Abstract
Background
Type 1 diabetes mellitus (TIDM) is a polygenic disorder with the involvement of several genetic and environmental risk factors. Mutation in genes namely ABCC8 and KCNJ11 disrupt the potentiality of KATP channel and regulates the secretion of insulin by detecting a change in the blood glucose level and consequently maintains glucose homeostasis. The present study was designed to investigate the association of ABCC8 and KCNJ11gene polymorphisms with type 1 diabetes. A case-control study was conducted enrolling 60 cases suffering from T1DM and 60 healthy controls of comparable age and sex. Gene variations were determined by PCR-RFLP and ARMS-PCR method.
Results
The ABCC8-3C > T (rs1799854) variation was found to be significantly associated with T1DM (p<0.01) and “CT” genotype was found to be predominant in T1DM with a threefold increased risk to diabetes and the association was statistically significant. However, we did not find any significant association of C>T (rs1801261) polymorphism of ABCC8 with T1DM. A significant association was observed for genetic variation at rs5219 C>T polymorphism and the frequency of TT genotype was found to be significantly higher in patients (46.7%) than in controls (21.7%), indicating the significant role of the KCNJ11 rs5219 variant in T1DM susceptibility (p<0.001), but we did not observe any significant association of G>A (rs5215) polymorphism of KCNJ11 with T1DM. In addition, haplotype analysis of the two genes revealed four haplotypes such as T-C-G-T, T-C-A-T, C-C-G-T, and T-T-G-T as risk haplotypes for type 1 diabetes (p<0.02) potentially making individual effects of these variants on the disease susceptibility, thereby indicating the synergistic role of these genes in the regulation of glucose homeostasis.
Conclusions
The present study highlights the importance of personalized medicine based on individual genetic profile.
Similar content being viewed by others
Background
Type 1 diabetes mellitus (T1DM) is characterized by auto immune destruction of pancreatic β cells leading to insulin deficiency [1]. It is one of the most common endocrine childhood diseases, usually presenting with characteristic symptoms of thirst, polyuria, blurred vision, and weight loss. In acute forms, ketoacidosis or a non-ketotic hyperosmolar state may arise and leads to stupors, coma, and death in the absence of effectual treatment [2].
The Diabetes Atlas estimates that there are 128,500 children and adolescents with diabetes in India, and International Diabetes Federation Atlas estimates that India has the second largest incidence and prevalence of children with type 1 diabetes worldwide [3]. A 30-year study conducted in Brazil in Bauru population suggests that the annual incidence of type 1 diabetes increased to 4% in children with ≤14 years of age [4]. A recent study conducted by Rosella et al. using a Diabetes Population Risk Tool in Canada showed that South Asians are at higher risk of developing diabetes [5]. Type 1 diabetes is a polygenic disorder with the high prevalence in South Asia compared to other developing countries. Environmental factors, sedentary lifestyle, and genetic susceptibility seem to be important, as a study suggests that 30–70% of the diabetes risk is attributed due to genetic variants [6].
The KCNJ11 and ABCC8 genes are located on the chromosome 11p15.1 and encode two subunits inwardly rectifying potassium channel (Kir6.2) and sulfonylurea receptor 1 (SUR1) and together forms KATP channel which is expressed in pancreatic ß-cells and plays a crucial role in the glucose-induced insulin secretion [7].
Structurally kir6.2 and SUR1 consists of four pore forming subunits that surrounds the pore of the KATP channel located at the plasma membrane of pancreatic beta cells and the secretion of insulin is initiated by closure of the channels and inhibited by their opening [8]. Both Kir6.2 and SUR1 are required for metabolic regulation of the channel: ATP closes the channel by binding to Kir6.2, and magnesium nucleotides (Mg-ADP and Mg-ATP) stimulate channel activity by interacting with SUR1. Activating heterozygous mutations in KCNJ11 and ABCC8 genes, encoding the two subunits of the ATP-sensitive potassium (KATP) channel, are the most common variants reported in neonates of type 1 diabetes [9].
Several SNPs of the KCNJ11 gene have been identified and among them, rs5219 polymorphism is most prominent in the regulation of glycemia [10]. The mutations in SUR1gene can cause various types of diabetes, such as hyperinsulinemic hypoglycemia of infancy. A -3C >T (rs1799854) and Thr759Thr (rs1801261) variants of the ABCC8 gene have been widely studied in association with type 2 diabetes and neonatal diabetes but no studies related to type 1 diabetes in Asian Indians with greater insulin resistance and a strong genetic background are reported [11, 12]. Hence, the present study was designed to investigate an association of KCNJ11 (rs5215 and rs5219) and ABCC8 (rs1799854 and rs1801261) gene polymorphisms with type 1 diabetes of Telangana cohort. The study could be of benefit to the children in alternative modality treatments from insulin injections to sulfonylurea drugs.
