Abstract
Background
Relative telomere length (RTL), the biological chronometer, varies considerably among individuals under the influence of multiple risk factors such as socioeconomic status (SES). It is anticipated that during fetal life, telomeres undergo reprogramming. The purpose of this study is to find the association between SES and telomere length of mother-newborn and genetic remodeling that occurs during fetal life.
Results
The mean telomere/single gene copy (T/S) ratio and RTL (base pairs) among 250 mother-newborn dyads were higher in cord blood of newborns (1.18 ± 0.23) (6765 ± 1350 bp) (95% confidence level) compared to maternal blood (1.13 ± 0.18) (6432 ± 1350 bp) of all SES of the Pakistani population. A positive association (r = 0.396, p < 0.05) (F (2,238) = 9.229, p < 0.05) was found between maternal and newborn telomere length by using Spearman’s correlation and regression analyses. Calculated RTL by Kruskal Wallis was found significant in low SES maternal and cord blood (5916 ± 754-6214 ± 596) compared to high SES maternal and cord blood (6818 ± 1248-7471 ± 1851).
Conclusion
Significantly longer RTL in cord blood than maternal blood was observed in the targeted Pakistani population, including the low socioeconomic group highlighting fetal telomere reprogramming. High education appears to have a strong determining factor for longer RTL.
Similar content being viewed by others
Background
Socioeconomic status (SES), the eminence of a person in society, is measured on an extensive range of parameters including occupation, income, wealth, education, and geographic location [1]. These measures generally viewed as broad indicators can be used interchangeably, but there is no “gold standard” on determining SES. During this era of science and development, people are progressively experiencing a different socioeconomic setback, leading to worldwide health deprivation especially in developing countries like Pakistan. According to WHO, Pakistan’s national poverty line rate is 24% and middle class (lower and upper) poverty line rate is 34% and 75% [2]. Age-related diseases and early mortality are linked to low SES. They can act as biological process activators that are interconnected to social disadvantage, exposing individuals to detrimental physical, mental, and behavioral insults and other multiple health problems [3].
Human telomeres, having almost 150 million base pairs, comprise less than 1% of the total genome. They are long non-coding tandem repeat sequences (TTAGGG) which shortens during every cell division due to an incomplete synthesis of the lagging strand by DNA polymerase [4]. After 50–70 cell divisions, the telomeres reach a critically short length (Hayflick limit) which is recognized by p53 protein to trigger cellular apoptosis [5].
Telomere, both in adults and children, acts as a mitotic clock and a risk predictor to the development and progression of different diseases [6]. Telomeropathies are now being recognized as an emerging problem due to compromised telomere structure and function. These can also be named as “telomere disorders” or “telomere syndromes.” It can be identified by DNA damage and shelterin (protein complex) disruption, but research is still ongoing in this area [7]. Older people with decreased telomere length have three to eight times increased risk of dying from heart disease or multiple illnesses [8]. Multiple diseases like blood pressure, glycated hemoglobin (HbA1C), decreased bone mineral density, and disrupted lipid profile can be biological age predictors. Telomere length has been extensively implicated with the risk of malignant tumors [9] and diseases like cardiovascular [10], metabolic [11], upper respiratory tract in children [12], chronic obstructive pulmonary disease (COPD) leading to emphysema [13, 14], and childhood diarrhea [15].
Prenatal stress and modified environmental factors cause a reduction in telomeres and play a significant role in aging and determining the quality of life [3]. Apart from genetic determinants, direct DNA damage can be caused by exposomes such as multiple environmental dynamics, cigarette smoking [16], physical activity, education [17], inflammation, and reactive oxygen species (ROS) [18]. The adverse intrauterine environment during pregnancy is caused by factors like psychosocial risk issues, lack of social support, low socioeconomic status, and childhood trauma [19, 20]. Association to the telomere and fetal programming and gene expression modifications has also been found linked with pre-eclampsia [21], intrauterine growth restriction (IUGR) [22], oxidative and nitrosative stress, and hypoxia [23, 24].
Maternal epigenetics can disturb the health and phenotype of the growing child, which can lead to adverse birth outcomes like preterm birth causing 16.33 g reduction in newborn birth weight [25] or large gestational age (SGA/LGA) and can also lead to postpartum depression [20, 26]. Progressing age and depression in mothers who have a high level of cortisol result in shorter telomeres in newborns [27, 28]. The association of education, ethnicity, and socioeconomic status with telomere has been considered as a biological indicator [17, 29, 30]. A retrospective study on archived neonatal dried blood from Michigan neonatal blood bank and maternal social status observed socioeconomic disparities reflect in telomere biology. The susceptibility to gene expression dysregulation and different health problems in neonates can be confirmed on the basis of telomere length size [31, 32].
