Abstract
Background
The angiotensin-converting enzyme-2 (ACE2) is recognized to be the fundamental receptor of severe acute respiratory syndrome coronavirus-2 (SARS-CoV2), responsible for the worldwide Coronavirus Disease-2019 (COVID-19) epidemic. However, genetic differences between people besides racial considerations and their relation to disease susceptibility are still not fully elucidated.
Main body
To uncover the role of ACE2 in COVID-19 infection, we reviewed the published studies that explore the association of COVID-19 with the functional characteristics of ACE2 and its genetic variations. Notably, emerging studies tried to determine whether the ACE2 variants and/or expression could be associated with SARS-CoV/SARS-CoV2 have conflicting results. Some researchers investigated the potential of “population-specific” ACE2 genetic variations to impact the SARS-CoV2 vulnerability and suggested no ethnicity enrichment for ACE2 polymorphisms that could influence SARS-CoV2 S-protein binding. At the same time, some studies use data mining to predict several ACE2 variants that could enhance or decline susceptibility to SARS-CoV. On the other hand, fewer studies revealed an association of ACE2 expression with COVID-19 outcome reporting higher expression levels of ACE2 in East Asians.
Conclusions
ACE2 gene variants and expression may modify the deleterious consequences of SARS-CoV2 to the host cells. It is worth noting that apart from the differences in gene expression and the genetic variations of ACE2, many other environmental and/or genetic factors could modify the disease outcome, including the genes for the innate and the adaptive immune response.
Similar content being viewed by others
Background
More than 24 months have passed since the first discovery of the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) cases in Wuhan, Hubei Province, China. However, it is still spreading enormously, causing a significant health issue in nearly all countries around the world, even those who have already confined the disease spread still worry about having many other waves. From the experience of the previous epidemics, understanding details of the disease pathophysiology could help by a significant way in its handling and control strategies, which we are in dire need to stop world losses from this pandemic. Although previous reports characterized the elderly age group as a risk factor for COVID-19, in particular, if associated with chronic diseases such as hypertension, heart disease, and/or diabetes mellitus [1], nowadays increasing the number of young cases with early complications necessitating ICU and multivisceral support becomes devastating which support the potential contribution of the genetic factors to this risk that warranted continuous research [2].
Given the essential roles of the renin-angiotensin system (RAS) in maintaining the balance of lung cell proliferation/apoptosis and mediating the intra-pulmonary blood pressure, inflammation, and fibrosis, its dysregulation has been linked to several pulmonary diseases, including COVID-19 [3, 4]. The angiotensin-converting enzyme 2 (ACE2), the homolog of ACE, is a catalytic component of RAS that has recently attracted global recognition [5]. ACE2 is reported to be the fundamental entry point of SARS-CoV [6]. It is required for host cell entry and subsequent viral replication after priming by the serine protease TMPRSS2 (transmembrane protease, serine 2) [7], as detailed in the following sections. Despite the spike proteins of SARS-CoV-2 and SARS-CoV are not identical, SARS-CoV2 spike protein has a much higher binding affinity to human ACE2 [8] and supports intense interaction with it [9], which signifies its enhanced pathogenicity.
Rather than the pulmonary expression, ACE2 is reported to be also distributed in the heart, the renal and luminal surface of intestinal epithelial cells, among others [10], explaining the SARS-CoV2 entry site in Wuhan patients and the multi-organ dysfunction observed in infected patients [11]. By using normal lung tissues, Zhao et al. have detected that about 83% of the lung ACE2 expression is situated in the alveolar epithelial cells type II, which may facilitate coronaviral invasion and harbor the virus for replication [12].
Accumulating evidence indicates that ACE2 genetic polymorphisms among populations and racial considerations may correlate with cellular susceptibility to SARS-CoV2 infection with controversial findings [13,14,15]. Also, the rationale for the genetic basis of ACE2 or coronavirus-resistant ACE2 mutant receptors is still mostly unknown in different populations. In this sense, this review aimed to explore some basics related to ACE2-SARS-CoV2 interaction and cell entry and the relation of different ACE2 variants to disease risk, severity, and progression among different populations worldwide.
Main text
Methods
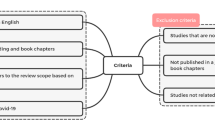
We screened the following medical electronic databases: PubMed, Web of Science, Scopus, and Cochrane CENTRAL for the relevant published data up to February 2022, using the keywords (“COVID-19” OR “SARS-CoV-2” OR “Coronavirus” OR “severe acute respiratory syndrome coronavirus-2” OR “coronavirus SARS-CoV-2” OR “2019-nCoV”) AND (“ACE II receptors” OR “angiotensin-converting enzyme 2” OR “angiotensin-converting enzyme-2” OR “ACE2” OR “angiotensin II receptor blockers” OR “Angiotensin-converting enzyme inhibitors” OR “ACE inhibitors”) AND (“Genetic Variations” OR “Genetic Diversity” OR “SNP” OR “polymorphism” OR “genotype” OR “single nucleotide polymorphism”). The identified records evaluated against the following inclusion criteria: studies are exploring the association of COVID-19 with ACE2 genetic variations, all types of studies, and the studies published in both peer-reviewed journals and as a preprint.
Different databases were applied to explore the structural and functional characteristics of the ACE2 gene. The data for gene structure and transcript splicing variant were obtained from Ensembl (www.ensembl.org). Predicted sequence of ACE2 protein and the essential structural motifs and the amino acid residues (in particular the amino acids required for virus binding) with their mutation outcomes were obtained by UniProt (https://www.uniprot.org/uniprot/Q9BYF1/). Protter (http://wlab.ethz.ch/protter/), a web application to visualize the sequence, annotations, and topology of the individual proteins, has been applied to visualize the amino acid residues of ACE2 and domains [16]. The signaling network of ACE is curated by the SIGNOR (SIGnaling Network Open Resource) v.2 [17]. Functional enrichment analysis and gene ontology were retrieved from (https://toppgene.cchmc.org/enrichment.jsp), and gene–gene interaction was retrieved from GeneMania (https://genemania.org/).