Methods
Ethical statement
The study was approved by the institutional ethics committee for conducting biomedical research. A written consent was obtained from the patients prior to the study and the objectives of the study were clearly explained.
Study population
A total of 60 children (< 15 years of age) with type 1 diabetes and an equal number of healthy children were included in the present study. The epidemiological variables like age, gender, nativity, occupation, life style habits, family history and clinical symptoms, and history of viral infections in the last 2 years were recorded with the help of structured questionnaire. The cases and control subjects were recruited on the basis of our inclusion and exclusion criteria. Clinically diagnosed type 1 diabetes mellitus (T1DM) children as defined by American Diabetes Association’s (ADA’s) criteria and Indian Diabetic Association were considered for the present study [13]. Patients with significant symptoms of polyuria/polydipsia and on regular treatment with human insulin therapy were included in the study. Patients with type 2 diabetes, renal disease, liver disease, thyroid disorders, or other endocrine or chronic diseases were excluded from study. The control subjects who were similar in age, gender, and ethnicity matched healthy individuals with no clinical or family history of DM in the first and second degree relatives or clinical symptoms of any other systemic diseases were included in the study. For controls, fasting glucose (FPG) < 126 mg/dl and glycated hemoglobin (HbA1c) < 6.0% criteria were considered as healthy individuals.
Molecular analysis
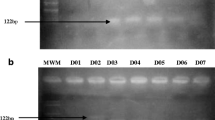
Genomic DNA was extracted from whole blood obtained from control and case group by salting out method of Lahiri et al. [14]. KCNJ11 (rs5215 and rs5219) (Figs. 1 and 2) and ABCC8 gene (rs1799854 and rs1801261) (Figs. 3, 4, 5, and 6) polymorphism genotyping was performed by appropriate ARMS-PCR and PCR-RFLP techniques (Table 1). PCR reaction was carried out in 0.2-ml tubes with a final volume of 25 μl, each containing 100 ng of genomic DNA, 50 picomoles reverse and forward primers, 200 μM of dNTPS, and 1× PCR buffer. Primers used for amplification [10, 15] were as described by Phani et al. and Venkatesan et al.
Statistical analysis
The demographic and clinical data were computed between controls and patients; allelic and genotypic frequencies and Hardy-Weinberg equilibrium were calculated by chi-square analysis. The association between genotypes and diabetes was evaluated by calculating the odds ratios (OR) at 95% confidence interval using open EPI6 software (Open Epi Version 2.3.1, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA 30322, USA). A two-tailed value of p < 0.05 was regarded as statistically significant.
Results
In the present study, the mean age of controls and diabetic children at the time of sample collection was10 ± 1.6 years and 8.4 ± 3.6 years respectively. With respect to gender, 43% of males and 56% of females were diabetic, while 46% males and 53% females were observed in controls (Table 2). The numbers of individuals with family history of T1DM were 23.3% while with past history of viral infections were 28.3% (Table 2).
ABCC8 -3C > T (rs1799854)
In the present study, the percentage distribution of CC, CT, and TT genotypes of rs1799854 was 43.3, 26.7, and 30 in controls and 21.7, 50, and 28.3 in the cases respectively. Table 3 represents the odds risk estimates of the -3C > T polymorphism in diabetic children compared to controls. The “CT” genotype was found to be predominant with a threefold increased risk to diabetes and the association was statistically significant (OR 3.75, 95% CI 1.52–9.23, p < 0.012). Indeed, the association was further tested in various combinations/models to authenticate the statistical significance, which also strengthened the “CT” genotypic association with diabetes (OR 2.75, 95% CI 1.28–5.90, p < 0.008). Further, when allele frequencies were compared, the frequency of recessive allele “T” was not found to be significantly associated with type 1 diabetic patients (OR 1.492, 95% CI 0.8964–2.492, p =0.15) (Figs. 1 and 2).
ABCC8 C>T (rs 1801261)
The percentage distribution of CC, CT, and TT genotypes was 36.7, 38.3, and 25 in controls and 40, 26.7, and 33.3 in the cases respectively. Table 4 results indicate that the rs 1801261 (C>T) ABCC8 variant was similar in distribution in both cases and controls [recessive (OR 1.50, 95% CI 0.68–3.32, p =0.31) and dominant models (OR 0.87, 95% CI 0.42–1.81, p =0.71)]. Further, when allele frequencies were compared, the frequency of recessive allele “T” was similar in distribution in both T1DM and control groups (OR 1.106, 95% CI 0.663–1.843, p =0.79). Odds test analysis also revealed no significant association between ABCC8 C>T (rs 1801261) polymorphism and diabetic children.