The hereditability of telomere length and its importance has been proved years ago worldwide and also in different parts of Asia. Thus, a respective study was required for the detection of altered genetics leading to comorbidities, mortality, or early aging in females and their newborns. Telomere length association with SES may cause health disparities and may implant long-term impressions on different socioeconomic status of Pakistan. The objective of this study was to find out the association between different maternal SES and telomere length of mothers-newborn as well as genetic remodeling during fetal life.
Method
Subjects and procedure
A total of 250 normal pregnant females between the age of 18–35 years and their newborns were included in the study from Ziauddin Medical Hospital, Karachi, from May 2018 to October 2018. It was a cross-sectional study, and samples were collected through the quota sampling technique after the approval from the Ethics Review Committee (ERC) of Ziauddin University and Hospital. Samples were distributed into different SES with defined income per month ($ rate October 2018): low < 15,000, lower middle 16,000–25,000, upper middle 26,000–100,000, and high < 100,000 [2]. A questionnaire was filled after taking the informed written consent from the patient or their attendants. Detailed information on maternal and newborn features was obtained from hospital record registers. Females with diabetes mellitus or known malignancies were not included in the study. Firstly, the reference DNA (pooled from 5 non-pregnant females) was used to draw a standard curve with concentrations ranging from 0.1 to 12 ng/μl. Before delivery, venous blood (3–5 ml) samples of the pregnant female were collected in ethylenediaminetetraacetic acid (EDTA) tubes. Umbilical cord vein blood (3–5 ml) samples were collected immediately postpartum into EDTA tubes from the cord. Samples were stored at − 20 °C. DNA extraction was done by EZ-10 spin column genomic DNA kit (BioBasic Canada Inc.). Qubit dsDNA HS Assay kit at 260 nm and 280 nm (Qubit 2.0 Invitrogen Life Technologies, USA) was used to measure the concentration of each DNA sample on Qubit spectrophotometer. For temperature optimization, SimpliAmp conventional thermal cycler (Applied Biosystem) was used. Primer sequences for telomere and single copy gene (β-globin) amplification were as follows: Tel (forward): 5′GGTTTTTGAGGGT-GAGGGTGAGGGTGAGGGTGAGGGT3′, Tel (reverse): 5′TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA3′, HBG (forward): 5′GCTTCTGACACAACTGTGTTCACTAGC3′, and HBG (reverse): 5′CACCAACTTC-ATCCACGTTCACC3′ [33]. All PCR products were analyzed on 2% agarose gel electrophoresis. Real-time PCR or quantitative PCR (qPCR) was done by using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen). In 30 μl reaction mixture, 15 μl of master mix, 2.5 μl of 10uM forward primers, 2.5 μl 10uM reverse primers, and 1 μl ROX dye 10 μl DNA (10 ng/μl) were used. This method was an extension of basic PCR, including relative quantification of target DNA using ct (cycle threshold) measurements by StepOne software (v2.3). For telomere amplification, qPCR temperatures were as follows: first holding stage for 50 °C for 2 min and 95 °C for 2 min, then 40 cycles were set at denaturation 95 °C for 15 s, annealing at 68 °C for 40 s and extension 75 °C for 3 min, and the second holding for 72 °C for 40 s. For reference gene (β-globin)/single gene copy amplification, PCR temperature was as follows: first holding stage for 50 °C for 2 min and 95 °C for 2 min, then 40 cycles were set at denaturation 95 °C for 15 s, annealing at 56 °C for 40 s and extension 75 °C for 3 min, and second holding for 72 °C for 40 s. To measure relative telomere length of maternal and cord blood samples, T/S ratio was calculated and then converted to base pairs (bp) by using the following equations[34]:
For interplate variation, reference DNA was loaded as a control in all runs of both telomere and reference gene. All samples were assayed in duplicate wells, and average values of two measurements were used for the statistical analyses. Fold method or delta delta ct (2-ΔΔCT) method was used to calculate the fold difference between the maternal and cord sample [35].
Statistical analysis
Data quality control analysis was done by using Statistical Package for Social Sciences (SPSS) version 20. Quantitative variables considered in the study were presented as mean ± standard deviation (SD), and qualitative variables were presented as frequency and percentages. Spearman’s correlation was used to check the correlation of maternal and cord blood RTL at 95% confidence level and p = < 0.05 significant. The Kruskal-Wallis test was used to examine the comparison of cord blood RTL with maternal demographics, SES, education, occupation, and ethnicity. The Mann-Whitney U test was also used to check the comparison between two groups. Multiple regression was used for the association of different variables.