COVID-19 Cell entry
About one-third of the viral genetic content is directed to encode four structural proteins, including spike glycoprotein (S), a small envelope protein (E), matrix protein (M), and the nucleocapsid protein (N) [18]. The glycoprotein (S) of the virus consists of two subunits named S1 and S2 [19]. S1 is mainly responsible for the virus-host interaction and cellular tropism with the critical function domain-receptor-binding domain (RBD), and S2 facilitates virus cell/host cell membrane fusion [20]. The infectivity assays on HeLa cells with or without ACE2 proteins extracted from bats, civets, pigs, mice, and humans, revealed that SARS-CoV-2 uses ACE2 to promote its entry into ACE2-expressing cells, but not from mouse species. It cannot enter those cells without ACE2, which can be considered specific receptors for this virus resembling SARS-CoV [21]. Additionally, the previous investigators excluded other receptors suspected of SARS-CoV-2 cell invasion, like dipeptidyl peptidase 4 and aminopeptidase N.
Coronavirus spike (S) glycoproteins facilitate viral entry and replication into cells through binding to ACE2 and its priming by the serine protease TMPRSS2 (Fig. 1) [22]. Tai and his research team discovered the presence of RBD in the SARS-CoV-2 S1 subunit and observed a robust binding ability to ACE2; moreover, they showed a significantly higher binding affinity than that to SARS-CoV, which may explain the higher infectious rate of SARS-CoV-2 over SARS-CoV [23, 24]. It was also found that the temperature-sensitivity for the SARS-CoV-2 binding affinity is much more than that for SARS-CoV, predicting that the SARS-CoV-2 infection rate would reduce with increased temperature much quicker than SARS-CoV [23]. Also, S glycoproteins give sanctuary to a furin cleavage site which enhances cell invasion and is considered a unique feature for SARS-CoV2 and could be targeted for antibodies [25]. S ectodomain trimer could be a beneficial target for designing vaccines and antiviral entry inhibitors. It was documented that murine polyclonal antibodies against SARS-CoV S effectively diminished SARS-CoV2 S mediated cell entry; this emphasizes the cross-neutralizing antibodies' role in conserving S epitopes upon vaccination [25].
SARS-CoV-2 cellular entry. The binding between the S-protein trimer of SARS-CoV-2 and ACE2 receptor. The figure demonstrated the receptor-binding domain (RBD) of the S1 subunit and the hydrophobic fusion peptide of the S2 subunit where the S-protein is bound to ACE2 and primed with the cellular protease TMPRSS2, and the fusion S2 subunit undergoes a conformational rearrangement. The affinity between ACE2 and the RBD of SARS-CoV-2 is higher than its affinity with the RBD of the SARS virus [27,28,29]
Although SARS-CoV-2 does not group inside SARS and SARS-related coronaviruses, structural investigation distinguished residues in the SARS-CoV-2 RBD that are basic for ACE2 binding; most of them share analogous side chain with that in the SARS-CoV RBD. Such structural similarity and succession unequivocally contend for the evolution between the SARS-CoV-2 and SARS-CoV RBDs to improve the binding ability to ACE2 receptors [26].
Angiotensin-converting enzyme 2 (ACE2): the hottest target of SARS-CoV-2 invasion
ACE2 (EC:3.4.17.23) is also termed as angiotensin-converting enzyme homolog (ACAH), ACE-related carboxypeptidase, and metalloprotease 15 (MPROT15), and was identified as the first reported ACE homolog in 2000. The protein is related to the ACE family of dipeptidyl-carboxypeptidases, which converts angiotensin I to angiotensin 1–9, and angiotensin II to angiotensin 1–7, which acts as a vasodilator and exerts important modulatory effects on the cardiovascular system [30,31,32] also effectively hydrolyzes apelin-13 and dynorphin-13 [32] (Fig. 2A). By cleavage, angiotensin II may be an essential regulator of heart function and may also have a protective role in acute lung injury [30, 31] (Fig. 2B). Furthermore, it plays a vital role in amino acid transport by acting as a binding partner of amino acid transporter SL6A19 in the intestine, regulating trafficking and the expression on the cell surface, and its catalytic activity [33].
ACE2 signaling network. A ACE2 interacts with several molecules, including S-protein. B The role of ACE2 in lung fibrosis. TGF-β1 is an essential mediator of fibrosis by activating downstream “SMAD” signaling, which triggers pro-fibrotic genes overexpression. Smad3 is the central canonical mediator for fibrogenesis, while Smad7 negatively regulates the fibrosis process. The RAS also controls the fibrotic process. Down-regulation of ACE2 decreases the angiotensin 1–7 level, upregulating the AT1R signaling cascade, which in turn triggers fibrosis (Data source: https://signor.uniroma2.it/)
ACE2 is a metalloproteinase with a total length of 805 amino acids (Fig. 3A) [34, 35]. It belongs to type I transmembrane glycoprotein (integral) and contains a single protruding extracellular catalytic domain. Like ACE, ACE2 has two domains: an amino-terminal catalytic domain and another carboxy-terminal domain. The catalytic domain has an active site called the zinc metallopeptidase domain (HEXXH motif) (Fig. 3B) [36].
