Abstract
Background
The present systematic review investigated the minimal clinically important difference (MCID), substantial clinical benefit (SCB), and patient-acceptable symptom state (PASS) of several frequent and established PROMs used to assess patients who have undergone TKA. This study was conducted according to the 2020 PRISMA statement.
Methods
In September 2023, PubMed, Web of Science, and Embase were accessed with no time constraint All clinical studies investigating tools to assess the clinical relevance of PROMs used to evaluate patients having received TKA were accessed. Only studies which evaluated the MCID, PASS, or SCB were eligible. The PROMs of interest were the Forgotten Joint Score-12 (FJS-12), the Oxford Knee Score (OKS), the Knee Injury and Osteoarthritis Outcome Score (KOOS) and its related subscales activity of daily living (ADL), pain, quality of life (QoL), sports and recreational activities, and symptoms, the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) score, the Knee Society Score (KSS) and related function score, and the Short Form-12 (SF-12) and Short Form-36 (SF-36).
Results
Data from 29,737 patients were collected. The overall risk of bias was low to moderate. The great variability of thresholds for MCID, SCB and PASS between questionnaires but also between investigated aspects was noted, whereby MCIDs for the SF-36 appear lower than for knee-specific questionnaires.
Conclusion
Despite its critical role from a patient’s perspective, the dimension of SCB is still neglected in the literature. Moreover, thresholds for the different concepts need to be condition-specific. We encourage authors to specifically report such data in future studies and to adhere to previously reported definitions to allow future comparison.
Level of evidence Level IV, systematic review and meta-analysis
Similar content being viewed by others
Introduction
Knee osteoarthritis (OA) affects large parts of the elderly population and is one of the leading causes of disability worldwide [1]. OA leads to reduced flexibility and mobility of the joint, and to load-dependent joint pain that can severely disable the patient, resulting in a high socio-economic burden [2]. If non-operative management of OA fails, partial or total knee arthroplasty (TKA) is performed. The affected joint surface is thereby replaced with prosthesis components to restore pain-free movement. While this highly standardised procedure achieves good to excellent results in most patients, 10–20% of them report persistent knee pain and functional limitations [3]. Great efforts are, therefore, still made to further improve outcomes after TKA.
Recent developments include the use of navigation and robotics [4], preoperative 3-dimensional planning [4, 5], the use of surface and insert geometries which allow more physiological joint kinematics [6,7,8] and the introduction of new alignment strategies that focus on the soft tissue needs than previous philosophies based solely on mechanical alignment [9].
The long-term effects of these new approaches may take up decades to become appreciable from registry data [4]. Patient satisfaction with the procedure, and the key dimension of success, is usually evaluated using patient-reported outcome measures (PROMs) [10]. Meaningful results can already be obtained during the first operative year, and a relatively steady state may be expected thereafter [11, 12]. PROMs are thus essential to evaluate the performance of TKAs and compare the performance of new techniques against established standards. When evaluating therapeutic success, a critical component of reporting medical data is the use of a p-value cut-off point of 0.05 [13]. In the scientific literature, results are routinely categorised as being either statistically significant or not significant [14]. Signalling the probability of error concerning a null hypothesis is a simple tool to characterise data and their potential relevance. This method, however, carries several pitfalls, which might be underrated in a clinical setting. First, clinically relevant differences might be missed because of false negative results (type II error) or falsely positively interpreted in case of a positive result (type I error), the likelihood of both being higher in underpowered studies [15, 16]. Second, even very small differences of no clinical relevance may reach a p < 0.05 when the sample size is simply large enough. Statistical significance, however, does not imply clinical relevance. Believing that statistically significant results always imply a clinically relevant finding can entail an erroneous application of study results [17].