Results
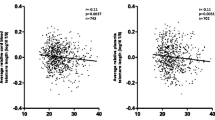
Characteristics of the 250 mother-newborn dyads are presented in Table 1. The maternal and paternal age (mean ± SD) of the samples was 27.17 ± 5.11 and 34.03 ± 4.90 years, respectively. The mean T/S ratio of cord blood (1.18 ± 0.23) was higher than maternal blood (1.13 ± 0.18) at 95% confidence level. A statistically significant (p = <0.05) association between mother and cord blood RTL was observed. The correlation (r = 0.395) between maternal and cord blood telomere presented in the scatter plot (Fig. 1) shows mean RLT in cord blood (6765 ± 1350 bp) was higher than maternal blood (6432 ± 1350 bp) across the samples.
In this study, SES was equally subgrouped (n = 63 (25%)) into 4 groups (low, lower middle, upper middle, and high) with defined income. In low and lower middle SES females, maternal RTL was shorter (5916 ± 754, 6477 ± 1064) than in cord RTL (6214 ± 596, 6492 ± 1065) (Table 1). On further analysis, consistently shorter maternal RTL was observed in upper middle and high SES females (6560 ± 1017, 6818 ± 1248) than their cord RTL (7471 ± 1851, 6936 ± 1326). (Table 1). The longest mean RTL was observed in mothers of high SES (Fig. 2a) and cord (newborns), p = < 0.05, of an upper middle SES (Fig. 2b).
The Kruskal-Wallis test was used to find mean difference between and maternal and cord blood RTLand SES and education. a Mean difference between SES and maternal blood RTL. b Mean difference between SES and cord blood RTL. c Mean difference between education and maternal blood RTL. d Mean difference between education and cord blood RTL. a Box plots depicted maternal RTL in low SES was shorter compared to upper middle and high SES. Nine outliers are lying in upper middle SES. Box plot (b) showed cord RTL was shortest in low SES and upper middle SES had longest with eight outliers (maximum number of outliers). c There were five categories of education groups; all the groups had outliers except masters group, whereas graduation group had longest RTL and no schooling group had smallest RTL. Plots (d) illustrated that all the groups had outliers except masters group. Graduation group had longest RTL whereas no schooling had smallest
In secondary analysis, when multiple comparisons were applied, statistically significant difference between various SES and maternal and cord RTL was observed. Maternal RTL for low SES was found significantly different from the other SES (p = 0.015, < 0.05, and < 0.05), respectively. Similarly, cord RTL also had a significant difference between the different maternal SES (p < 0.05, p < 0.05) (Table 2). Regression analysis highlighted a positive association between mother SES and cord RTL (F (2, 238) = 9.229, p < 0.05).
Regarding education, mothers were divided into different educational levels: no schooling (n = 70 (28%)), less than high school (n = 64 (25.6%)), high school (n = 40 (16%)), graduation (n = 16 (24.4%)), and master’s group (n = 15 (6%)). Longer RTL (6596 ± 887) was observed in females with a graduation degree compared to females with no schooling (6228 ± 116) (Fig. 2c), but the results were not significant (p = 0.089) (Table 1). However, significantly (p = 0.04) longer telomeres were observed in cord RTL of women with graduation and master’s degree (7264 ± 1634, 6709 ± 1324) compared to newborns of no schooling group (6549 ± 1174) (Tables 1 and 3, Fig. 2d). But regression analysis showed no association between education and RTL (B0 = − 91.922, T = − 0.966, p = 0.335).
Major occupational groups in the study females were housewives (n = 222 (89%)), teacher (n = 15 (06%)), and doctors (n = 9 (3.6%)), respectively. Longer RTL (6991 ± 726) was noted in doctors and shortest (6331 ± 903) in teachers. Our results also draw attention to cord telomeres which were longest (7675 ± 186) in newborns of females with banking occupation compared to housewives (6741 ± 1414) and teachers (6778 ± 542) (Table 1). Among father’s occupation, unemployed and laborer’s newborns showed decreased telomere length (6167 ± 473, 6229 ± 856) in comparison to other occupational groups (businessman 6776 ± 1062, driver 6897 ± 1352, shopkeeper 7027 ± 1866). Statistically significant (p = 0.012) results with longest RTL (8075 ± 751) were observed in newborns of father’s working for private jobs.
We also examined different ethnic groups in our study (Table 1): Pathan (n = 83 (33%)), Urdu speaking (n = 62 (25%)), Punjabi (n = 46 (18%)), Sindhi (n = 37 (15%)), and others (Hindu, Christian, Memon) (n = 20 (08%)). Research highlighted longest maternal RTL in Punjabi ethnicity (6618 ± 1120) and shortest in Urdu speaking (6319 ± 1008). Whereas in cord RTL, Urdu speaking had the longest (7042 ± 1654) and Memon ethnic groups had the shortest (6402 ± 1063). Therefore, large telomere length difference between mother and newborn was seen in Urdu speaking ethnic group (6319–7042) (p = 0.327, 0.192).