Sequence and aa residues of ACE2. A ACE2 gene encoding a deduced 805-aa protein. The unprocessed ACE2 (1–17) and ACE2 chain (18–805). Functional roles are illustrated based on [34] and [35]. (https://www.uniprot.org/uniprot/Q9BYF1/protvista). B ACE2 sequence contains an N-terminal signal (1 to 18), a TM domain (740 to 763), and a metalloprotease zinc-binding consensus (374 to 378, HEMGH). Blue aa residues are required for RBD interaction with the extracellular catalytic domain of ACE2 [36]. C. 3D structure of the ACE2 protein (https://www.uniprot.org/uniprot/Q9BYF1/protvista)
Based on a recent study by Yan et al., the RBD Gln 498, Thr 500, and Asn 501 of the SARS-CoV-2 configure a connecting net of hydrogen bonds with ACE2 structured Tyr 41, Gln 42, Lys 3535, and Arg 357, respectively. Furthermore, Lys 417, Tyr 453, and Gln 474 of RBD interact with Asp 30, His 34, and Gln 24 of ACE2, respectively. Through Vander Waals forces, Phe 486 of RBD interacts with Met 82 of ACE2 to ensure binding of the virus to the receptor and subsequent internalization (Fig. 3) [36]. Interestingly, the TMPRSS2 cleaves the ACE2 residues 697 to 716 to facilitate the S-protein-driven viral entry [37]. The impact of some experimental mutation of one or more amino acids on the biological properties of the ACE2 protein, in particular, the binding to SARS-CoV (by similarity could be SARS-CoV2), is summarized in Table 1 [33, 35, 38, 39]. It is worth noting that ACE2 can also be trimmed by “a disintegrin and metalloproteinase domain-containing protein 17 (ADAM 17),” which releases an extracellular fragment called soluble ACE2 (sACE2) and is measured as sACE2 plasma activity [40]. It has been considered a possible candidate for monitoring the evolution of COVID-19 [41]. The sACE2 retains an intact SARS-CoV-2 interaction site, suggesting its ability to bind to SARS-CoV-2. Kornilov and colleagues have observed that COVID-19-related regulatory pathways may induce ACE2 shedding, and the sACE2 concentrations may correlate with the level of systemic inflammation associated with COVID-19 [42]. Furthermore, the calmodulin–calcium signaling pathway which contributes to ACE2 release has been suggested to add new insights for clinical/therapeutic applications of ACE2 for COVID-19 [43].
Structural and functional analysis of ACE2
Human ACE2 (NCBI_Gene ID:59,272), a protein-coding gene, is located along the short arm of the Chromosome X (Xp22.2), spanning 41,116 bases long on the reverse strand from 15,561,033 to 15,602,148, according to the “Human Genome Assembly GRCh38” (Fig. 4A). It comprises 18 exons that can be transcribed into five different splice variants (ACE2-201 to ACE2-205); only two are protein-coding, as depicted in Fig. 4A [44].
ACE2 genomic structure and interactions. A The ACE2 gene is mapped to X chromosome Xp22.2. It contains 18 exons and has five transcripts; from the top to down: protein-coding transcripts ACE2-202 (3507 bp; 805aa) and ACE-201 (3339 bp; 805aa), in addition to three noncoding processed transcripts; ACE2-203/4/5 (998/786/599 bp, respectively). B Gene–gene network analysis for ACE2 gene. (https://genemania.org/search/homo-sapiens/ACE2/)
A recent study by Fujikura and Uesaka REF has identified 349 single nucleotide variants (SNVs) in the coding regions and splice sites. SNVs were found in multiple protein-coding regions, including those in the contact residues between SARS-COV2 and human ACE2. There were 247 missense SNVs (70.8%) and 94 synonymous SNVs (26.9%). The residual 2% of SNVs, stop-gained (n = 2), splice site variants (n = 2), start-loss (n = 1), and indels (n = 3) were recorded. The majority of these SNVs were rare or quite rare, with allele frequency < 1% or < 0.001%, respectively. The frequency of deleterious SNVs is higher for rare SNVs than for SNVs with a high allele frequency [45].
Gene–gene network analysis reveals the implication of ACE2 in angiotensin maturation, regulation of systemic arterial blood pressure, peptide hormone metabolism, proteolysis, and regulation of cytokine production, among others (Fig. 4B).
There is an expression of this gene in endothelial cells in small/large arteries, arterial smooth muscle cells, the heart, the alveolar epithelial cells, the small intestine enterocytes, Leydig cells, and Sertoli cells [46,47,48,49,50] (Fig. 5). Recently, it was discovered to be expressed in the proximal renal tubules and the small intestine [51]. According to its organ- and cell-specific expression, this gene regulates both cardiovascular and renal function, in addition to fertility (https://www.ncbi.nlm.nih.gov/gene/59272).
ACE2 expression in different human tissues. Expression values are shown in transcripts per Million (TPM), calculated from a gene model with isoforms collapsed to a single gene. Each box plot represents the median, 25th percentile, and 75th percentile. The points below or above 1.5 times the interquartile range are considered outliers. Data Source: GTEx Analysis Release V8 (dbGaP Accession phs000424.v8.p2) [51]
Gene ontology (GO) annotations related to this gene include virion attachment to host cell (receptor-mediated) among others in biological process category (Fig. 6A), host cell surface binding, virion binding, and virus receptor activity in the molecular function category (Fig. 6B), membrane region and raft, cell projection membrane, microvillus, and brush border membrane in the cellular component group (Fig. 6C).
Functional annotation of ACE2. A Biological process, B Molecular functions, and C Cellular components for ACE2 with p-values set at < 0.01 for (A) and < 0.05 for (B and C). The processes and molecular functions related to coronavirus binding and infection are encircled by red broken line rectangles (https://toppgene.cchmc.org/)
Association of ACE2 gene variants with SARS-CoV2 infection
Since virus receptors are essential for cellular pathogen entry, they can influence the development and/or progression of viral diseases [52]; previous studies tried to determine whether the ACE2 variants and/or expression could be associated with SARS-CoV/SARS-CoV2 with conflicting results.
Although an earlier report demonstrated no association between ACE2 variants and SARS-CoV susceptibility or outcomes with no difference related to sex [13], Gemmati et al. reported a higher incidence of COVID-19 infection in males with more severe representations. Death rates from SARS-CoV2 infection were 65 percent higher in males than in females. Part of the reason for these observations is the location of ACE2 on chromosome Xp22.22. This X-linked association renders the heterozygous females with higher ACE2 expression more protected than the hemizygous males [53].