KCNJ11 G>a (rs 5215)
The percentage distribution of GG, GA, and AA genotypes was 63.3, 20, and 16.7 in controls and 55, 18.3, and 26.7 in the cases respectively. No significant association was detected under recessive (OR 1.82, 95% CI 0.75–4.42, p =0.18), dominant (OR 1.41, 95% CI 0.68–2.94, p =0.35), and over dominant genetic models (OR 0.90, 95% CI 0.36–2.23, p =0.82). Further, when allele frequencies were compared, the frequency of recessive allele “A” was also found to be not significantly associated with type 1 diabetics (OR 1.533, 95% CI 0.8838–2.675, p =0.16). Odds test analysis revealed no significant association between KCNJ11 G>A (rs 5215) polymorphism and diabetic children (Table 5).
KCNJ11 C>T (rs 5219)
The present study suggests a significant association between rs5219 C>T polymorphism and patients with diabetes. The frequency of TT genotype was found to be significantly higher in patients (46.7%) than in controls (21.7%). In both co-dominant model CC vs TT [odds ratio = 4.59, 95%; CI= (1.87, 11.29); p < 0.001] and recessive model TT vs CC+CT [odds ratio = 3.16, 95% confidence interval (1.43, 7.02); p < 0.003] (Table 6), a significant difference was observed. Further, the allele frequencies when compared, a strong association was found with recessive allele “T” in diabetic patients [odds ratio = 2.978, 95% confidence interval (1.764, 5.076); p <0.006] compared to controls (Table 6). The HWE analysis showed no deviation either in case or in controls in both the SNPs in the present study which could be due to the small sample size
Haplotype analysis
In the present study, sixteen haplotypes based on the four polymorphisms were constructed and analyzed for the possible association with diabetes by using SNP stat program (https://www.snpstats.net/start.htm, Table 7). The T-C-G-T haplotype was found to be significantly associated with 3.98-fold risk (95% CI 1.02–15.57, p < 0.05), T-C-A-T haplotype with 9.65 fold risk (95% CI 0.88–106.14, p < 0.06), C-C-G-T haplotype with 4.48 fold risk (95% CI 0.98–20.56, p < 0.05), and T-T-G-T haplotype with 6.70 fold risk (95% CI 1.04–43.02, p < 0.04) in diabetes compared to controls. Further the global haplotype association test was performed and confirmed that the T-C-G-T, T-C-A-T, C-C-G-T, and T-T-G-T are the risk haplotypes for diabetes (p<0.02) with respect to ABCC8 and KCNJ11 gene polymorphisms.
Linkage disequilibrium
Linkage disequilibrium analysis (LD), defined by the delta coefficient (D′), was determined in TID and controls for four SNPs, ABCC8-3C > T (rs1799854), ABCC8 C>T (rs 1801261), KCNJ11 G>A (rs 5215), and KCNJ11 C>T (rs 5219). D′ < 0:2 indicate no linkage disequilibrium between two loci, D′ > 0: 5 indicate a weak linkage disequilibrium, D′ 0:8 indicate a linkage imbalance between the loci, and D′ =1 signifies complete linkage disequilibrium. In the present study, pair-wise linkage disequilibrium (LD) for 4 single nucleotide polymorphisms was assessed in controls and T1DM. The analysis has generated 6 marker combinations out of 4 SNPs. Most of the SNP marker combinations exhibited low LD scores with a D′ 0.20 and r2 0.022 (Tables 8 and 9). Thus, these observations indicate absence of linkage disequilibrium between the four SNP polymorphisms in patients and control groups (Fig. 7).
Discussion
T1DM is a chronic disease in children caused by insufficient insulin production because of the autoimmune destruction of pancreatic islet beta cells (β cells), and as a result, the glucose levels in the blood cannot be maintained at normal concentrations [16]. The higher prevalence of type 1 diabetes was identified in relatives which implies a genetic risk and degree of genetic identity. Roughly 50 additional genes contribute individually to smaller effects (INS, IPF1, GLIS3, HNF1B, RFX6, FOXP3, EIF2AK3, SLC19A1, NEUROD1, and PTF1ATP) in the etiology of T1DM. These will include gene variants that regulate immune tolerance and immuneregulation. Some variants influence and modify viral responses to functions of endocrine and environmental signals as well as expressed in some of the β-cells of the pancreas (Fig. 8).The SUR-1 and Kir 6.2 proteins of ABCC8 and KCNJ11 genes play a crucial role in the function of the KATP channel and the mutations in this gene can disrupt their activity and lead to type 1 DM. It is apparent from the literature that several variants of the ABCC8 and KCNJ11genes are associated with different types of DM. Heterozygous mutations in either Kir6.2 or SUR1 lower KATP channel activity and result in familial persistent hyperinsulinemia that presents as hypoglycemia of infancy, a rare genetic disorder [17]. This brings into the question that which of those ABCC8 and KCNJ11gene polymorphisms and their composites play an important role in the development of T1DM.
