune-regulatory effects. However, extensive randomized clinical trials, and clinical and prospective research in this area are required to demonstrate the safety and therapeutic efficacy of DIP and adenosine modulators in the treatment of Covid-19.
Similar content being viewed by others
Background
Coronavirus disease 2019 (Covid-19) is a recent worldwide infection that was recognized for the first time in late December 2019 in Wuhan, China [1], triggered by severe acute respiratory coronavirus 2 (SARS-CoV-2). This disease may lead to critical instabilities systemically, including pro-inflammatory activation, cytokine storm, and associated damage to various organs [2]. Covid-19 affects various body systems, predominantly the respiratory system. The main presentation of the disease is acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Besides, acute kidney, pancreatic, and cardiac injury, neurological disorders, and endothelial dysfunction might occur as extra-pulmonary manifestations [3]. These multiple impacts of Covid-19 are attributed to the presence of angiotensin-converting enzyme 2 (ACE2), a receptor for SARS-CoV-2, on the cells of multiple organs, which facilitates its entry into various host cells [3, 4]. ACE2 receptor is principally articulated in the lung alveolar cells type II and proximal renal tubules. When SARS-CoV-2 binds to the ACE2, these defending receptors will be down-regulated. Consequently, the level of vasoconstrictors angiotensin II (Ang II) would increase, and the vasodilator angiotensin (Ang 1–7) (Ang 1–9) would decrease, accompanied by the production of the pro-inflammatory cytokines [5].
Dipyridamole (DIP) is a nucleoside transport and phosphodiesterase (PDE) inhibitor that is used as an antiplatelet agent [6]. The primary mechanisms of DIP are inhibition of adenosine reuptake in the red blood cells (RBCs), platelets, and endothelial cells, as well as inhibition of PDE. DIP has diverse clinical effects. It reduces pulmonary hypertension, improves coronary blood flow, myocardial function and perfusion, mild peripheral vasodilator effect, and endothelial functions by inhibiting the discharge of the pro-inflammatory cytokines and preventing the sub-endothelial thrombogenicity.
As well, SARS-CoV-2 infection is usually interrelated with inhibition of adenosine (AD) as well as activation of PDE [7]. Since Covid-19 is linked with cardiovascular complications and coagulopathy, the main target of our mini-review study was to elucidate the potential impacts of DIP on the adenosinergic pathway in Covid-19.
Adenosinergic pathway and DIP in Covid-19
It is known that AD is a primary nucleoside for building RNA and DNA, and it has different derivatives, including adenosine monophosphate (AMP), adenosine diphosphate (ADP), and adenosine triphosphate (ATP). These derivatives act as signal transductions for the modulation of different physiological processes. AD acts on the specific receptors subtypes, including A1, A2A, A2B, and A3, broadly found in different tissues. It acts as a cytoprotective signal against tissue injury. AD has immunosuppressive and anti-inflammatory effects (A2A, A2B) and is upregulated during ischemia and tissue hypoxia [8].
Cellular AD concentration is controlled by specific regulators, which are adenosine kinase (ADK), adenosine deaminase (ADA), and equilibrative nucleoside transporter-1 (ENT-1). ADA metabolizes AD to inosine when AD is present at a higher concentration owing to its low binding capacity. At the same time, ADK metabolizes AD, at baseline concentration, to 5-inosine monophosphate due to its higher affinity and capacity [9, 10]. In addition, ADA regulates the expression of ADA-binding protein (ADA-BP) on CD26 and dipeptidyl dipeptidase 4 (DPP-4). Interestingly, besides to ACE2 receptor, which has a low expression in the lung, ADA-BP of DPP-4/CD26 is considered as a potential receptor for binding and entrance of SARS-CoV-2. Thus, ADA competes with SARS-CoV-2 for binding to ADA-BP. Moreover, ADA activators like pegademase ADA or recombinant ADA have been effectively used in the management of the human immunodeficiency virus (HIV) [11]. It has been proposed that ADA regulates and fine-tunes AD's immunosuppressive and anti-inflammatory effects. Besides, the early administration of recombinant ADA attenuates the binding of Middle East respiratory syndrome coronavirus (MERS-CoV) to its entry point, the DPP-4 receptor [12]. Since there is a 50% similarity in the genome sequence between SARS-CoV-2 and MERS-CoV, the recombinant ADA may minimize the severity of Covid-19 by inhibiting the binding between SARS-CoV-2 and DPP-4 [13]. Therefore, DPP-4 inhibitors may diminish SARS-CoV-2 pathogenesis and Covid-19 severity in diabetic people via modulation of SARS-CoV-2 entry and the accompanied inflammations [14]. Expressions of DPP-4 receptors are higher in patients with diabetes mellitus, nicotine smoking, chronic obstructive pulmonary disease (COPD), and obesity. This issue might explain the susceptibility of Covid-19 patients to the development of ALI and ARDS [15].