According to newborn gender, variations among newborn RTL in different SES were explored. There was n = 133 (53%) girls and n = 117 (47%) boys born to different SES females. Birth rate of girls was higher in lower and lower middle SES (Fig. 3). Overall, girls had longer RTL than boys; however, boys from low SES had longer telomere (6229 ± 551) compared to counterpart girls (6203 ± 635) (Fig. 3). Longest RTL (7651 ± 2210) was noted in girls of upper middle SES females (p = 0.768).
The results showed that there was more average delta ct (cycle threshold) in cord blood (3.57 ± 4.51) compared to the maternal (2.40 ± 3.66) with coefficient of variance (CV) at 14% (Fig. 4). The fold difference outcome showed less gene expression in mothers compared to the cord (maternal RTL 10.1 ± 2.26 (CVs = 11–15%), cord blood RTL 12.4 ± 3.63 (CVs = 15–27%)).
Discussion
Socioeconomic disparities are a well-known cause for the shortening of telomere length. There is a scarcity of studies on the association between mothers and newborns from these strata (low, lower middle, upper middle, and high). This study showed positive (p < 0.05) association (F (2, 238) = 9.229) between cord blood RTL and mother’s socioeconomic status (SES), hence revealing a statistically significant difference among all the subgroups. The RTL of low SES had a significant relationship with all the other groups (p < 0.05) [17].
Mothers from low SES in our study had the shortest RTL (5916 ± 754) compared to the lower middle, upper middle, and higher group. Previously, mixed results have been observed on SES and telomere length [36,37,38,39]. However, few studies on children’s telomere genetics revealed a shorter telomere length in adult CD8+ CD28− T cells under adverse SES and hence can increase the probability of developing an illness in the upper respiratory tract infection during childhood [40]. Researchers working on the influence of low socioeconomic status on child health concluded that intergenerational conduction of telomere length can act as markers of biological versus chronological age especially at birth [41].
The results supporting our objective of fetal telomere reprogramming during pregnancy demonstrated that mothers from low SES had shorter telomeres than mothers living under well-privileged life conditions. This may cause further fabrication of stable epigenetic adaptations in newborns in the form of different altered features [42]. A cross-sectional study of the USA, by Rehkopf et al., is in accordance with our results, showing shorter RTL as a risk factor for cardiovascular diseases [43]. Other age-related diseases have also been linked to low SES [44]. However, on the contrary, pregnancy-related diseases that we studied such as gestational diabetes mellitus (GDM) and hypertension were found in high SES females.
Contradictory to our positive association between SES and RTL some researchers demonstrated no association between RTL and social adversity (p > 0.05) [45, 46]. A study on 65-year-old Chinese men also showed shorter telomere length associated with self-rated higher economic status. Scientists justified such results by considering social factor more influencing than lifestyle. Their observations regarding unhealthy lifestyles were in terms of smoking, alcohol, physical activity, and antioxidant intake in high SES [37].
In this study, we also found that qPCR is a cost-effective and efficient technique for calculating DNA telomere length [29, 47]. The results highlighted a moderate correlation between maternal and cord blood RTL which is in accordance with another study (p = 0.34) with other reported risk factors [48]. Our study cord blood T/S ratio (0.81–2.29) calculated results are in accordance with the T/S ratio of Belgium (0.51 to 1.75) [49] and US population (1.36–3.19) [32].
We also observed that during fetal development, telomere length improves in cord blood; thus, mothers had a shorter length than a cord in our study which has also been observed by Weng et al. (p < 0.001) [50].
Inconsistent with the view that educational attainment and income are the most important indicators of SES of a person, the stronger association was also seen between education and SES measures. The studies used the levels of education, health, residence, and income to evaluate SES. Our study reported significant (p = 0.04) association among cord RTL and education. These results may reflect that education has a strong impact on telomere attrition. A study among different universities showed a wide range of telomere length: 6.3 kbp (University of Texas), 6.4 kbp (University of Pennsylvania), and 8.7 kbp (Ohio State University). Therefore, as the education level increases, the telomere length also increases [51]. The data from the National Health and Nutrition Examination Survey, 1999–2002, reported significantly (− 0.042; p < .01) shorter telomeres 5.49 kbp in adults with less than a high school degree compared to college graduates 5.63 kbp [29, 30]. These results are similar to our study in which females with graduate degrees had longer telomeres along with their newborns (6.5 kbp, 7.2 kbp) compared to groups with less education.