The KCNJ11 gene affects the insulin secretion pathway by lowering the channel activity recognized in familial hyperinsulinemia hypoglycemia T2DM. According to the findings by Hamming et al. [18], the rs5219 variant display decreased ATP inhibition, which may contribute to the observed increased risk for T2DM. According to Qiu et al., a systematic meta-analysis was performed for rs5219 variant and T2DM in different genetic models which revealed a strong relationship between the rs5219 polymorphism and susceptibility to T2DM risk with a per-allele odds ratio (OR) of 1.12 (95% CI 1.09–1.16; P<10−5) and by ethnicity, notably increased risks were found for the polymorphism in Caucasians and East Asians [19].
Several studies have been carried out to evaluate the effects on the risk of T1DM (Cejková et al.; Qu et al.; Raj et al.; Ko et al.), and none of them has observed association in Caucasians and Asians. However, these results may vary depending upon the ethnicity of the study population. The genotype frequency for rs5219 reported in the present study was higher (47%) for recessive allele than the rate reported for Euro-Brazilian [(14%) Souza et al., 2017], Korean [(26 %) Ko et al., 2012], and Czech [(25%) Cejková et al., 2007] populations [20, 21]. In the present study, the significant role of the KCNJ11 rs5219 variant in TID susceptibility was observed. In another study, the rs5215 polymorphism was associated with blood pressure among subjects with T2DM [22] whereas the remaining studies showed no association with T2DM, T1DMM, or GDM [23, 24]. However, the present study did not reveal any such significant association with the rs5215 variant of KCNJ11 with T1DMM.
The Kir 6.2 Protein of KCNJ11gene has a significant function in insulin secretion, consequently making it a possible susceptibility gene for T2DM. The rs5219 variant of KCNJ11 has been largely described to be associated with T2DM in diverse ethnic populations [25]. However, the study conducted in the Korean population suggests no association of the rs5219 variant polymorphism with T1DM. Since the mutations in the KCNJ11 gene are responsible for permanent neonatal diabetes mellitus [26], a study carried out by Lo in the Taiwan population found heterozygous missense mutation in the KCNJ11 gene to be significantly associated [27]. Furthermore, the present study represents a similar observation of the significant association of KCNJ11 (rs5219) gene polymorphism. Most of the KCNJ11-related neonatal diabetes patients experience an improvement with oral SU treatment, which regulates the KATP channel [27].
According to Thurber et al., the initiation of SU treatment at a juvenile age is associated with improved response to SU therapy. Decreased responsiveness to SU could be due to the loss of β-cell mass over time in those treated with insulin. Consequently, initial genetic diagnosis and significant treatment are pivotal in neonates and T1DM [28]. Extensive studies have been shown that the non-synonymous polymorphism rs5219 (Lys23Glu) is more recurrent in T2DM [29]. In vitro experiments in human pancreatic islets exhibited depletion in response to SUF in presence of the non-synonymous polymorphism 23Lys, which has been confirmed in studies performed on T2DM patients of Chinese ethnicities undergoing SUF therapy [30].
The SUR-1 protein of the ABCC8 gene accounts for the common genetic etiology of permanent and transient DM. In recent times, ABCC8 has been conceding as MODY12 subtype with a rising in the number of relatives being affected [31,32,33].The -3C >T (rs1799854) polymorphism and Thr759Thr (rs 1801261) were found to be associated with type 2 diabetes in Caucasians, Danish, and French Caucasian diabetic individuals [12]. Additionally, in the ABCC8 gene, three variants were studied within the Japanese population, and out of the three variants, only exon16 -3C > T was most significantly related to type 2 diabetes [34]. In the study by Venkatesan et al., no such association of both the genetic variants (rs1799854 and rs1801261) was reported with T2DM respectively.