To better interpret the clinical impact of study findings, new criteria have been proposed [18]. When using PROMS, minimum thresholds can be determined that still represent a clear or important benefit. Jaeschke et al. proposed the term “minimal clinically important difference (MCID)” (later also termed minimal important difference, MID) [19]. Acknowledging the relevance of such an approach, additional clinically oriented concepts have been introduced which can be used to better interpret PROM data. The MCID describes the smallest difference a patient can perceive in a specific questionnaire. The magnitude of a treatment-associated improvement that a patient recognises as a meaningful benefit is termed the substantial clinical benefit (SCB) [20]. The former two parameters are relative to the initial symptomatic state before treatment. A helpful concept to rate a cohort’s condition in absolute terms is the patient-acceptable symptom state (PASS), defined as the value on a PROM scale beyond which patients with a specific condition consider themselves well or in a satisfactory state [21]. Using all these parameters in the interpretation of study data, a better and patient-oriented description of obtained success rates in therapeutic approaches can be provided.
The results of surgical procedures depend on numerous factors. In the case of TKA, this is certainly the surgery itself, but also patient expectations before surgery, the degree of improvement in knee function and pain relief and potentially also socioeconomic domains [3]. Therefore, parameters such as MCID, PASS, or SCB may need to be defined as condition-specific and possibly also context-specific. To date, reference values for these thresholds are scarce and scattered in the literature, and at the same time highly necessary to better interpret the findings arising from clinical studies. The present systematic review investigated the MCID, PASS, and SCB of several frequent and established PROMs used to assess patients who have undergone TKA.
Methods
Eligibility criteria
All clinical studies investigating tools to assess the clinical relevance of PROMs used to assess patients having received TKA were accessed. Only studies which evaluated the MCID, PASS, or SCB were eligible. According to the authors’ language capabilities, articles in English, German, Italian, French, and Spanish were eligible. Only studies with levels I to IV of evidence, according to the Oxford Centre of Evidence-Based Medicine [22], were considered. Reviews, opinions, letters, and editorials were not considered. Missing quantitative data under the outcomes of interests warranted the exclusion of the study.
Search strategy
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the 2020 PRISMA statement [23]. The PICOD algorithm was preliminarily established:
-
P (Problem): Endstage knee OA;
-
I (Intervention): TKA;
-
C (Comparison): Tool to assess the clinical efficacy of surgery;
-
O (Outcomes): MCID, PASS, SCB;
-
D (Design): Clinical study.
In September 2023, the following databases were accessed: PubMed, Web of Science, and Embase. No time constraint was set for the search. The Medical Subject Headings (MeSH) used for the database search are reported in the Appendix. No additional filters were used in the database search.
Selection and data collection
Two authors (****) independently performed the database search. All the resulting titles were screened by hand and, if suitable, the abstract was accessed. The full text of the abstracts which matched the topic of interest was accessed. If the full text was not accessible or available, the article was not considered for inclusion. A cross reference of the bibliography of the full-text articles was also performed for inclusion. Disagreements were debated and mutually solved by the authors. In case of further disagreements, a third senior author (**) made the final decision.
Data items
Two authors (****) independently performed data extraction. The following data at baseline were extracted: author, year of publication, country, study design, journal, type of analysis performed, type of PROMs investigated, and number of patients included. Data on the MCID, PASS, and SCB were collected. The PROMs of interest were the Forgotten Joint Score-12 (FJS-12) [24], the Oxford Knee Score (OKS) [25], the Knee Injury and Osteoarthritis Outcome Score (KOOS) and its related subscales activities of daily living (ADL), pain, quality of life (QoL), sports and recreational activities, and symptoms [26], the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) score [27, 28], the Knee Society Score (KSS) and related function score [29], and the Short Form-12 (SF-12) [30] and Short Form-36 (SF-36) [30,31,32,33]. Data were extracted in Microsoft Office Excel version 16.72 (Microsoft Corporation, Redmond, USA).