Telomere elongation during fetal life is due to the dramatic increase of telomerase enzyme during fertilization; however, it is downregulated under cell differentiation [52]. Therefore, longer telomere in cord blood can be due to an increase in activity and remodeling of genetic material. Epigenetic regulation also plays a substantial role in the length elongation of cord blood; however, sometimes master epigenetic regulators (DNA methyltransferases and histone methyltransferases) may also interfere with telomere loss [42].
Occupation, among other demographics, highly correlates with education and has a strong association with telomere length slow destruction [29]. The majority of females in our study were unemployed (housewives) and had shorter telomeres (6741 bp) compared to working women (doctors and bankers). Research showing longer telomeres of retired, working women (5972 bp) than homemakers (5567 bp) (p = 0.023) endorses our results [53]. Thus, it can be concluded that exhaustion-related work has a high tendency to accelerate biological aging. Stress related to heightened cortisol levels in females has shown differences in RTL, and this has also been found associated with increased risk of hypertension and accelerated aging [54]. Conflicting to the current study, research on working ladies indicated decrease in 472 bp telomeres compared to non-employed ladies [55].
There is a direct association between length of telomeres with work-related stress resulting in acceleration of biological aging, coronary heart disease, and breast cancer [56, 57]. Moreover, cancer susceptibility is known to be caused due to the accumulation of de novo mutations that increase proliferative potential of the somatic cells by escalating telomerase enzyme activity resulting in longer telomeres [58].
Father’s occupation showed a strong association with cord telomere depicting that it may have wear and tear effects on newborn health [56]. Shorter telomeres were observed in newborns with unemployed and laborer fathers compared to ones with private jobs (p = 0.012). A study on welders in Sweden supports our findings showing association with oxidative stress and DNA methylation (p = 0.033). Hence, occupational exposures can cause telomere alterations and have a strong impact on the quality of newborn life [59].
Ethnicity has been found associated with occupation. Fujishrio et al. observed that duration of job < 10 years was connected with the ethnicity of African-American, Latino, and white population [60]. Different telomere lengths among whites (5.55 kbp), African-American (5.68 kbp), and Latino (5.49 kbp) emphasized the ethnicity advantage among people along with occupation [51]. Another cohort study highlighted longer telomeres in blacks compared to white ethnicity despite having the same standard of education in both groups [3]. Looking into the ethnicity of our respective study, mothers from Urdu speaking ethnic group had smallest (6319 bp) and Sindhis had longest (6598 bp) telomeres. However, cord telomere of the Urdu speaking group was found longest (7042 bp) among all ethnic groups. Therefore, we can relate that there is a strong fetal programming among Urdu speaking followed by Sindhi and Pathan ethnicities. A study found a strong relation of ethnicity (Urdu speaking and Pathan) with oral cancer having an incidence of 19 % among the Pakistani population. In this study, a high prevalence of Pathan and Urdu speaking ethnicity with shorter telomere length may be at high risk of oral cancer [61].
Gender (newborn) association with RTL pointed out 29 bp longer telomeres in newborn girls compared to newborn boys. This evidence was supported by many studies which confirmed longer telomeres in girls (0.181 kb, 6.83%, 50–100 bp) [49, 60, 61]. An unexpected 26 bp shorter telomere was observed in low SES newborn girls, whereas girls belonging to upper middle SES had 308 bp longer telomeres. Similar to our study, Mitchell et al. also discovered longer telomeres in children with lower-middle SES than low SES (p = 0.007) [41]. Hence, social status plays a crucial role in the transmission of telomere length in future generations.
Gene expression capacity of telomeres was calculated by fold difference (2^-∆∆ ct) method which provides more accurate estimates of relative gene expression [35]. Our study results showed more telomere gene expression in cord than maternal having coefficient of variance CVs (11–15%, 15–27%) and standard deviation (SD) ± 3.66. In this respective study, the results of average ∆ct signify that the telomere qPCR needs more cycles in both maternal (2.4 times) and cord samples (3.5 times) to produce as much fluorescent signal as the reference gene qPCR. The positive Δct indicated that reference gene is longer than telomere, whereas inverse results were seen in the Cawthon qPCR analysis (− 9.05 ± 1. 48) [33].
A significant association (p < 0.05) has been found in the relationship between newborn RTL and mother SES and education which was evaluated for the first time in the population of Karachi, Pakistan. These findings emphasized that genetic remodeling in pregnant women from low SES leads to improved RTL in the newborn. Fetal programming of the telomere biology may signify health disparities and lifespan of the targeted female population and their offspring. Thus, telomere can act as a biological aging marker in both mothers and newborns.