In terms of pharmacogenetics, certain studies have found that the TT genotype of the ABCC8 gene may be associated with an increase in HBA1C and triglyceride levels in SU-treated diabetics, and according to Zychma et al., the rs1799854 variant was most significantly linked with β-cell dysfunction, further tested with sulfonylurea [35]. In the present study, a significant association of rs1799854 variant in type 1 diabetic children was reported and the difference in the allelic and genotypic frequencies was observed in most reported cases, which designate a substantial stipulation to assess their implications in diabetes progression and drug response. The ABCC8 rs1801261 polymorphism was associated with the risk of T2DM in Canadian and Danish population [36]. In contrast, two preceding studies comprising North Indian and Finnish populations have put forth that the ABCC8 rs1801261 gene is not associated with a risk to T2DM [37]. However, no significant association of rs1801261 variant with type 1 diabetic children was reported in our study.
In neonates, inactivating mutations in genes encoding Kir6.2 (KCNJ11) and SUR1 (ABCC8) are accountable for T2DM while activating mutations given on to hypoglycemia [27]. Some studies have reported that diabetic patients having KCNJ11 gene variants respond better to pharmacotherapy with SUs as compared to insulin [38]. In one study, the genetic variants of ABCC8 were disclosed for a significant reduction in HBA1C concentration [39]. The rs1799854 (-3G >T) often combined with the closely linked non-synonymous variant rs1801261 (Thr759Thr) was associated with reduced insulin secretion after tolbutamide infusion in non-diabetic relatives of T2DM patients [40]. T2DM patients on SUF treatment carrying the rs1799854G/G genotype exhibited significantly lower HBA1C levels compared with the patients with T/T genotype and improved insulin sensitivity determined by HOMA index in response to repaglinide [41, 42].
Several studies of European and other Western populations have revealed an association of KNCJ11 and ABCC8 gene polymorphisms with Neonatal Diabetes [43]. Stanik et al. confirmed an association of the two gene polymorphisms (KCNJ11 and ABCC8) in neonatal diabetes in the Slovakian population and have successfully switched from insulin to SU which leads to a decrease of HBA1C from 9.3 to 11.0% on insulin to 5.7 to 6.6% on SU treatment [44]. Flanagan et al. established that of all the patients tested, KATP channel mutations (ABCC8 gene mutations) accounted to 89%, and thus, ABCC8 can be one of the casual gene implicated in TNDM and sequel in a distinct clinical subtype that comprise biphasic diabetes and specifically can be treated with sulfonylureas [45]. According to Ellard et al., fifty-nine patients with PNDM were studied and 16 patients were identified with ABCC8 gene variants with a recessive mode of inheritance observed in 8 patients. Functional studies showed a reduced response to ATP consistent with an activating mutation that results in reduced insulin secretion [46].
In the present study, to elucidate the contribution of genetic variants in the KCNJ11-ABCC8 gene region to T1DM susceptibility in the South Indian population, haplotype analysis was performed and revealed several different risk haplotypes. Multiple risk alleles for the SUR1 gene exist in distinct Caucasian populations and suggested that some ABCC8 haplotypes represented a higher diabetes risk than others [47]. The exon 16-3C/exon 18T allele of the ABCC8 variant was increased in the diabetic group in most Caucasian studies whereas the exon 16-T/exon 18C haplotype was elevated in two studies in Denmark and Netherlands with a similar association in the present study. Furthermore, when the haplotype association test was performed in both KCNJ11-ABCC8 gene region four at-risk haplotypes (T-C-G-T, C-C-G-T, T-T-G-T, and T-C-A-T) in the present study revealed significance in T1DM patients. The haplotype combination of T-T-G-T consisted of the recessive allele combination of ABCC8 (exon16-3T/exon18T) gene and wild type, recessive allele combination of KCNJ11 (rs5215 and rs5219) gene with 6.70 fold risk (95% CI 1.04–43.02, p < 0.04) in diabetes compared to controls. Although rs1801261 of the ABCC8 gene and rs5215 of the KCNJ11 gene were not significantly associated with the disease, the haplotype analysis showed the synergistic action of these variants in disease susceptibility.
Thus, the regulation of insulin release is mediated by KCNJ11 in association with distinct genes such as ABCC8, ABCC9, and CACNA1A-G. Nonetheless, the accurate functional relationship of these genes in the regulation of insulin release remains to be adjudged. Upcoming studies are propounded to discover the exact role of KCNJ11 and ABCC8 gene variants and their relation with further genes in T1DM for the potential development of suitable therapeutic strategies.
Conclusions
In conclusion, the present study revealed a significantly increases frequency of KCNJ11 rs5219 (C > T) and ABCC8 rs1799854 (C > T) among type 1 diabetic children. As sulfonylureas drugs stimulates insulin secretion by closing KATP channels in pancreatic β cells, mutations in KCNJ11 and ABCC8 often respond to sulfonylureas, allowing transition from insulin therapy. Thus, the present study highlights the importance of personalized medicine based on individual genetic profile which would accurately predict which individuals with a specific medical condition would respond to a specific medical therapy. However, more number of studies needs to be employed for the accurate diagnostic and treatment strategies.