DIP doesn't affect ADA activity or expression of ADA-BP; however, a higher intra-lymphocytic concentration is linked with immune suppression in patients with chronic kidney diseases. Also, high interferon-gamma (INF-γ) activates ADA activity in patients with HIV, thus, both INF-γ and ADA are regarded as prognostic and diagnostic factors for disease severity [16]. Tan et al. [17] experimental study demonstrated that DIP reduces the discharge of the pro-inflammatory cytokines and activation of T cells via modulation of the AD pathway. Indeed, ADA activity is negatively correlated with DPP-4 activity [17]. Therefore, AD elevation by DIP may increase ADA activity, reducing DPP-4 expression and interaction with SARS-CoV-2. However, ADA might be a possible target for SARS-CoV-2, causing a significant reduction in the cellular concentration of AD and the development of ALI and ARDS. Alongside this suggestion, a preclinical study proposed that AD protects against ALI and ARDS in a mouse model [18].
Augmentation of AD through inhibition of ADA and ADK may enhance the clinical results in Covid-19 patients. This can be attained via its immunosuppressive and anti-inflammatory effects, especially in the late phase, to counteract the exaggerated immune response that usually occurs in this phase of the disease [19, 20]. However, the immunosuppressive effect mediated by AD may affect viral clearance and increase viral replication in the initial phase of infection as ADA controls the negative impact of AD on the immune cells and immune response [21].
It has been reported that ADA inhibitors like pentostatin improves ARDS and its associated chronic inflammatory reactions [22]. Besides, ADK inhibitors like iodotubercidin attenuate ALI and ARDS via inhibition of neutrophil migration and improvement of the lung capillary-alveolar barrier. Nevertheless, the preclinical studies didn't recommend using ADK inhibitors due to the dangerous adverse effects such as liver toxicity and cerebral hemorrhage [23, 24].
Furthermore, AD, through the A2A receptor, activates the regulatory T cell (Treg), which regulates the immune response to hypoxia through the reduction of neutrophil infiltrations, cytokine production, and protein extravasations in the lung alveoli [25]. Similarly, AD, through activation of lung peroxisome proliferator-activated receptor gamma (PPARγ), attenuates lung inflammations and interstitial fluid accumulation with the improvement of the alveolar gas exchange [25]. AD, through the A2B receptor, constrains the discharge of the pro-inflammatory cytokines and chemokines in the lung during hypoxia and mechanical ventilation injury [26]. Consequently, through its immune-modulating effect, AD might be an efficient agent in managing Covid-19-induced ARDS. Falcone et al. [27] reported a case with Covid-19 and ARDS treated using standard therapy and oxygen (21%) mixed with AD. They noticed that within one month, there was a dramatic improvement in clinical and radiological outcomes of this patient. Likewise, a retrospective analysis reported by Correale et al. [28] showed that standard therapy and oxygen (21%) mixed with AD could substantially enhance the consequences of 14 Covid-19 patients suffering from ALI. Furthermore, a docking analysis study illustrated that AD has anti-SARS-CoV-2 by interfering with the main viral protease and its inhibition [29, 30]. Therefore, AD has a critical role in managing Covid-19 by suppressing SARS-CoV-2 and associated lung inflammations.