Conclusions
Positive associations between mother and cord RTL were observed in the targeted Pakistani population. Significantly longer cord blood RTL than maternal blood was observed, including the low socioeconomic group highlighting fetal reprogramming. However, the high SES group had longer telomere (bp) than low SES. In this study, education was found a strong determinant for longer RTL and has a shielding effect against telomere shortening.
Limitations
Small sample size could be responsible for insignificant results of mother and newborn blood RTL. Further investigations on maternal health condition and lifestyle could also be helpful in revising the data.
Future recommendations
Evaluation of the telomerase level in the blood and father’s blood samples could be taken for the detection of telomere inheritance. Longitudinal cohort studies should be done to detect all the risk factors. Sequencing could be done for phylogenetic analysis.
Abbreviations
- bp:
-
Base pairs
- ct:
-
Cycle threshold
- CV:
-
Coefficient of variance
- GDM:
-
Gestational diabetes mellitus
- kbp:
-
kilo base pair
- qPCR:
-
Quantitaive polymerase chain reaction
- RTL:
-
Relative telomere length
- SD:
-
Standard deviation
- SES:
-
Socioeconomic status
- T/S ratio:
-
Telomere/single copy gene
- US:
-
United States of America
References
Aslam HM, Alvi AA, Mughal A, Haq Z, Qureshi WA, Haseeb A, Aziz S (2013) Association of socioeconomic classes with diet, stress and hypertension. J Pak Med Assoc. 63(2):289–294
Global_POVEQ_PAK.pdf (2018):http://databank.worldbank.org/data/download/poverty/33EF03BB-9722-4AE2-ABC7-AA2972D68AFE/
Adler NE, Stewart J (2010) Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci 1186(1):5–23
Blackburn E, Epel E, Lin J (2015) Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350(6265):1193–1198
Zakian VA (2012) Telomeres: the beginnings and ends of eukaryotic chromosomes. Exp Cell Res. 318(12):1456–1460
Bojesen SE (2013) Telomeres and human health. J Intern Med. 274(5):399–413
Stella GM, Balestro E, Lacedonia D, Baraldo S (2016) Telomeropathies: an emerging spectrum of disorders with important implications for patients with interstitial lung disease. Minerva Medica. 107(1 Suppl 1):9–14
Shammas MA (2011) Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 14(1):28
Zhu X, Han W, Xue W, Zou Y, Xie C, Du J, Jin G (2016) The association between telomere length and cancer risk in population studies. Scientific reports. 6:22243
D’Mello MJ, Ross SA, Briel M, Anand SS, Gerstein H, Pare G (2015) Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circulation: cardiovascular genetics. 8(1):82–90
Révész D, Milaneschi Y, Verhoeven JE, Penninx BW (2014) Telomere length as a marker of cellular aging is associated with prevalence and progression of metabolic syndrome. J Clin Endocrinol Metab. 99(12):4607–4615
Cohen S, Janicki-Deverts D, Turner RB, Casselbrant ML, Li-Korotky HS, Epel ES, Doyle WJ (2013) Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. Jama. 309(7):699-705.
Houben JM, Mercken EM, Ketelslegers HB, Bast A, Wouters EF, Hageman GJ, Schols AM (2009) Telomere shortening in chronic obstructive pulmonary disease. Respir Med 103(2):230–236
Berndt A, Leme AS, Shapiro SD (2012) Emerging genetics of COPD. EMBO Mol Med. 4(11):1144–1155
Eisenberg DT, Borja JB, Hayes MG, Kuzawa CW (2017) Early life infection, but not breastfeeding, predicts adult blood telomere lengths in the Philippines. Am J Hum Biol. 29(4):e22962
Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD (2005) Obesity, cigarette smoking, and telomere length in women. Lancet. 366(9486):662–664
Adler N, Pantell MS, O’Donovan A, Blackburn E, Cawthon R, Koster A, Opresko P, Newman A, Harris TB, Epel E (2013) Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. Brain Behav Immun. 27:15–21
Kordinas V, Ioannidis A, Chatzipanagiotou S (2016) The telomere/telomerase system in chronic inflammatory diseases. Cause or effect? Genes. 7(9):60
Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA (2010) Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. 39(3):263–272
Dunkel Schetter C (2011) Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 62:531–558
Sukenik-Halevy R, Amiel A, Kidron D, Liberman M, Ganor-Paz Y, Biron-Shental T (2016) Telomere homeostasis in trophoblasts and in cord blood cells from pregnancies complicated with preeclampsia. Am J Obstet Gynecol. 214(2):283–2e1
Biron-Shental T, Sukenik-Halevy R, Sharon Y, Laish I, Fejgin MD, Amiel A (2014) Telomere shortening in intra uterine growth restriction placentas. Early Hum Dev. 90(9):465–469
Fragkiadaki P, Tsoukalas D, Fragkiadoulaki I, Psycharakis C, Nikitovic D, Spandidos DA, Tsatsakis AM (2016) Telomerase activity in pregnancy complications. Mol Med Rep. 14(1):16–21
Marciniak A, Patro-Małysza J, Kimber-Trojnar Ż, Marciniak B, Oleszczuk J, Leszczyńska-Gorzelak B (2017) Fetal programming of the metabolic syndrome. Taiwan J Obstet Gynecol. 56(2):133–138
Smith MV, Gotman N, Yonkers KA (2016) Early childhood adversity and pregnancy outcomes. Matern Child Health J. 20(4):790–798
Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel Schetter C (2015) Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. 11:99–137
Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, Bommarito K, Madden T, Olsen MA, Subramaniam H, Peipert JF, Bierut LJ (2015) Maternal age and risk of labor and delivery complications. Matern Child Health J. 19(6):1202–1211
Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, Lin J, Wolkowitz OM (2015) Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry. 20(5):615
Alexeeff SE, Schaefer CA, Kvale MN, Shan J, Blackburn EH, Risch N, Ranatunga DK, Jorgenson E, Hoffmann TJ, Sakoda LC, Quesenberry CP (2019) Telomere length and socioeconomic status at neighborhood and individual levels among 80,000 adults in the Genetic Epidemiology Research on Adult Health and Aging cohort. Environmental Epidemiology. 3(3):e049
Needham BL, Rehkopf D, Adler N, Gregorich S, Lin J, Blackburn EH, Epel ES (2015) Leukocyte telomere length and mortality in the National Health and Nutrition Examination Survey, 1999–2002. Epidemiology (Cambridge, Mass.). 26(4):528
Farrukh S, Baig S, Hussain R, Lucky MH. Association of cord blood telomere biology with mother’s education. International Journal of Biochemistry Research & Review. 2019;1-9.
Needham BL, Hicken MT, Govia IO, Mitchell C, Abdou CM (2017) Maternal social disadvantage and newborn telomere length in archived dried blood spots from the michigan neonatal biobank. Biodemography Soc Biol. 63(3):221
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30(10):e47
Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, Epel ES (2013) Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc Sci Med. 85:1–8
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostatistics Bioinformatics Biomathematics. 3(3):71
Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD (2006) The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 5(5):361–365
Steptoe A, Hamer M, Butcher L, Lin J, Brydon L, Kivimäki M, Marmot M, Blackburn E, Erusalimsky JD (2011) Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav Immun. 25(7):1292–1298
Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A (2008) The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 168(2):154–158
Robertson T, Batty GD, Der G, Green MJ, McGlynn LM, McIntyre A, Shiels PG, Benzeval M (2012) Is telomere length socially patterned? Evidence from the West of Scotland Twenty-07 Study. PloS One. 7(7):e41805
Cohen S, Janicki-Deverts D, Turner RB, Marsland AL, Casselbrant ML, Li-Korotky HS, Epel ES, Doyle WJ (2013) Childhood socioeconomic status, telomere length, and susceptibility to upper respiratory infection. Brain Behav Immun. 34:31–38
Mitchell AM, Kowalsky JM, Epel ES, Lin J, Christian LM (2018) Childhood adversity, social support, and telomere length among perinatal women. Psychoneuroendocrinology. 87:43–52
Entringer S, de Punder K, Buss C, Wadhwa PD (2018) The fetal programming of telomere biology hypothesis: an update. Philos Trans R Soc B Biol Sci. 373(1741):20170151
Rehkopf DH, Needham BL, Lin J, Blackburn EH, Zota AR, Wojcicki JM, Epel ES (2016) Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: a cross-sectional study of US adults. PLoS Med. 13(11):e1002188
Geifman-Holtzman O, Xiong Y, Holtzman EJ, Hoffman B, Gaughan J, Liebermann DA (2010) Increased placental telomerase mRNA in hypertensive disorders of pregnancy. Hypertens Pregnancy. 29(4):434–445
Harris SE, Martin-Ruiz C, von Zglinicki T, Starr JM, Deary IJ (2012) Telomere length and aging biomarkers in 70-year-olds: the Lothian Birth Cohort 1936. Neurobiol Aging. 33(7):1486–14e3
Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, Dunning AM (2011) Life stress, emotional health, and mean telomere length in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 66(11):1152–1162
Wolkowitz OM, Mellon SH, Epel ES et al (2011) Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress—preliminary findings. PLoS One. 6(3):e17837. https://doi.org/10.1371/journal.pone.0017837
Nordfjall K, Eliasson M, Stegmayr B et al (2008) Increased abdominal obesity, adverse psychosocial factors and shorter telomere length in subjects reporting early ageing; the MONICA Northern Sweden Study. Scand J Public Health. 36(7):744–752
Martens DS, Plusquin M, Gyselaers W, De Vivo I, Nawrot TS (2016) Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 14(1):148
Weng Q, Du J, Yu F, Huang T, Chen M, Lv H, Ma H, Hu Z, Jin G, Hu Y, Shen H (2016) The known genetic loci for telomere length may be involved in the modification of telomeres length after birth. Sci Rep. 6:38729
Lynch SM, Mitra N, Ravichandran K, Mitchell J, Spangler E, Zhou W, Paskett ED, Gehlert S, DeGraffinreid C, Stowe R, Dubowitz T (2017) Telomere length and neighborhood circumstances: Evaluating biological response to unfavorable exposures
Hallows SE, Regnault TR, Betts DH (2012) The long and short of it: the role of telomeres in fetal origins of adult disease. J Pregnancy.