Availability of data and materials
All the data is available in the manuscript.
Abbreviations
- ARMS-PCR:
-
Amplification-refractory mutation system polymerase chain reaction
- ABCC8:
-
ATP-binding cassette transporter sub-family C member 8
- EIF2AK3:
-
Eukaryotic Translation Initiation Factor 2 Alpha Kinase 3
- FOXP3:
-
Forkhead box P3
- GLIS3:
-
Transcription factor Gli-similar 3
- HBA1C:
-
Glycated hemoglobin
- HNF1B:
-
Hepatocyte nuclear factor 1 homeobox B
- INS:
-
Insulin protein coding gene
- IPF1:
-
Insulin promoter factor 1
- MODY:
-
Maturity-onset diabetes of the young
- NEUROD1:
-
Neurogenic differentiation 1
- KCNJ11:
-
Potassium Inwardly Rectifying Channel Subfamily J Member 11
- PCR:
-
Polymerase chain reaction
- PTF1ATP:
-
Pancreas Associated Transcription Factor 1a
- RFLP:
-
Restriction fragment length polymorphism
- RFX6:
-
Regulatory factor X, 6
- SNP:
-
Single nucleotide polymorphisms
- SLC19A1:
-
Solute Carrier Family 19 Member 1
- T1DM:
-
Type 1 diabetes mellitus
References
Ahmed RG (2011) Evolutional interactions between diabetes and development. Diabetes Res Clin Pract 92:153–167
Mohan V, Sandeep S, Deepa R, Shah B, Varghese C (2007) Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res 125:217–230
Mapa-Tassou C, Katte JC, Maadjhou CM, Mbanya JC (2019) Economic impact of diabetes in Africa. Curr Diab Rep 19(2):5
Negrato CA, Lauris JR, Saggioro IB, Corradini MC, Borges PR, Crês MC, Junior AL, Guedes MF, Gomes MB (2017) Increasing incidence of type 1 diabetes between 1986 and 2015 in Bauru, Brazil. Diabetes Res Clin Pract 127:198–204
Rosella LC, Mustard CA, Stukel TA, Corey P, Hux J, Roos L, Manuel DG (2012 Aug 1) The role of ethnicity in predicting diabetes risk at the population level. Ethn Health 17(4):419–437
Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte RO, Tuomilehto J (2000) Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) project group. Diabetes Care 23(10):1516–1526
Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G et al (2003) Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 52:568–572
McTaggart JS, Clark RH, Ashcroft FM (2010) The role of the KATP channel in glucose homeostasis in health and disease: more than meets the islet. J Physiol 588(17):3201–3209
Ashcroft FM (2006) KATP channels and insulin secretion: a key role in health and disease. Biochem Soc Trans 34(2):243–246
Phani NM, Guddattu V, Bellampalli R, Seenappa V, Adhikari P, Nagri SK, Sydney CD, Mundyat GP, Satyamoorthy K, Rai PS (2014) Population specific impact of genetic variants in KCNJ11 gene to type 2 diabetes: a case-control and meta-analysis study. PLoS One 9(9):e107021
Inoue H, Ferrer J, Welling CM, Elbein SC, Hoffman M, Mayorga R et al (1996) Sequence variants in the sulfonylurea receptor (SUR) gene are associated with NIDDM in caucasians. Diabetes. 45:825–831
Hani EH, Clément K, Velho G, Vionnet N, Hager J, Philippi A et al (1997) Genetic studies of the sulfonylurea receptor gene locus in NIDDM and in morbid obesity among French caucasians. Diabetes. 46:688–694
(2018) American Diabetes Association’s standards of medical Care in Diabetes—2019. Diabetes Care 42(Suppl. 1):S1–S194. https://doi.org/10.2337/cd18-0105
Lahiri D, Nurnberger J (1991) A rapid non enzymatic method for preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res 19:5444
Venkatesan R, Bodhini D, Narayani N, Mohan V (2014) Association study of the ABCC8 gene variants with type 2 diabetes in south Indians. Indian J Human Genet 20(1):37
Wilkin TJ (2009 Jul) The accelerator hypothesis: a review of the evidence for insulin resistance as the basis for type I as well as type II diabetes. Int J Obes (Lond) 33(7):716–726
Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J et al (2004) Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 350:1838–1849
Hamming KS, Soliman D, Matemisz LC, Niazi O et al (2009) Coexpression of the type 2 diabetes susceptibility gene variants KCNJ11 E23K and ABCC8 S1369A alter the ATP and sulfonylurea sensitivities of the ATP-sensitive K+ channel. Diabetes 58:2419–2424