Of note, Covid-19 complications are linked with coagulopathy and thrombosis owing to endothelial dysfunction and platelet activation by SARS-CoV-2 as evident through the elevated D-dimer serum levels [31]. The reduction of AD during SARS-CoV-2 and cytokine storm is due to augmentation of the intracellular transport of AD through ENT-1. The reduction of AD contributes to platelet activation and thrombosis through the reduction of cAMP [32]. DIP inhibits ENT-1 and ENT-2, so the extracellular AD would be increased through attenuation of the intracellular transport. Elevation of the extracellular AD is linked with platelet inhibition via activation of cAMP, thus, it will reduce the risk of intravascular thrombosis [33, 34]. Previously, it has been shown that DIP attenuates ALI in experimental rats through modulation of the lung AD via blocking ENT-1 and ENT-2 pathways [35].
Besides, DIP, through inhibition of PDE3, may prevent coagulopathy and ALI in Covid-19 patients through modulation of platelet function and lung inflammation [34]. The interactions between DIP and AD in SARS-CoV-2 infection might be beneficial, as shown in Fig. 1.
Therefore, DIP, through modulation of the AD/PDE axis, has a critical contribution to the management of Covid-19. DIP inhibits the pro-inflammatory cytokines and lung inflammation during Covid-19. Also, DIP, through escalation of AD, leads to potent anti-inflammatory effects which participate in the reduction of cytokine storm and the associated ALI [36, 37]. Moreover, the important contribution of DIP to Covid-19 is correlated with its wide-spectrum potential antiviral effects [38]. Interestingly, docking studies revealed that DIP blocks SARS-CoV-2 main protease (Mpro), leading to attenuation of Covid-19 pneumonia [39, 40]. Liu et al. [41] showed that when DIP was administered in severely ill Covid-19 patients, it led to a significant clinical improvement with the reduction of D-dimer. Similarly, DIP improves immune recovery and inhibits thrombosis and coagulation disorders in cases having Covid-19 [41].
Into the bargain, activation of nod-like receptor pyrin 3 (NLRP3) inflammasome usually accompanies SARS-CoV-2 infection. This causes the discharge of interleukins (IL-1β and IL-18) and the development of ALI and ARDS. Thus, DIP, by blocking ENT-1 and ENT-2 pathways, may reduce NLRP3 inflammasome-mediated ALI and ARDS [42]. In addition, DIP down-regulates inflammatory signaling pathway such as NF-κB, matrix metalloproteinase (MMP1, MMP9), and cyclooxygenase-2 (COX-2). This can be accomplished by suppressing the macrophage-1 gene (Mac-1) [43], which is enormously activated in Covid-19 infection. It has been proposed that COX-2 inhibitors have a remarkable outcome in the management of Covid-19 by reduction of lung inflammation and IL-6 [44]. Similarly, MMPs inhibitors like aprotinin reduce the pro-inflammatory cytokines-mediated ALI and ARDS [45, 46].
Renin-angiotensin system (RAS) is highly affected in Covid-19 owing to the down-regulation of ACE2 and interconnected with the progress of ALI, ARDS, and injury in multiple organs [47]. It has been reported recently that DIP reduces RAS and circulating AngII serum levels through an AD-dependent pathway [46, 47] or PDE inhibition pathway [48, 49]. Furthermore, DIP also mitigates Covid-19-induced complications like acute kidney injury [50], acute coronary syndrome [51], acute brain injury [52], and cytokine storm-mediated multi-organ injury [53, 54].
Therefore, this study highlighted that DIP has potential pleiotropic effects (Fig. 2) that mitigate SARS-CoV-2 infection and the accompanying extra-pulmonary disorders. Thereby, DIP might be a "Holy Grail" for Covid-19 patients who are severely ill.
Conclusion
Hence, DIP has a possible therapeutic impact in the managing of Covid-19. This effect can be achieved either directly, through anti-SARS-CoV-2, anti-inflammatory, and anti-platelets consequences, or indirectly, through augmentation of the extracellular AD, which has immune-regulatory and anti-inflammatory outcomes. However, extensive randomized clinical trials and clinical and prospective studies are necessary to declare the safety and clinical effectiveness of DIP and AD modulators in the management of Covid-19.