Methodological quality assessment
The risk of bias was evaluated following the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions [34]. Two reviewers (****) evaluated the risk of bias in the extracted studies. The Risk of Bias in Nonrandomised Studies of Interventions (ROBINS-I) tool was used [35]. The tool is completed in three phases. During phase one, the relevance of the research question is evaluated (optional). Phase two considers four domains through which risks of bias can be introduced into systematic reviews: study eligibility criteria, identification and selection of studies, data collection, summary, and results. Phase three assesses the overall risk of bias in the interpretation of the review findings, and whether the interpretation has taken into account any limitations identified in any of the domains of phase two. Seven domains of potential bias in non-RCTs were assessed. Possible confounding and the nature of patient selection before the start of the comparative intervention are assessed by two domains. A further domain is used to assess bias in the classification during the intervention. The final four domains assess the methodological quality after the start of the intervention: biases from deviations from originally intended interventions, missing data, erroneous measurement of outcomes, or bias in the selection of the reported outcomes are evaluated. The figure of the ROBINS-I was elaborated using the Robvis Software (Risk-of-bias VISualization, Riskofbias.info, Bristol, UK) [36].
Synthesis methods
The statistical analyses were performed by the main author (**) following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [37]. For descriptive statistics, the arithmetic mean was used using the IBM SPSS software version 25 (International Business Machines Corporation, Armonk, USA).
Results
Study selection
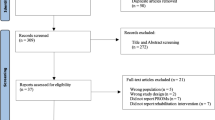
The systematic literature search resulted in 714 articles. A total of 285 were identified as duplicates and, therefore, excluded. A further 393 investigations were discarded as they did not meet the predefined inclusion criteria. The reasons for exclusion were inadequate study design (N = 291), low level of evidence (N = 17), missing implementation of at least one tool to determine the clinical relevance of PROMs (MCID, PASS, or SCB) (N = 31), not reporting data from at least one PROM of interest (FJS-12, OKS, KOOS, WOMAC, KSS, SF-12 or SF-36) (N = 35), and language limitations (N = 9). After full-text evaluation, an additional 22 investigations were excluded because they did not offer any quantitative data on the outcomes of interest. In conclusion, 14 studies were available for inclusion. All of them were non-RCTs. The results of the literature search are shown in Fig. 1.
Methodological quality assessment
The ROBINS-I was applied to investigate the risk of bias in all studies included in the present systematic review. Confounding could be ruled out in most articles. One study was rated with a serious risk of confounding, as there were differences in the intervention groups at baseline. Patient selection was described in detail in all studies. Exclusion of patients or differences in follow-up time of individual patients were predominantly not detected, which indicates an overall low to moderate risk of bias based on participant selection. The risk of bias in the classification of interventions resulted mainly low, as there were neither nondifferential misclassification nor differential misclassification identified. Furthermore, systematic differences between the experimental and comparison groups were not found, leading to a low to moderate risk of deviations from the intended interventions. The lack of assessor blinding in all studies reviewed indicated a predominantly moderate risk of bias in the measurement of outcomes. Given the mainly good quality of the included studies, the overall risk of bias was low to moderate. The ROBINS-I is reported in Fig. 2.
Study characteristics and results of individual studies
Data from 29,737 patients were collected. The generalities and demographics of the included studies are shown in Table 1.
Results syntheses
An overview of the results of MCID, PASS, and SCB about the FJS, OKS, KOOS, WOMAC, KSS, SF-12 and SF-36 is reported in Table 2.
Discussion
This study systematically investigated the MCID, PASS, and SCB of several frequent and established PROMs used to assess patients who have undergone TKA. Fourteen studies were eventually analysed including 29,737 patients.
When examining the different magnitudes of MCIDs in the various questionnaires, a relatively large variability between questionnaires can be observed. Very low MCIDs were reported for the different dimensions of the SF-36 (most values clearly below 10). In contrast, in the knee-specific questionnaires, the MCID was much greater, with mostly values clearly above 10, and in the case of the WOMAC, even above 20 in some instances. For KOOS-ADL, -Pain, and -QoL values were almost identical for TKA, for example, compared to chondral procedures of the knee. In contrast, values for the dimensions of sports/recreational activities and symptoms were much lower for TKA than for cartilage repair procedures [52,53,54]. The reason for this phenomenon can only be speculated upon. One possible explanation might be that values for sports/recreational activities might be already so low in patients scheduled for TKA that even minor improvements exert a strong impact on the patient’s perception of the condition. This is in contrast to cartilage repair procedures, where a relatively high activity level is present preoperatively. Of note, the MCIDs for the KOOS (total score) described in the present review are in agreement with those reported in a systematic review displaying both distribution and anchor-based derivation of MCIDs [55]. Their study included 18 studies—two of which were included in the present investigation.