Similarly, through analysis of the “1000 Genomes Project,” which contains samples from almost all ethnicities, a study has suggested the possibility of “population-specific” ACE2 genetic variations that impact the susceptibility to SARS-CoV2 infection [54]. Also, the genetic analysis by Cao and co-workers did not find any mutation difference in ACE2 that would influence SARS-CoV2/ S-protein binding [15]. Cao et al., however, were criticized by some researchers for focusing merely on a limited population variation data set [52]. Using data mining in several data sets and applying “structural predictions,” Suryamohan et al. could predict several ACE2 variants (i.e., “E23K, S19P, I21V, K26R, T27A, N64K, T92I, K26E, H378R, Q102P, and M383T”) which have the potential to increase the sensitivity of the host to SARS-CoV. Alternatively, “N33I, K31R, D38V, H34R, E35K, E37K, N51S, K68E, Y50F, F72V, G326E, G352V, Y83H, D355N and Q388L” variants were predicted to decrease S-protein-ACE2 binding affinity with a subsequent decline in infection susceptibility [55]. Interestingly, most of the previously predicted variants were clustered in the N-terminal region (extracellular catalytic domain) of ACE2 (Fig. 3B) that interacts with the S-protein. However, the latter investigators confirmed that the above-identified variants are present in the general population with rare allele frequencies without any significant observable frequencies among different populations or even when stratified by sex. Another Italian research group has explored some ACE2 variants that could impact protein stability and SARS-CoV-2 binding. They found that c.1517 T > C p. (Val506Ala) had the highest disturbance effect, c.631G > A p.(Gly211Arg) and c.77A > G p. (Lys26Arg) had a high frequency of allele as well as c.1166C > A p.(Pro389His), and c.1051C > G p.(Leu351- Val) were predicted to affect the interaction of spike protein [14]. Furthermore, through comparative genetic analysis of nearly 81,000 human genomes across eight populations, Hou et al. explored 63 potentially deleterious ACE2 variants that could affect the genetic susceptibility to COVID-19 [56].
ACE2 gene variants and COVID-19 outcome
The ACE2 gene variants may modify the deleterious consequences of SARS-CoV2 to the host cells [56, 57]. The first COVID-19 genome-wide association study identified the 3p21.31 gene cluster, including “SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6, and XCR1” as a genetic susceptibility locus in severe patients with COVID-19 and respiratory failure [58]. Previous genetic studies indicated that ACE2 polymorphisms are related to the rate of hypertension progression in different populations [59]. ACE 2 variants were also found to be associated with cardiovascular and pulmonary conditions through altering the angiotensinogen-ACE2 interactions [56]. Several mutations have been speculated to modify the ACE2 protein expression level, as reported previously in a murine model [60]. Also, ACE2 deletion in the mice model was associated with increased tissue/circulation Ang II levels and cardiovascular damage [61, 62]. The mechanisms by which ACE2 gene variants could impact the structural and/or the catalytic activity of the gene product could be at the transcriptional (mRNA expression), post-transcriptional modifications (such as N-glycosylation), or ACE2 protein levels that influence the outcome of COVID-19 by acting on blood pressure through the RAS and possible impact on lung/heart damages through the Ang II-triggered oxidative stress [44].
Furthermore, the recent study by Khayat et al. has unraveled at least ten ACE2-related variants in coding, noncoding, and regulatory sites that can offer a plausible biological explanation for the epidemiological differences related to COVID-19 [57]. They have identified the rs182366225 and rs2097723 variants associated with ACE2 upregulation to be more prevalent (30% to 180% more frequently) in the East Asian population, whereas rs1027571965 and rs889263894 variants were exclusively found in indigenous populations from Amazon. In contrast, the later population had higher frequencies of “rs2285666 and rs35803318” than other populations. Furthermore, Africans were identified to have higher rates of three relevant polymorphisms (rs147311723, rs142017934, and rs4646140), in which “rs142017934” was exclusive to this population and associated with gene upregulation. However, Europeans and some Africans have a higher frequency of an (rs5934250) allele that seems to downregulate ACE2 in some tissues [57]. The ACE insertion/deletion (I/D) variant, which influences enzyme levels with subsequent change in the “ACE/Ang II/AT1R axis” function, also showed many correlations with SARS-CoV-2 infection and appeared to impact the outcome of COVID-19 disease [4]. It has been observed that the (II) genotype (that is associated with the least ACE plasma levels compared to the ID/DD variants) is the most prevalent genotype among asymptomatic COVID-19 cases. However, the (DD) genotype is predominate in COVID-19 patients, particularly in the European elderly population who present with severe disease phenotype and could increase the risk of COVID-19-related mortality [63, 64]. More detailed associations of ACE I/D variant with COVID-19 severity and comorbidities have been covered in the interesting review by Gintoni et al. [4].
ACE2 gene expression and COVID-19 outcome
Higher ACE2 expression was reported in men's lungs more than women, while serum activities appear higher in females than males, supporting the hypothesis related to the observed gender-related differences in disease severity/outcome [65,66,67]. The putative role of estrogen in upregulating the ACE2 expression/plasma activity was suggested as a possible cause for relative female protection against COVID-19 infection compared to males [68]. Also, given the site of ACE2 locus on the X chromosome, these could explain in part the severe phenotype of COVID-19 in males compared to females [69]. Moreover, several differences in the ACE2 expressions have been observed between different countries, which correlate with genetic variations[70, 71]. ACE2 expression in Asian individuals was reported to be more significant, in healthy human lung samples, than in Caucasians and African Americans [12, 72].
Similarly, an ACE2 quantitative expression analysis study on East Asians, Europeans, Africans, South Asians, and mixed Americans reported higher expression levels of ACE2 in East Asians [15] that could partly explain the variations in disease outcome among different populations. Osman et al. reported a decrease in the expression of circulating ACE2 mRNA and cell surface ACE2 during COVID-19, and prolonged viral shedders of COVID-19 were associated with low sACE2 plasma concentrations [41]. As a result, they concluded that ACE2 no longer metabolizes Ang II with increased plasma concentrations associated with worse outcomes. Furthermore, the soluble forms of ACE2 have recently been shown to inhibit SARS-CoV-2 infection [43]. In the context of enhanced ACE2 deficiency produced by the viral invasion, the significant “ACE2/AT1-7/Mas axis” dysregulation could contribute to augmenting the inflammatory/thrombotic processes progression [73]. Even the ACE2 expression/activity has been found to change rapidly in response to certain food items [74] and many food components are reported to be useful for the treatment of COVID-19, and these may act through altering ACE2 expression and/or its activity as detailed in the recent Sahu et al. review article [67].