Availability of data and materials
All data are involved in the manuscript.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- AD:
-
Adenosine
- ADA:
-
Adenosine deaminase
- ADA-BP:
-
Adenosine deaminase-binding protein
- ADK:
-
Adenosine kinase
- ADP:
-
Adenosine diphosphate
- ALI:
-
Acute lung injury
- AMP:
-
Adenosine monophosphate
- AngII:
-
Angiotensin II
- ARDS:
-
Acute respiratory distress syndrome
- ATP:
-
Adenosine triphosphate
- COPD:
-
Chronic obstructive pulmonary disease
- COX-2:
-
Cyclooxygenase-2
- Covid-19:
-
Coronavirus disease 2019
- DIP:
-
Dipyridamole
- DPP-4:
-
Dipeptidyl dipeptidase 4
- ENT:
-
Equilibrative nucleoside transporter
- HIV:
-
Human immunodeficiency virus
- IL:
-
Interleukins
- INF-γ:
-
Interferon-gamma
- RAS:
-
Renin-angiotensin system
- RBCs:
-
Red blood cells
- SARS-CoV-2:
-
Severe acute respiratory coronavirus 2
- Treg:
-
Regulatory T cell
- MERS-CoV:
-
Middle east respiratory syndrome coronavirus
- MMP:
-
Metalloproteinase
- NLRP3:
-
Nod-like receptor pyrin 3
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- PDE:
-
Phosphodiesterase
References
Al-Kuraishy HM, Al-Naimi MS, Lungnier CM, Al-Gareeb AI (2020) Macrolides and COVID-19: An optimum premise. Biomed Biotechnol Res J (BBRJ) 4:189
Dousari AS, Moghadam MT, Satarzadeh N (2020) COVID-19 (Coronavirus disease 2019): a new coronavirus disease. Infect Drug Resist 13:2819
Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, Hou C, Wang H, Liu J, Yang D (2020) Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care 24:1–10
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46:586–590
Bank S, De SK, Bankura B, Maiti S, Das M, Khan GA (2021) ACE/ACE2 balance might be instrumental to explain the certain comorbidities leading to severe COVID-19 cases. Biosci Rep 41:
Bakola V, Karagkiozaki V, Tsiapla A, Pappa F, Moutsios I, Pavlidou E, Logothetidis S (2018) Dipyridamole-loaded biodegradable PLA nanoplatforms as coatings for cardiovascular stents. Nanotechnology 29:275101
Lakkas L, Naka KK, Bechlioulis A, Girdis I, Duni A, Koutlas V, Moustakli M, Katsouras CS, Dounousi E, Michalis LK (2020) The prognostic role of myocardial strain indices and dipyridamole stress test in renal transplantation patients. Echocardiography 37:62–70
Draper-Joyce CJ, Khoshouei M, Thal DM, Liang Y-L, Nguyen AT, Furness SG, Venugopal H, Baltos J-A, Plitzko JM, Danev R (2018) Structure of the adenosine-bound human adenosine A1 receptor–Gi complex. Nature 558:559–563
Elekhnawy E, Sonbol F, Abdelaziz A, Elbanna T (2021) An investigation of the impact of triclosan adaptation on proteus mirabilis clinical isolates from an Egyptian university hospital. Braz J Microbiol 52:927–937
Geiger JD, Khan N, Murugan M, Boison D (2020) Possible role of adenosine in COVID-19 pathogenesis and therapeutic opportunities. Front Pharmacol 11:594487
Eleftheriou P, Amanatidou D, Petrou A, Geronikaki A (2020) In silico evaluation of the effectivity of approved protease inhibitors against the main protease of the novel SARS-CoV-2 virus. Molecules 25:2529
Raj VS, Smits SL, Provacia LB, van den Brand JM, Wiersma L, Ouwendijk WJ, Bestebroer TM, Spronken MI, van Amerongen G, Rottier PJ (2014) Adenosine deaminase acts as a natural antagonist for dipeptidyl peptidase 4-mediated entry of the middle east respiratory syndrome coronavirus. J Virol 88:1834–1838
Rabaan AA, Al-Ahmed SH, Haque S, Sah R, Tiwari R, Malik YS, Dhama K, Yatoo MI, Bonilla-Aldana DK, Rodriguez-Morales AJ (2020) SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med 28:174–184
Du H, Wang DW, Chen C (2020) The potential effects of DPP-4 inhibitors on cardiovascular system in COVID-19 patients. J Cell Mol Med 24:10274–10278