Qiu L, Na R, Xu R, Wang S, Sheng H et al (2014) Quantitative assessment of the effect of KCNJ11 gene polymorphism on the risk of type 2 diabetes. PLoS One 9(4):e93961. https://doi.org/10.1371/journal.pone.0093961
Cejková P, Novota P, Cerná M, Kolostová K et al (2007) KCNJ11 E23K polymorphism and diabetes mellitus with adult onset in Czech patients. Folia Biol (Praha) 53:173–175
Raj SM, Howson JM, Walker NM, Cooper JD et al (2009) No association of multiple type 2 diabetes loci with type 1 diabetes. Diabetologia 52:2109–2116. https://doi.org/10.1007/s00125-009-1391-y
Koo BK, Cho YM, Park BL et al (2007) Polymorphisms of KCNJ11 (Kir6.2 gene) are associated with type 2 diabetes and hypertension in the Korean population. Diabet Med 24(2):178–186. https://doi.org/10.1111/j.1464-5491.2006.02050.x
Odgerel Z, Lee HS, Erdenebileg N et al (2012) Genetic variants in potassium channels are associated with type 2 diabetes in a Mongolian population. J Diabetes 4(3):238–242. https://doi.org/10.1111/j.1753-0407.2011.00177.x
Cho HJ, Lee SY, Kim YG et al (2011) Effect of genetic polymorphisms on the pharmacokinetics and efficacy of glimepiride in a Korean population. Clin Chim Acta 412(19):1831–1834
Zhou D, Zhang D, Liu Y, Zhao T, Chen Z, Liu Z, Yu L, Zhang Z, Xu H, He L (2009) The E23K variation in the KCNJ11 gene is associated with type 2 diabetes in Chinese and east Asian population. J Hum Genet 54:433–435. https://doi.org/10.1038/jhg.2009.54
Kanakatti Shankar R, Pihoker C, Dolan LM, Standiford D, Badaru A, Dabelea D, Rodriguez B, Black MH, Imperatore G, Hattersley A, Ellard S (2013) Permanent neonatal diabetes mellitus: prevalence and genetic diagnosis in the SEARCH for diabetes in youth study. Pediatr Diabetes 14(3):174–180
Lo FS (2018) Mutation screening of INS and KCNJ11 genes in Taiwanese children with type 1B diabetic onset before the age of 5 years. J Formos Med Assoc 117(8):734–737
Thurber BW, Carmody D, Tadie EC, Pastore AN, Dickens JT, Wroblewski KE, Naylor RN, Philipson LH, Greeley SA (2015) United States neonatal diabetes working group. Age at the time of sulfonylurea initiation influences treatment outcomes in KCNJ11-related neonatal diabetes. Diabetologia. 58(7):1430–1435
Liu Z, Zhang Y, Feng Q et al (2006) Association analysis of 30 type 2 diabetes candidate genes in Chinese Han population. Zhongguo Yi XueKeXue Yuan Xue Bao 28(2):124–128
Sesti G, Laratta E, Cardellini M et al (2006) The E23K variant of KCNJ11 encoding the pancreatic beta-cell adenosine 5'-triphosphate-sensitive potassium channel subunit Kir6.2 is associated with an increased risk of secondary failure to sulfonylurea in patients with type 2 diabetes. J Clin Endocrinol Metab 91(6):2334–2339. https://doi.org/10.1210/jc.2005-2323
Ovsyannikova AK, Rymar OD, Shakhtshneider EV, Klimontov VV, Koroleva EA, Myakina NE, Voevoda MI (2016) ABCC8-related maturity-onset diabetes of the young (MODY12): clinical features and treatment perspective. Diabetes Therapy 7(3):591–600
Bowman P, Flanagan SE, Edghill EL, Damhuis A, Shepherd MH, Paisey R, Hattersley AT, Ellard S (2012) Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia. 55(1):123–127
Dallali H, Pezzilli S, Hechmi M, Sallem OK, Elouej S, Jmel H, Halima YB, Chargui M, Gharbi M, Mercuri L, Alberico F (2019) Genetic characterization of suspected MODY patients in Tunisia by targeted next-generation sequencing. Acta Diabetol 56(5):515–523
Yokoi N, Kanamori M, Horikawa Y, Takeda J, Sanke T, Furuta H et al (2006) Association studies of variants in the genes involved in pancreatic beta-cell function in type 2 diabetes in Japanese subjects. Diabetes 55:2379–2386
Zychma MJ, Gumprecht J, Strojek K, Grzeszczak W, Moczulski D, Trautsolt W et al (2002) Sulfonylurea receptor gene 16-3 polymorphism-association with sulfonylurea or insulin treatment in type 2 diabetic subjects. Med Sci Monit 8:512–515