Although probably the most important parameter from a patient’s perspective, the SCB was rarely evaluated in the included studies. In the FJS-12 and the KOOS, values were, however, twice as high for the SCB than for the MCID except QoL (where MCID and SCB were almost identical). In the KSS, SCB values were even 4 times higher for the MCID. This illustrates the fact that both parameters—MCID and SCB—have their justification and that a single presentation of an improvement at the size of the MCID does not necessarily imply a sufficient improvement for the patient. SCB levels after TKA were also calculated by Lyman et al. [56], who reported a range from 15 to 36 for the different KOOS dimensions. Interstingly, Haydel et al. observed in a cohort of TKA that better preoperative KOOS-Symptoms, -QoL, and -ADL living subscale scores were statistically significantly associated with failing to meet the MCID and SCB on each respective subscale [57].
For the PASS, a great variability between questionnaires but also between investigated aspects was noted. In the SF-36, for example, the PASS scoring for mental health was over twice as high (69) than for physical functioning (34). Values for a PASS were highest in the KOOS with QoL (83) and Pain (85), whereas they were low in the FSJ-12 with only 30 of 100 points. This low value for the FSJ-12 (33.3) was, however, also reported by Singh et al. 2022 [58], using a receiver operating characteristic curve point to calculate the value. In another study, the PASS in the KOOS ranged from 80 to 88 except QoL with 66 points [12]. Similar to the MCID, PASS values seem to be strongly dependent on the condition they are applied to. In a recent systematic review, PASS thresholds in KOOS-ADL for ACL tears were 92 to 100, and KOOS-Symptoms (73–78) and KOOS-QoL (53–57) in meniscus injuries [59].
When interpreting PASS values, it needs to be considered from which baseline values in questionnaires patients started. A retrospective registry study observed that patients suffering from OA and treated with conservative means defined lower PASS values post-intervention when they also had lower baseline values [60]. Caution also needs to be exercised when relating to PASS values reported in the literature. While for MCID and SCB it is somehow clear that threshold values are discussed, for the PASS it is often the rate of patients having achieved such a symptom state that is related to (e.g. [61]). Such a rate can be calculated as a ratio of patients having met a certain threshold [61], the universal definition of which is still a matter of debate as can be seen by the data presented in this systematic review. Alternatively, a PASS is directly evaluated simply by anchor questions. Depending on the wording, chosen increments and context of these anchor questions, completely different results might be obtained.
The limitations of the present study are mostly related to the low number of available studies reporting quantitative data on MCID, SCB, and PASS in the context of TKA (n = 14). While at least the number of patients included is quite substantial in some of these studies, not all values were reported for all three values in all questionnaires. The lack of a mix of data from several studies per item makes the presented available data prone to selection, recruitment or reporting bias. Moreover, it was not specifically reported whether the data presented were derived by distribution or anchor-based methods. The mode of data derivation influences the thresholds calculated, while although an anchor-based calculation may seemingly be more intuitive, it is of difficult application, especially in retrospective studies when the necessary anchor questions are missing [39, 55]. Distribution methods, on the other hand, result in values that describe statistical significance and do not capture clinical changes as directly perceived by the patient [55]. For this reason, probably both derivation methods have their justification. Anchor-based techniques will, however, have to be standardised, and for both techniques, their independent threshold values will need to be established.
Despite these imprecisions, we are convinced of the concept of judging PROM data on their clinical relevance by applying MCID, SCB, or PASS. We encourage authors to specifically report such data in future studies and to adhere to previously reported definitions to allow future comparison.
Conclusion
We found substantial variability of thresholds for MCID, SCB and PASS between questionnaires but also between investigated aspects. Thresholds thus need to be condition-specific in patients undergoing TKA. Although clinically important, SCB is still neglected in the literature. We encourage authors to report such data in future studies and to adhere to previously reported definitions to allow future comparison.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available throughout the manuscript.