Solerte SB, Di Sabatino A, Galli M, Fiorina P (2020) Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19. Acta Diabetol 57:779–783
Hu X, Xing B, Wang W, Yang P, Sun Y, Zheng X, Shang Y, Chen F, Liu N, Yang L (2020) Diagnostic values of Xpert MTB/RIF, T-SPOT. TB and adenosine deaminase for HIV-negative tuberculous pericarditis in a high burden setting: a prospective observational study. Sci Rep 10:1–10
Tan MKX, Heng TYJ, Mak A (2019) The potential use of metformin, dipyridamole, N-acetylcysteine and statins as adjunctive therapy for systemic lupus erythematosus. Cells 8:323
Kahraman S, Altinova AE, Elgun S, Yalcin MM, Yilmaz BA, Ozkan C, Akturk M, Toruner FB (2019) Serum activities of dipeptidyl peptidase-4 and adenosine deaminase in polycystic ovary syndrome: association with obesity. Gynecol Endocrinol 35:714
Abouelkhair MA (2020) Targeting adenosinergic pathway and adenosine A2A receptor signaling for the treatment of COVID-19: a hypothesis. Med Hypotheses 144:110012
Olugbodi JO, Olaleye MT, Mostafa-Hedeab G, Alqarni M, Ilesanmi OB, Batiha GE-S, Akinmoladun AC (2022) Glyphaeaside C-enriched extract of glyphaea brevis restored the antioxidant and reproductive integrity of 1, 4-dinitrobenzene-intoxicated rats. Biomed Pharmacother 145:112359
Herrera-Calderon O, Chacaltana-Ramos LJ, Huayanca-Gutiérrez IC, Algarni MA, Alqarni M, Batiha GE-S (2021) Chemical constituents, in vitro antioxidant activity and in silico study on NADPH oxidase of Allium sativum L. (Garlic) essential oil. Antioxidants 10:1844
Kempin S, Sun Z, Kay NE, Paietta EM, Mazza JJ, Ketterling RP, Frankfurt O, Claxton DF, Saltzman JN, Srkalovic G (2019) Pentostatin, cyclophosphamide, and rituximab followed by alemtuzumab for relapsed or refractory chronic lymphocytic leukemia: a phase 2 trial of the ECOG-acrin cancer research group (E2903). Acta Haematol 142:224–232
Caetano D, Gonçalves Lima CM, Lima Sanson A, Faria Silva D, De Souza Hassemer G, Verruck S, Gregorio SR, Da Silva GA, De Cassia Franco RJ, Xavier Coutrim M (2022) Chemical fingerprint of non-aged artisanal sugarcane spirits using Kohonen artificial neural network. Food Anal Methods 15:890–907
Onikanni AS, Lawal B, Olusola AO, Olugbodi JO, Sani S, Ajiboye BO, Ilesanmi OB, Alqarni M, Mostafa-Hedeab G, Obaidullah AJ (2021) Sterculia tragacantha lindl leaf extract ameliorates STZ-induced diabetes, oxidative stress, inflammation and neuronal impairment. J Inflamm Res 14:6749
He X, Hu J-L, Li J, Zhao L, Zhang Y, Zeng Y-J, Dai S-S, He F-T (2013) A feedback loop in PPARγ–adenosine A2A receptor signaling inhibits inflammation and attenuates lung damages in a mouse model of LPS-induced acute lung injury. Cell Signal 25:1913–1923
Hoegl S, Brodsky KS, Blackburn MR, Karmouty-Quintana H, Zwissler B, Eltzschig HK (2015) Alveolar epithelial A2B adenosine receptors in pulmonary protection during acute lung injury. J Immunol 195:1815–1824
Falcone R, Colì E, Felletti S, Sapienza A, Castelfranchi C, Paglieri F (2020) All we need is trust: How the COVID-19 outbreak reconfigured trust in Italian public institutions. Front Psychol 11:561747
Correale P, Caracciolo M, Bilotta F, Conte M, Cuzzola M, Falcone C, Mangano C, Falzea AC, Iuliano E, Morabito A, Foti G (2020) Therapeutic effects of adenosine in high flow 21% oxygen aereosol in patients with Covid19-pneumonia. PLoS One, 15(10):e0239692
Eyer L, Zouharová D, Širmarová J, Fojtíková M, Štefánik M, Haviernik J, Nencka R, De Clercq E, Růžek D (2017) Antiviral activity of the adenosine analogue BCX4430 against West Nile virus and tick-borne flaviviruses. Antivir Res 142:63–67
Venugopal PP, Chakraborty D (2020) Molecular mechanism of inhibition of COVID-19 main protease by β-adrenoceptor agonists and adenosine deaminase inhibitors using in silico methods. J Biomol Struct Dyn 1–16.