Wooster et al. have suggested in their preprint article that five ACE2-related variants “rs4240157, rs6632680, rs4830965, rs1476524, and rs2048683” might be associated with higher ACE2 tissue-specific expression, resulting in hospitalization, whereas the “rs1548474” variant showed association with low tissue expression and lesser severity [75]. Also, the rs2106809 variant has been suggested to be associated with variable circulating ACE2 levels, whereas CC/CT genotype resulted in greater levels when compared with the TT genotype. Therefore, “quantification of soluble ACE2 (sACE2) in body fluids was suggested as a protective biomarker for a rapid test screening,” as concluded by Chaudhary [40]. Additionally, a combined effect of genetic variants in genes responsible for the synthesis of proinflammatory cytokines/chemokines along with ACE2 has been suggested to be responsible for differences in patients' response to COVID-19 in terms of hypercytokinemia/cytokine storm that characterized by excessive proinflammatory cytokine production associated with multiple organ failure [76].
ACE2 role in emerging COVID-19-related treatment
In seeking a suitable treatment for COVID-19, recently, the RBD of SARS-CoV-2 spike glycoprotein (S-protein) was modeled in 242 structural models with variations of human ACE2 binding [77]. Several ACE2 variants have been speculated in the African and American populations, including “p.Met383Thr, p.Pro389His, and p.Asp427Tyr,” which may influence the clinical efficacy of hydroxychloroquine or chloroquine [56]. This could explain why therapeutic use of hydroxychloroquine was not significantly associated with differences in “in-hospital mortality” [78].
Also, one of the proposed strategies for COVID-19 treatment was the soluble ACE2 and ACE2-Fc fusion protein that work as decoy receptors to SARS-CoV2 [79]. Using “Clinical-Grade sACE2,” in vitro study showed that the human recombinant soluble ACE2 (hrsACE2) could significantly block early stages of SARS-CoV-2 infections [80]. Also, it has been suggested that designing a recombinant non-functioning form of sACE2, which carries one or more of the specified variants that show a gain of function activity and permit binding to the viral RBD more avidly, could have a potential virus neutralization and COVID-19 treatment [79]. Similarly, by using functional models and molecular dynamics simulations, Zhang et al. could point to the broad efficacy of an engineered sACE2 decoy (has three amino acid substitutions) against SARS-CoV-2 variants in mice by markedly augmenting the affinity for the S-protein of several SARS-CoV-2 variants, supporting its therapeutic potential [81]. Recently, Vitiello and Ferrara demonstrated the significant pharmacological synergism of the triple therapy baricitinib (immunomodulator)/remdesivir (antiviral)/rhACE2 (a soluble recombinant human form of ACE2) for the effective treatment of COVID-19. The “rhACE2” could activate the Ang 1–7 and Ang 1–9 biosynthesis pathway of the RAS system by decreasing Ang II levels; this could be associated with a decline in cytokine proinflammatory concentration [82]. Thus, the rhACE2 could prove “useful as a trap effect for circulating SARS-CoV2 and decrease viral load and hinder infection,” as the investigators concluded [82].
Interestingly, El-Shennawy et al. reported “an increase about 135-fold higher potency in blocking the binding of the viral spike protein RBD, and a 60- to 80-fold higher efficacy in preventing infections by SARS-CoV-2” for their newly identified circulating extracellular vesicles that express ACE2 (evACE2) compared to vesicle-free rhACE2 [83]. They proved that evACE2 could protect the hACE2 transgenic mice from SARS-CoV-2-induced lung injury and mortality and proposed its application as a treatment modality to existing and/or future coronaviruses that use ACE2 receptors. Another therapeutic modality based on the potential use of the intranasal “ACE2-overexpressing A549 cell-derived microparticles (AO-MPs)” that are taken up by alveolar macrophages, in which these particles increase the endosomal pH with a decrease in the lysosomal pH in these cells, thus directing the bound SARS-CoV-2 from phago-endosomes to lysosomes for subsequent degradation. In this way, these particles could also inhibit the proinflammatory phenotype of the alveolar macrophages, increasing the treatment efficacy against the virus in the mice model with few (if any) side effects [84].
Another emerging proposal for COVID-19 treatment has been assumed by Bakry et al., in which they suggested the use of the mesenchymal stem cells that are coated with anti-ACE2 antibodies to help in the achievement of better cell attachment to SARS-CoV2- infected cells and competing with the virus for the same receptor [85]. They proposed that the attached antibodies be targeted to the metallopeptidase domain (19–611 a.a.) of ACE2 that interacts with the S-protein. Additionally, Wang et al. developed an “inhaled microfluidic microsphere” with a genetically engineered membrane from ACE2 receptor-overexpressing cells/macrophages. As this system competes with the virus for ACE2 binding, it can significantly reduce the viral infectivity along with the respiratory system in vitro and in vivo, as well as can efficiently alleviate the proinflammatory cytokine storm [86]. Although all the studies mentioned above open a new era in COVID-19 treatment and management, Hou et al. recommended that “further pharmacogenomic studies that integrate drug response and genetic data from patients with COVID-19 are urgently needed” [56] to help future targeted and personalized therapy applications in clinics.
Conclusions
It is worth noting that apart from the differences in ACE2 genetic variations and gene expression, many other genetic and/or environmental factors, including, for example, the genes related to the innate and adaptive immunity, the viral load, the preventive precautions that are taken at the level of the individuals and the countries, among others, could influence COVID-19 virulence and modify disease outcome. Most of the studies mentioned above have limitations, including the non-reproducibility of genetic variant studies among different ethnic groups [40]. So much is yet to be known.