Westerlund H, Kivimäki M, Singh-Manoux A, Melchior M, Ferrie JE, Pentti J, Jokela M, Leineweber C, Goldberg M, Zins M, Vahtera J (2009) Self-rated health before and after retirement in France (GAZEL): a cohort study. Lancet. 374(9705):1889–1896
Steptoe A, Hamer M, Lin J, Blackburn EH, Erusalimsky JD (2016) The longitudinal relationship between cortisol responses to mental stress and leukocyte telomere attrition. J Clin Endocrinol Metab. 102(3):962–969
Parks CG, DeRoo LA, Miller DB, McCanlies EC, Cawthon RM, Sandler DP (2011) Employment and work schedule are related to telomere length in women. Occup Environ Med. 68(8):582–589
Ahola K, Sirén I, Kivimäki M, Ripatti S, Aromaa A, Lönnqvist J, Hovatta I (2012) Work-related exhaustion and telomere length: a population-based study. PLoS One. 7(7):e40186
Samulin Erdem J, Notø HØ, Skare Ø, Lie JA, Petersen-Øverleir M, Reszka E, Pepłońska B, Zienolddiny S (2017) Mechanisms of breast cancer risk in shift workers: association of telomere shortening with the duration and intensity of night work. Cancer Med. 6(8):1988–1997
Jafri MA, Ansari SA, Alqahtani MH, Shay JW (2016) Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 8(1):69
Li H, Hedmer M, Wojdacz T, Hossain MB, Lindh CH, Tinnerberg H, Albin M, Broberg K (2015) Oxidative stress, telomere shortening, and DNA methylation in relation to low-to-moderate occupational exposure to welding fumes. Environ Mol Mutagen. 56(8):684–693
Fujishiro K, Needham BL, Landsbergis PA, Seeman T, Jenny NS, Roux AV (2018) Selected occupational characteristics and change in leukocyte telomere length over 10 years: the Multi-Ethnic Study of Atherosclerosis (MESA). PloS One. 13(9):e0204704
Idrees R, Fatima S, Abdul-Ghafar J, Raheem A, Ahmad Z (2018) Cancer prevalence in Pakistan: meta-analysis of various published studies to determine variation in cancer figures resulting from marked population heterogeneity in different parts of the country. World J Surg Oncol. 16(1):129
Acknowledgements
The corresponding author would like to acknowledge the participants and the supporting staff in this study for their immense support.
Availabilty of data and materials
The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Funding
This study was supported by Ziauddin University. The research was approved by the Research Committee (RAC) (Bio.125.7/3/18) of Ziauddin University, Karachi, Pakistan. The corresponding author would like to thank the participants and the supporting staff in this study.
Author information
Authors and Affiliations
Contributions
FS contributed to the research design and research approval processes, the sample collection, the laboratory work, and the article writing till the proofreading of the article. BS is the main supervisor of the research, designed the main concept of the research, and helped in the laboratory and writing of the research article. HR was the co-supervisor and helped in the sample collection from hospitals and finalizing of the research. SA helped in the sample handling and laboratory extraction procedures. KTS helped in the sample handling and laboratory extraction procedures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Review Committee (ERC) of Ziauddin University and Hospital (Reference Code: 0070218SFBIO) in accordance with the Helsinki Declaration. Written informed consent was obtained from all individual participants or their attendants included in the study.
Consent for publication
Consent to publish the data was obtained from all individual participants or their attendants included in the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Farrukh, S., Baig, S., Hussain, R. et al. Telomere reprogramming during fetal life in low socioeconomic mothers. Egypt J Med Hum Genet 20, 9 (2019). https://doi.org/10.1186/s43042-019-0007-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-019-0007-4