Manolis AS, Manolis TA, Manolis AA, Papatheou D, Melita H (2021) COVID-19 infection: viral macro-and micro-vascular coagulopathy and thromboembolism/prophylactic and therapeutic management. J Cardiovasc Pharmacol Ther 26:12–24
Abdelhamid AM, Saber S, Youssef ME, Gaafar AGA, Eissa H, Abd-Eldayem MA, Alqarni M, Batiha GE-S, Obaidullah AJ, Shahien MA (2022) Empagliflozin adjunct with metformin for the inhibition of hepatocellular carcinoma progression: emerging approach for new application. Biomed Pharmacother 145:112455
Attallah NG, El-Kadem AH, Negm WA, Elekhnawy E, El-Masry TA, Elmongy EI, Altwaijry N, Alanazi AS, Al-Hamoud GA, Ragab AE (2021) Promising antiviral activity of agrimonia pilosa phytochemicals against severe acute respiratory syndrome coronavirus 2 supported with in vivo mice study. Pharmaceuticals 14:1313
Garcia-Borreguero D, Guitart X, Malo CG, Cano-Pumarega I, Granizo JJ, Ferré S (2018) Treatment of restless legs syndrome/Willis-Ekbom disease with the non-selective ENT1/ENT2 inhibitor dipyridamole: testing the adenosine hypothesis. Sleep Med 45:94–97
Eckle T, Hughes K, Ehrentraut H, Brodsky KS, Rosenberger P, Choi DS, Ravid K, Weng T, Xia Y, Blackburn MR (2013) Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J 27:3078–3089
Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Al-Buhadily AK, Al-Harchan NA, Lugnier C (2020) COVID-19 and phosphodiesterase enzyme type 5 inhibitors. J Micros Ultrastruct 8:141
Elekhnawy E, Negm WA (2022) The potential application of probiotics for the prevention and treatment of COVID-19. Egypt J Med Hum Genet 23:1–9
Zirintunda G, Biryomumaisho S, Kasozi KI, Batiha GE-S, Kateregga J, Vudriko P, Nalule S, Olila D, Kajoba M, Matama K (2021) Emerging anthelmintic resistance in poultry: can ethnopharmacological approaches offer a solution? Front Pharmacol. https://doi.org/10.3389/fphar.2021.774896
Aly O (2020) Molecular docking reveals the potential of aliskiren, dipyridamole, mopidamol, rosuvastatin, rolitetracycline and metamizole to inhibit COVID-19 virus main protease.
Thomé MP, Borde C, Larsen AK, Henriques JA, Lenz G, Escargueil AE, Maréchal V (2019) Dipyridamole as a new drug to prevent Epstein-Barr virus reactivation. Antiviral Res 172:104615
Liu X, Li Z, Liu S, Chen Z, Sun J, Zhao Z, Huang Y-Y, Zhang Q, Wang J, Shi Y (2020) Therapeutic effects of dipyridamole on COVID-19 patients with coagulation dysfunction. MedRxiv.