Availability of data and materials
The authors declare that all data used to elaborate this project is available for consultation by emailing the correspondent author.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- AAs:
-
Amino acids
- ACAH:
-
Angiotensin-converting enzyme homolog
- AECII:
-
Alveolar epithelial type II cells
- AO-MPs:
-
ACE2-overexpressing A549 cell-derived microparticles
- E:
-
A small envelope protein
- evACE2:
-
Extracellular vesicles that express ACE2
- hrsACE2:
-
Human recombinant soluble ACE2
- I/D:
-
Insertion/deletion
- M:
-
Matrix protein
- MPROT15:
-
Metalloprotease 15
- N:
-
Nucleocapsid protein
- RAS:
-
Renin-angiotensin system
- RBD:
-
Receptor-binding domain
- S:
-
Spike glycoprotein
- sACE2:
-
Soluble ACE2
- SARS-COV:
-
Severe acute respiratory syndrome coronavirus
- SARS-COV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SIGNOR:
-
SIGnaling network open resource
- Smad:
-
Small mother against decapentaplegic
- TGF-β1:
-
Transforming growth factor-β1
- TMPRSS:
-
Transmembrane protease, serine 2
References
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Salvatore PP, Sula E, Coyle JP, Caruso E, Smith AR, Levine RS et al (2020) Recent increase in COVID-19 cases reported among adults aged 18–22 years - United States, May 31-September 5, 2020. MMWR Morb Mortal Wkly Rep 69(39):1419–1424. https://doi.org/10.15585/mmwr.mm6939e4
Tan WSD, Liao W, Zhou S, Mei D, Wong WF (2018) Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol 40:9–17. https://doi.org/10.1016/j.coph.2017.12.002
Gintoni I, Adamopoulou M, Yapijakis C (2022) The impact of ACE and ACE2 gene polymorphisms in pulmonary diseases including COVID-19. In Vivo 36(1):13–29. https://doi.org/10.21873/invivo.12672
Scialo F, Daniele A, Amato F, Pastore L, Matera MG, Cazzola M et al (2020) ACE2: the major cell entry receptor for SARS-CoV-2. Lung 198(6):867–877. https://doi.org/10.1007/s00408-020-00408-4
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B et al (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11(8):875–879. https://doi.org/10.1038/nm1267
Asselta R, Paraboschi EM, Mantovani A, Duga S (2020) ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 12(11):10087–10098. https://doi.org/10.18632/aging.103415
Amin M, Sorour MK, Kasry A (2020) Comparing the binding interactions in the receptor binding domains of SARS-CoV-2 and SARS-CoV. J Phys Chem Lett 11(12):4897–4900. https://doi.org/10.1021/acs.jpclett.0c01064
Xu X, Chen P, Wang J, Feng J, Zhou H, Li X et al (2020) Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 63(3):457–460. https://doi.org/10.1007/s11427-020-1637-5
Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M et al (2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487(7408):477–481. https://doi.org/10.1038/nature11228
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan China. JAMA 323(11):1061–1069. https://doi.org/10.1001/jama.2020.1585
Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W (2020) Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med 202(5):756–759. https://doi.org/10.1164/rccm.202001-0179LE
Chiu RW, Tang NL, Hui DS, Chung GT, Chim SS, Chan KC et al (2004) ACE2 gene polymorphisms do not affect outcome of severe acute respiratory syndrome. Clin Chem 50(9):1683–1686. https://doi.org/10.1373/clinchem.2004.035436
Benetti E, Tita R, Spiga O, Ciolfi A, Birolo G, Bruselles A et al (2020) ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur J Hum Genet 28(11):1602–1614. https://doi.org/10.1038/s41431-020-0691-z
Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X et al (2020) Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov 6:11. https://doi.org/10.1038/s41421-020-0147-1
Omasits U, Ahrens CH, Müller S, Wollscheid B (2014) Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 30(6):884–886. https://doi.org/10.1093/bioinformatics/btt607
Licata L, Lo Surdo P, Iannuccelli M, Palma A, Micarelli E, Perfetto L et al (2020) SIGNOR 2.0, the SIGnaling network open resource 2.0: 2019 update. Nucleic Acids Res 48(D1):D504–D510. https://doi.org/10.1093/nar/gkz949
Cui J, Li F, Shi ZL (2019) Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17(3):181–192. https://doi.org/10.1038/s41579-018-0118-9
Xu Y, Lou Z, Liu Y, Pang H, Tien P, Gao GF et al (2004) Crystal structure of severe acute respiratory syndrome coronavirus spike protein fusion core. J Biol Chem 279(47):49414–49419. https://doi.org/10.1074/jbc.M408782200
He Y, Zhou Y, Liu S, Kou Z, Li W, Farzan M et al (2004) Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun 324(2):773–781. https://doi.org/10.1016/j.bbrc.2004.09.106
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273. https://doi.org/10.1038/s41586-020-2012-7
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–80.e8. https://doi.org/10.1016/j.cell.2020.02.052
He J, Tao H, Yan Y, Huang SY, Xiao Y (2020) Molecular mechanism of evolution and human infection with SARS-CoV-2. Viruses. https://doi.org/10.3390/v12040428
Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S et al (2020) Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 17(6):613–620. https://doi.org/10.1038/s41423-020-0400-4
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D (2020) Structure, function, and antigenicity of the SARS-CoV-2 Spike glycoprotein. Cell 181(2):281–92.e6. https://doi.org/10.1016/j.cell.2020.02.058
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S et al (2020) Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581(7807):215–220. https://doi.org/10.1038/s41586-020-2180-5
Tortorici MA, Veesler D (2019) Structural insights into coronavirus entry. Adv Virus Res 105:93–116. https://doi.org/10.1016/bs.aivir.2019.08.002
Tortorici MA, Walls AC, Lang Y, Wang C, Li Z, Koerhuis D et al (2019) Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol 26(6):481–489. https://doi.org/10.1038/s41594-019-0233-y
Wan Y, Shang J, Graham R, Baric RS, Li F (2020) Receptor Recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. https://doi.org/10.1128/JVI.00127-20
Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N et al (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87(5):E1-9. https://doi.org/10.1161/01.res.87.5.e1
Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ (2000) A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275(43):33238–33243. https://doi.org/10.1074/jbc.M002615200
Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J et al (2002) Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277(17):14838–14843. https://doi.org/10.1074/jbc.M200581200
Camargo SM, Singer D, Makrides V, Huggel K, Pos KM, Wagner CA et al (2009) Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology 136(3):872–882. https://doi.org/10.1053/j.gastro.2008.10.055
Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T et al (2004) ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem 279(17):17996–18007. https://doi.org/10.1074/jbc.M311191200
Li W, Zhang C, Sui J, Kuhn JH, Moore MJ, Luo S et al (2005) Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J 24(8):1634–1643. https://doi.org/10.1038/sj.emboj.7600640
Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367(6485):1444–1448. https://doi.org/10.1126/science.abb2762
Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S (2014) TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 88(2):1293–1307. https://doi.org/10.1128/JVI.02202-13
Rushworth CA, Guy JL, Turner AJ (2008) Residues affecting the chloride regulation and substrate selectivity of the angiotensin-converting enzymes (ACE and ACE2) identified by site-directed mutagenesis. FEBS J 275(23):6033–6042. https://doi.org/10.1111/j.1742-4658.2008.06733.x
Guy JL, Jackson RM, Jensen HA, Hooper NM, Turner AJ (2005) Identification of critical active-site residues in angiotensin-converting enzyme-2 (ACE2) by site-directed mutagenesis. FEBS J 272(14):3512–3520. https://doi.org/10.1111/j.1742-4658.2005.04756.x
Chaudhary M (2020) COVID-19 susceptibility: potential of ACE2 polymorphisms. Egypt J Med Hum Genet 21(1):54. https://doi.org/10.1186/s43042-020-00099-9
Osman IO, Melenotte C, Brouqui P, Million M, Lagier JC, Parola P et al (2021) Expression of ACE2, soluble ACE2, angiotensin I, angiotensin II and angiotensin-(1–7) is modulated in COVID-19 patients. Front Immunol 12:625732. https://doi.org/10.3389/fimmu.2021.625732
Kornilov SA, Lucas I, Jade K, Dai CL, Lovejoy JC, Magis AT (2020) Plasma levels of soluble ACE2are associated with sex, Metabolic Syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19. Crit Care 24(1):452. https://doi.org/10.1186/s13054-020-03141-9
García-Escobar A, Vera-Vera S, Jurado-Román A, Jiménez-Valero S, Galeote G, Moreno R (2022) Calcium signaling pathway is involved in the shedding of ACE2 catalytic ectodomain: new insights for clinical and therapeutic applications of ACE2 for COVID-19. Biomolecules. https://doi.org/10.3390/biom12010076
Devaux CA, Rolain JM, Raoult D (2020) ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect 53(3):425–435. https://doi.org/10.1016/j.jmii.2020.04.015
Fujikura K, Uesaka K (2021) Genetic variations in the human severe acute respiratory syndrome coronavirus receptor. J Clin Pathol 74(5):307–313. https://doi.org/10.1136/jclinpath-2020-206867
Harmer D, Gilbert M, Borman R, Clark KL (2002) Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 532(1–2):107–110. https://doi.org/10.1016/s0014-5793(02)03640-2
Douglas GC, O’Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI et al (2004) The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 145(10):4703–4711. https://doi.org/10.1210/en.2004-0443
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637. https://doi.org/10.1002/path.1570
Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S et al (2005) Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 26(4):369–375. https://doi.org/10.1093/eurheartj/ehi114
Kowalczuk S, Bröer A, Tietze N, Vanslambrouck JM, Rasko JE, Bröer S (2008) A protein complex in the brush-border membrane explains a Hartnup disorder allele. FASEB J 22(8):2880–2887. https://doi.org/10.1096/fj.08-107300
Consortium G (2020) The GTEx consortium atlas of genetic regulatory effects across human tissues. Science 369(6509):1318–1330. https://doi.org/10.1126/science.aaz1776
Itoyama S, Keicho N, Hijikata M, Quy T, Phi NC, Long HT et al (2005) Identification of an alternative 5’-untranslated exon and new polymorphisms of angiotensin-converting enzyme 2 gene: lack of association with SARS in the Vietnamese population. Am J Med Genet A 136(1):52–57. https://doi.org/10.1002/ajmg.a.30779
Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V (2020) COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int J Mol Sci. https://doi.org/10.3390/ijms21103474
Simsek FI, Colapkulu N, Leblebici IM, Alimoglu O (2020) Another perspective for COVID-19 pandemic: angiotensin-converting enzyme 2 and ethnicity. North Clin Istanb 7(6):636–638. https://doi.org/10.14744/nci.2020.62144
Suryamohan K, Diwanji D, Stawiski EW, Gupta R, Miersch S, Liu J et al (2021) Human ACE2 receptor polymorphisms and altered susceptibility to SARS-CoV-2. Commun Biol 4(1):475. https://doi.org/10.1038/s42003-021-02030-3
Hou Y, Zhao J, Martin W, Kallianpur A, Chung MK, Jehi L et al (2020) New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med 18(1):216. https://doi.org/10.1186/s12916-020-01673-z
Khayat AS, de Assumpção PP, MeirelesKhayat BC, ThomazAraújo TM, Batista-Gomes JA, Imbiriba LC et al (2020) ACE2 polymorphisms as potential players in COVID-19 outcome. PLoS ONE 15(12):e0243887. https://doi.org/10.1371/journal.pone.0243887
Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P et al (2020) genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med 383(16):1522–1534. https://doi.org/10.1056/NEJMoa2020283
Bosso M, Thanaraj TA, Abu-Farha M, Alanbaei M, Abubaker J, Al-Mulla F (2020) The two faces of ACE2: the role of ACE2 receptor and its polymorphisms in hypertension and COVID-19. Mol Ther Methods Clin Dev 18:321–327. https://doi.org/10.1016/j.omtm.2020.06.017
Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE et al (2006) ACE and ACE2 activity in diabetic mice. Diabetes 55(7):2132–2139. https://doi.org/10.2337/db06-0033
Yamamoto K, Ohishi M, Katsuya T, Ito N, Ikushima M, Kaibe M et al (2006) Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 47(4):718–726. https://doi.org/10.1161/01.HYP.0000205833.89478.5b