Abbreviations
- ADL:
-
Activities of daily living
- FJS-12:
-
Forgotten Joint Score-12
- KOOS:
-
Knee Injury and Osteoarthritis Outcome Score
- KSS:
-
Knee society score
- MCID:
-
Minimal clinically important difference
- MID:
-
Minimal important difference
- OA:
-
Osteoarthritis
- OKS:
-
Oxford knee score
- PASS:
-
Patient-acceptable symptom state
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROM:
-
Patient-reported outcome measure
- QoL:
-
The quality of life
- RCT:
-
Randomised controlled trial
- SCB:
-
Substantial clinical benefit
- SF:
-
Short form
- TKA:
-
Total knee arthroplasty
- WOMAC score:
-
Western Ontario and McMaster Universities Osteoarthritis score
References
Hunter DJ, March L, Chew M (2020) Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet 396:1711–1712
Hardenberg M, Spekle EM, Coenen P, Brus IM, Kuijer P (2022) The economic burden of knee and hip osteoarthritis: absenteeism and costs in the Dutch workforce. BMC Musculoskelet Disord 23:364
Gunaratne R, Pratt DN, Banda J, Fick DP, Khan RJK, Robertson BW (2017) Patient dissatisfaction following total knee arthroplasty: a systematic review of the literature. J Arthroplasty 32:3854–3860
Pailhe R (2021) Total knee arthroplasty: latest robotics implantation techniques. Orthop Traumatol Surg Res 107:102780
Kobayashi A, Ishii Y, Takeda M, Noguchi H, Higuchi H, Toyabe S (2012) Comparison of analog 2D and digital 3D preoperative templating for predicting implant size in total knee arthroplasty. Comput Aided Surg 17:96–101
Clary CW, Fitzpatrick CK, Maletsky LP, Rullkoetter PJ (2013) The influence of total knee arthroplasty geometry on mid-flexion stability: an experimental and finite element study. J Biomech 46:1351–1357
Koh YG, Nam JH, Kang KT (2018) Effect of geometric variations on tibiofemoral surface and post-cam design of normal knee kinematics restoration. J Exp Orthop 5:53
Wakelin E, Walter W, Bare J, Theodore W, Twiggs J, Miles B (2019) Implant geometry and alignment-driven variability in post-total knee arthroplasty kinematics. Orthopaedic Proc 101-B:140
Karasavvidis T, Pagan Moldenhauer CA, Haddad FS, Hirschmann MT, Pagnano MW, Vigdorchik JM (2023) Current concepts in alignment in total knee arthroplasty. J Arthroplasty 38:S29–S37
Choi YJ, Ra HJ (2016) Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res 28:1–15
Kennedy DM, Stratford PW, Riddle DL, Hanna SE, Gollish JD (2008) Assessing recovery and establishing prognosis following total knee arthroplasty. Phys Ther 88:22–32
Connelly JW, Galea VP, Rojanasopondist P et al (2019) Patient acceptable symptom state at 1 and 3 years after total knee arthroplasty: thresholds for the knee injury and osteoarthritis outcome score (KOOS). J Bone Joint Surg Am 101:995–1003
Fisher RA (1992) Statistical methods for research workers. In: Kotz S, Johnson NL (eds) Breakthroughs in statistics: methodology and distribution. Springer New York, New York, pp 66–70
Sterne JA, Davey SG (2001) Sifting the evidence-what’s wrong with significance tests? BMJ 322:226–231
Button KS, Ioannidis JP, Mokrysz C et al (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14:365–376
Halpern SD, Karlawish JH, Berlin JA (2002) The continuing unethical conduct of underpowered clinical trials. JAMA 288:358–362
Dettori JR, Norvell DC, Chapman JR (2019) P-value worship: is the idol significant? Global Spine J 9:357–359
Guyatt G, Walter S, Norman G (1987) Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis 40:171–178
Jaeschke R, Singer J, Guyatt GH (1989) Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 10:407–415
Glassman SD, Copay AG, Berven SH, Polly DW, Subach BR, Carreon LY (2008) Defining substantial clinical benefit following lumbar spine arthrodesis. J Bone Joint Surg Am 90:1839–1847
Tubach F, Ravaud P, Baron G et al (2005) Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis 64:34–37
Howick J CI, Glasziou P, Greenhalgh T, Carl Heneghan, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M. The 2011 Oxford CEBM Levels of Evidence. Oxford Centre for Evidence-Based Medicine Available at https://www.cebmnet/indexaspx?o=5653 2011.