Freeman TL, Swartz TH (2020) Targeting the NLRP3 inflammasome in severe COVID-19. Front Immunol 11:1518
Guo S, Stins M, Ning M, Lo EH (2010) Amelioration of inflammation and cytotoxicity by dipyridamole in brain endothelial cells. Cerebrovasc Dis 30:290–296
Ong SWX, Tan WYT, Chan YH, Fong SW, Renia L, Ng LF, Leo YS, Lye DC, Young BE (2020) Safety and potential efficacy of cyclooxygenase-2 inhibitors in coronavirus disease 2019. Clin Trans Immunol 9:e1159
Batiha G, Shaheen H, Al-Kuraishy H, Teibo J, Akinfe O, Al-Garbee A, Teibo T, Kabrah S (2021) Possible mechanistic insights into iron homeostasis role of the action of 4-aminoquinolines (chloroquine/hydroxychloroquine) on COVID-19 (SARS-CoV-2) infection. Eur Rev Med Pharmacol Sci 25:7565–7584
Solun B, Shoenfeld Y (2020) Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19. Med Drug Discov 7:100052
Al-Kuraishy HM, Al-Niemi MS, Hussain NR, Al-Gareeb AI, Al-Harchan NA, Al-Kurashi AH (2020) The potential role of renin angiotensin system (RAS) and dipeptidyl peptidase-4 (DPP-4) in COVID-19: navigating the uncharted. In: Kibel A (ed) Selected chapters from the reninangiotensin system. IntechOpen, London, pp 151–165
Hanif A, Agba SO, Ledent C, Tilley SL, Morisseau C, Nayeem MA (2021) Adenosine A2A receptor and vascular response: role of soluble epoxide hydrolase, adenosine A1 receptor and angiotensin-II. Mol Cell Biochem 476:1965–1978
Islam MT, Quispe C, Herrera-Bravo J, Sarkar C, Sharma R, Garg N, Fredes LI, Martorell M, Alshehri MM, Sharifi-Rad J (2021) Production, transmission, pathogenesis, and control of dengue virus: a literature-based undivided perspective. BioMed Res Int 2021:1
Wu T, Wang H, Xin X, Zhang T, Hou Y, Fang M, Lu X, Xu Y (2020) An MRTF-A–Sp1–PDE5 axis mediates angiotensin-II-induced cardiomyocyte hypertrophy. Front Cell Dev Biol 8:839
Balakumar P, WitnessKoe WE, Gan YS, JemayPuah SM, Kuganesswari S, Prajapati SK, Varatharajan R, Jayachristy SA, Sundram K, Bahari MB (2017) Effects of pre and post-treatments with dipyridamole in gentamicin-induced acute nephrotoxicity in the rat. Regul Toxicol Pharmacol 84:35–44
Aagaard E, Aalen J, Afilalo J, Agmon Y Agoston Vas Coldea LN P1341 Agostoni P. P638 Agostoni PG 410 Agricola E. 1183, 428, P1251, P218, P271, P300, P755 Agrifoglio M. Agrifoglio 624: 1000, 624.
Lana D, Ugolini F, Melani A, Nosi D, Pedata F, Giovannini MG (2017) The neuron-astrocyte-microglia triad in CA3 after chronic cerebral hypoperfusion in the rat: protective effect of dipyridamole. Exp Gerontol 96:46–62
Rangwani C (2020) Possible relationship of mast cell degranulation and cytokin e storm related COVID19 morbidity in young and old population. SSRN Electron J. https://doi.org/10.2139/ssrn.3563062
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
HMA-K, AIA-G, EE, and GEB contributed to the study conception, design, material preparation, data collection, data analysis, and writing the first draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-kuraishy, H.M., Al-Gareeb, A.I., Elekhnawy, E. et al. Dipyridamole and adenosinergic pathway in Covid-19: a juice or holy grail. Egypt J Med Hum Genet 23, 140 (2022). https://doi.org/10.1186/s43042-022-00354-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-022-00354-1