Hansen T, Echwald SM, Hansen L et al (1998) Decreased tolbutamidestimulated insulin secretion in healthy subjects with sequence variants in the high-affinity sulfonylurea receptor gene. Diabetes 47:598–605
Matharoo K, Arora P, Bhanwer AJS (2013) Association of adiponectin (AdipoQ) and sulphonylurea receptor (ABCC8) gene polymorphisms with type 2 diabetes in north Indian population of Punjab. Gene 527:228–234
Dupont J, Pereira C, Medeira A, Duarte R, Ellard S, Sampaio L (2012) Permanent neonatal diabetes mellitus due to KCNJ11 mutation in a Portuguese family: transition from insulin to oral sulfonylureas. J Pediatr Endocrinol Metab 25:367–370
Zhang H, Liu X, Kuang H, Yi R, Xing H (2007) Association of sulfonylurea receptor 1 genotype with therapeutic response to gliclazide in type 2 diabetes. Diabetes Res Clin Pract 77:58–61
Elbein SC, Sun J, Scroggin E, Teng K, Hasstedt SJ (2001) Role of common sequence variants in insulin secretion in familial type 2 diabetic kindreds: the sulfonylurea receptor, glucokinase, and hepatocyte nuclear factor 1alpha genes. Diabetes Care 24(3):472–478
He Y, Zhang R, Shao X et al (2008) Association of KCNJ11 and ABCC8 genetic polymorphisms with response to repaglinide in Chinese diabetic patients. Acta Pharmacol Sin 29(8):983–989. https://doi.org/10.1111/j.1745-7254.2008.00840.x
Nikolac N, Simundic A-M, Katalinic D, Topic E, Cipak A, Zjacic RV (2009) Metabolic control in type 2 diabetes is associated with sulfonylurea receptor-1 (SUR-1) but not with KCNJ11 polymorphisms. Arch Med Res 40(5):387–392. https://doi.org/10.1016/j.arcmed.2009.06.006
Remedi MS, Friedman JB, Nichols CG (2017) Diabetes induced by gainof-function mutations in the Kir6.1 subunit of the K-ATP channel. J Gen Physiol 149:75–84
Stanik J, Gasperikova D, Paskova M et al (2007) Prevalence of permanent neonatal diabetes in Slovakia and successful replacement of insulin with sulfonylurea therapy in KCNJ11 and ABCC8 mutation carriers. J Clin Endocrinol Metab 92(4):1276–1282. https://doi.org/10.1210/jc.2006-2490
Flanagan SE, Patch AM, Mackay DJ et al (2007) Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood [published correction appears in diabetes. 2008 Feb;57(2):523]. Diabetes. 56(7):1930–1937. https://doi.org/10.2337/db07-0043
Ellard S, Flanagan SE, Girard CA et al (2007) Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet 81(2):375–382. https://doi.org/10.1086/519174
t Hart LM, de Knijff P, Dekker JM, Stolk RP, Nijpels G, van der Does FEE, Ruige JB, Grobbee DE, Heine RJ, Maassen JA (1999) Variants in the sulphonylurea receptor gene: association of the exon 16±3t variant with type II diabetes mellitus in Dutch Caucasians. Diabetologia 42:617–662
Acknowledgements
The authors acknowledge Niloufer Hospital, Institute of Child Health Hyderabad for providing samples.
Funding
No funding was granted for the present study.
Author information
Authors and Affiliations
Contributions
The concept of the present study was given by “SR” and “SK.” “MK,” “NC,” and “SP” diagnosed the patients and provided clinical history and samples. “SM” has done literature search and drafted the original manuscript. “PN” revised the manuscript. “VA” approved the final version to be published. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutional ethics committee for conducting biomedical research. Ethics Committee - Institute of Genetics and Hospital for Genetic Diseases belongs to Osmania University with reference No: 30/IEC/IOG/OU/18 dated: 05-02-18.
A written consent was obtained from the parents of the patients prior to the study and the objectives of the study were clearly explained.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reddy, S., Maddhuri, S., Nallari, P. et al. Association of ABCC8 and KCNJ11 gene variants with type 1 diabetes in south Indians. Egypt J Med Hum Genet 22, 27 (2021). https://doi.org/10.1186/s43042-021-00149-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-021-00149-w