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Behrend H, Giesinger K, Giesinger JM, Kuster MS (2012) The “forgotten joint” as the ultimate goal in joint arthroplasty: validation of a new patient-reported outcome measure. J Arthroplasty 27(430–6):e1
Dawson D (2014) Oxford Knee Score. In: Michalos AC (ed) Encyclopedia of quality of life and well-being research. Springer Netherlands, Dordrecht, pp 4554–4555
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD (1998) Knee Injury and Osteoarthritis Outcome Score (KOOS)–development of a self-administered outcome measure. J Orthop Sports Phys Ther 28:88–96
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Bellamy N, Buchanan WW (1986) A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol 5:231–241
Insall JN, Dorr LD, Scott RD, Scott WN (1989) Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 248:13–14
Ware J Jr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233
Patel AA, Donegan D, Albert T (2007) The 36-item short form. J Am Acad Orthop Surg 15:126–134
Tarlov AR, Ware JE Jr, Greenfield S, Nelson EC, Perrin E, Zubkoff M (1989) The Medical Outcomes Study. An application of methods for monitoring the results of medical care. JAMA 262:925–930
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
Cumpston M, Li T, Page MJ et al (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10:CD000142
Sterne JA, Hernan MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
McGuinness LA, Higgins JPT (2021) Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12:55–61
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10:ED000142. https://doi.org/10.1002/14651858.ED000142
Ayers DC, Yousef M, Yang W, Zheng H (2023) Age-related differences in pain, function, and quality of life following primary total knee arthroplasty: results from a FORCE-TJR (Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement) cohort. J Arthroplasty 38:S169–S176
Carender CN, Glass NA, De A, Bozic KJ, Callaghan JJ, Bedard NA (2022) Outcomes vary significantly using a tiered approach to define success after total knee arthroplasty. J Arthroplasty 37:1266–1272
Clement ND, MacDonald D, Simpson AH (2014) The minimal clinically important difference in the Oxford knee score and Short Form 12 score after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 22:1933–1939
Clement ND, Bardgett M, Weir D, Holland J, Gerrand C, Deehan DJ (2018) What is the minimum clinically important difference for the WOMAC index after TKA? Clin Orthop Relat Res 476:2005–2014
Clement ND, Scott CEH, Hamilton DF, MacDonald D, Howie CR (2021) Meaningful values in the Forgotten Joint Score after total knee arthroplasty. Bone Joint J 103-B:846–854
Clement ND, Weir D, Deehan D (2022) Meaningful values in the Short Form Health Survey-36 after total knee arthroplasty—an alternative to the EuroQol five-dimension index as a measure for health-related quality of life: minimal clinically important difference, minimal important change, patient-acceptable symptom state thresholds, and responsiveness. Bone Joint Res 11:477–483
Escobar A, Quintana JM, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I (2007) Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cartilage 15:273–280
Escobar A, Garcia Perez L, Herrera-Espineira C et al (2013) Total knee replacement; minimal clinically important differences and responders. Osteoarthritis Cartilage 21:2006–2012
Gousopoulos L, Dobbelaere A, Ratano S et al (2023) Custom total knee arthroplasty combined with personalised alignment grants 94% patient satisfaction at minimum follow-up of 2 years. Knee Surg Sports Traumatol Arthrosc 31:1276–1283
Heijbel S, Dahl AW, Nilsson KG, Hedstrom M (2022) Substantial clinical benefit and patient acceptable symptom states of the Forgotten Joint Score 12 after primary knee arthroplasty. Acta Orthop 93:158–163
Ingelsrud LH, Terluin B, Gromov K, Price A, Beard D, Troelsen A (2021) Which Oxford Knee Score level represents a satisfactory symptom state after undergoing a total knee replacement? Acta Orthop 92:85–90
Lizaur-Utrilla A, Gonzalez-Parreno S, Martinez-Mendez D, Miralles-Munoz FA, Lopez-Prats FA (2020) Minimal clinically important differences and substantial clinical benefits for Knee Society Scores. Knee Surg Sports Traumatol Arthrosc 28:1473–1478
Maxwell JL, Felson DT, Niu J et al (2014) Does clinically important change in function after knee replacement guarantee good absolute function? The multicenter osteoarthritis study. J Rheumatol 41:60–64
Nishimoto J, Tanaka S, Inoue Y, Tanaka R. Minimal clinically important differences in short-term postoperative Knee injury and Osteoarthritis Outcome Score (KOOS) after total knee arthroplasty: a prospective cohort study. J Orthop Trauma Rehabil 2023;0.