Rabelo LA, Todiras M, Nunes-Souza V, Qadri F, Szijártó IA, Gollasch M et al (2016) Genetic deletion of ACE2 induces vascular dysfunction in C57BL/6 mice: role of nitric oxide imbalance and oxidative stress. PLoS ONE 11(4):e0150255. https://doi.org/10.1371/journal.pone.0150255
Gunal O, Sezer O, Ustun GU, Ozturk CE, Sen A, Yigit S et al (2021) Angiotensin-converting enzyme-1 gene insertion/deletion polymorphism may be associated with COVID-19 clinical severity: a prospective cohort study. Ann Saudi Med 41(3):141–146. https://doi.org/10.5144/0256-4947.2021.141
Ristić S, Pavlić SD, Nadalin S, Čizmarević NS (2021) ACE I/D polymorphism and epidemiological findings for COVID-19: one year after the pandemic outbreak in Europe. J Infect 83(3):381–412. https://doi.org/10.1016/j.jinf.2021.06.002
Gwathmey TM, Shaltout HA, Nixon PA, O’Shea TM, Rose JC, Washburn LK et al (2008) Gender differences in urinary ACE and ACE2 activities in adolescents. Wiley Online Library
Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF et al (2020) Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J 41(19):1810–1817. https://doi.org/10.1093/eurheartj/ehaa373
Sahu S, Patil CR, Kumar S, Apparsundaram S, Goyal RK (2022) Role of ACE2-Ang (1–7)-Mas axis in post-COVID-19 complications and its dietary modulation. Mol Cell Biochem 477(1):225–240. https://doi.org/10.1007/s11010-021-04275-2
Ciaglia E, Vecchione C, Puca AA (2020) COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr 8:206. https://doi.org/10.3389/fped.2020.00206
Vausort M, Wagner DR, Devaux Y (2014) Long noncoding RNAs in patients with acute myocardial infarction. Circ Res 115(7):668–677. https://doi.org/10.1161/CIRCRESAHA.115.303836
Li MY, Li L, Zhang Y, Wang XS (2020) Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 9(1):45. https://doi.org/10.1186/s40249-020-00662-x
Al-Eitan LN, Alahmad SZ (2021) Pharmacogenomics of genetic polymorphism within the genes responsible for SARS-CoV-2 susceptibility and the drug-metabolising genes used in treatment. Rev Med Virol 31(4):e2194. https://doi.org/10.1002/rmv.2194
Sun P, Lu X, Xu C, Sun W, Pan B (2020) Understanding of COVID-19 based on current evidence. J Med Virol 92(6):548–551. https://doi.org/10.1002/jmv.25722
Verdecchia P, Cavallini C, Spanevello A, Angeli F (2020) The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med 76:14–20. https://doi.org/10.1016/j.ejim.2020.04.037
Quiles JL, Rivas-García L, Varela-López A, Llopis J, Battino M, Sánchez-González C (2020) Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19? Environ Res 191:110053. https://doi.org/10.1016/j.envres.2020.110053
Wooster L, Nicholson C, Sigurslid H, Lino Cardenas C, Malhotra R (2020) Polymorphisms in the ACE2 locus associate with severity of COVID-19 Infection. medRxiv
Badawi A (2020) Hypercytokinemia and pathogen-host interaction in COVID-19. J Inflamm Res 13:255–261. https://doi.org/10.2147/JIR.S259096
Sorokina M, Teixeira JMC, Barrera-Vilarmau S, Paschke R, Papasotiriou I, Rodrigues JPGL et al (2020) Structural models of human ACE2 variants with SARS-CoV-2 Spike protein for structure-based drug design. Sci Data. 7(1):309. https://doi.org/10.1038/s41597-020-00652-6
Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J et al (2020) Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA 323(24):2493–2502. https://doi.org/10.1001/jama.2020.8630
Li G, De Clercq E (2020) Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 19(3):149–150. https://doi.org/10.1038/d41573-020-00016-0
Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M et al (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181(4):905–13.e7. https://doi.org/10.1016/j.cell.2020.04.004
Zhang L, Dutta S, Xiong S, Chan M, Chan KK, Fan TM et al (2022) Engineered ACE2 decoy mitigates lung injury and death induced by SARS-CoV-2 variants. Nat Chem Biol 18(3):342–351. https://doi.org/10.1038/s41589-021-00965-6
Vitiello A, Ferrara F (2022) Association and pharmacological synergism of the triple drug therapy baricitinib/remdesivir/rhACE2 for the management of COVID-19 infection. Naunyn Schmiedebergs Arch Pharmacol 395(1):99–104. https://doi.org/10.1007/s00210-021-02169-0
El-Shennawy L, Hoffmann AD, Dashzeveg NK, McAndrews KM, Mehl PJ, Cornish D et al (2022) Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2. Nat Commun 13(1):405. https://doi.org/10.1038/s41467-021-27893-2
Wang Z, Lv J, Yu P, Qu Y, Zhou Y, Zhou L et al (2022) SARS-CoV-2 treatment effects induced by ACE2-expressing microparticles are explained by the oxidized cholesterol-increased endosomal pH of alveolar macrophages. Cell Mol Immunol 19(2):210–221. https://doi.org/10.1038/s41423-021-00813-6
Bakry NS, Abdelgawad M, Abdel-Latif A, Lotfy A (2022) Mesenchymal stromal cells coated with anti-ACE2 antibodies might improve efficacy against COVID-19. Hum Cell 35(1):418–420. https://doi.org/10.1007/s13577-021-00620-1
Wang Z, Xiang L, Lin F, Cai Z, Ruan H, Wang J et al (2022) Inhaled ACE2-engineered microfluidic microsphere for intratracheal neutralization of COVID-19 and calming of the cytokine storm. Matter 5(1):336–362. https://doi.org/10.1016/j.matt.2021.09.022
Acknowledgements
To Covid-19 MENA Response Research Team, all authors are members of the project; besides that, we do thank Dr. Ahmed Negida, MBBS, Zagazig University, Egypt, for his support and establishment of the MENA team.
Funding
This work does not receive any funds.
Author information
Authors and Affiliations
Contributions
Study design and team management were done by NBHD, database search was done by AAS, SA, ASE, and HA, analysis was done by HA, FMF, and AMF, manuscript elaboration was done by MSF and AMF, and manuscript editing was done by MSF, NBHD, and AMF. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval and Consent to participate
Ethics approval is not applicable since the publication is based on data that was previously published and not on its own investigations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fawzy, M.S., Ashour, H., Shafie, A.A.A. et al. The role of angiotensin-converting enzyme 2 (ACE2) genetic variations in COVID-19 infection: a literature review. Egypt J Med Hum Genet 23, 97 (2022). https://doi.org/10.1186/s43042-022-00309-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-022-00309-6