Chahal J, Lansdown DA, Davey A, Davis AM, Cole BJ (2021) The clinically important difference and patient acceptable symptomatic state for commonly used patient-reported outcomes after knee cartilage repair. Am J Sports Med 49:193–199
Ogura T, Ackermann J, Mestriner AB, Merkely G, Gomoll AH (2021) The minimal clinically important difference and substantial clinical benefit in the patient-reported outcome measures of patients undergoing osteochondral allograft transplantation in the knee. Cartilage 12:42–50
Wang D, Chang B, Coxe FR et al (2019) Clinically meaningful improvement after treatment of cartilage defects of the knee with osteochondral grafts. Am J Sports Med 47:71–81
Beiene ZA, Tanghe KK, Kahlenberg CA, McLawhorn AS, MacLean CH, Gausden EB (2023) Defining a successful total knee arthroplasty: a systematic review of metrics of clinically important changes. Arthroplasty 5:25
Lyman S, Lee YY, McLawhorn AS, Islam W, MacLean CH (2018) What are the minimal and substantial improvements in the HOOS and KOOS and JR versions after total joint replacement? Clin Orthop Relat Res 476:2432–2441
Haydel A, Guilbeau S, Roubion R, Leonardi C, Bronstone A, Dasa V (2019) Achieving validated thresholds for clinically meaningful change on the knee injury and osteoarthritis outcome score after total knee arthroplasty: findings from a university-based orthopaedic tertiary care safety net practice. J Am Acad Orthop Surg Glob Res Rev 3:e00142
Singh V, Fiedler B, Huang S, Oh C, Karia RJ, Schwarzkopf R (2022) Patient acceptable symptom state for the forgotten joint score in primary total knee arthroplasty. J Arthroplasty 37:1557–1561
Macri EM, Young JJ, Ingelsrud LH et al (2022) Meaningful thresholds for patient-reported outcomes following interventions for anterior cruciate ligament tear or traumatic meniscus injury: a systematic review for the OPTIKNEE consensus. Br J Sports Med 56:1432–1444
Cronstrom A, Ingelsrud LH, Nero H et al (2023) Interpretation threshold values for patient-reported outcomes in patients participating in a digitally delivered first-line treatment program for hip or knee osteoarthritis. Osteoarthr Cartil Open 5:100375
Murphy GT, Shatrov J, Duong J, Fritsch BA (2023) How does the use of quantified gap-balancing affect component positioning and limb alignment in robotic total knee arthroplasty using functional alignment philosophy? A comparison of two robotic platforms. Int Orthop 47:1221–1232
Acknowledgements
None.
Registration and protocol
The present review was not registered.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
FM: conception and design, drafting, statistical analysis; FS: literature search, data extraction, risk of bias assessment; NM: supervision, revision; AB: drafting; LS: literature search, data extraction, risk of bias assessment; UKH: drafting. All authors have agreed to the final version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complies with ethical standards. Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have any competing interests in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Migliorini, F., Maffulli, N., Schäfer, L. et al. Minimal clinically important difference (MCID), substantial clinical benefit (SCB), and patient-acceptable symptom state (PASS) in patients who have undergone total knee arthroplasty: a systematic review. Knee Surg & Relat Res 36, 3 (2024). https://doi.org/10.1186/s43019-024-00210-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43019-024-00210-z