Abstract
Bottle gourd is a good source of health-beneficial bioactive substances. This research aimed at evaluating the effect of different cooking methods (pressure cooking, microwaving, and frying) and extraction solvents (methanol, ethanol and butanol) on bottle gourd fruit phenolic compounds and antioxidant capacity. The quantitative estimation of polyphenolic compounds, flavonoids and tannins was estimated by spectrophotometric methods. The antioxidative properties were evaluated using ferric thiocyanate (FTC), thiobarbituric acid (TBA), ferric reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) tests using the standard methods. Moreover, quantification of individual polyphenols was carried out by high-performance thin layer chromatography (HPTLC) technique. Frying and pressure-cooking thermal treatments were observed to be the best methods for retaining bioactive molecules. When compared to the raw counterpart, the retention level of total phenol content (TPC) in pressure cooked and fried samples was 23.8% and 13.3%, respectively. Similarly, antioxidant capacity in terms of FRAP was observed to increase by 47.26% after pressure cooking and 28.50% after microwave cooking in respect to the raw sample. The HPTLC results showed that this fruit has high antioxidant capacity and is rich in phenolic acid [gallic(17.83 mg g−1dwb), p-coumaric (6.70 mg g−1dwb)and vanillic (60.66 mg g−1dwb] and flavonoids [quercetin (24.64 mg g−1dwb) and myrecetin (20.73 mg g−1dwb].The chromatography indicated destruction and transformation of new phenolic compounds due to heat treatments. The correlation analysis revealed that flavonoids were much more responsible for their antioxidant activity. Cooking methods and extraction solvents affected the retention and recovery of polyphenolic compounds available in bottle gourd fruit. These findings offer valuable information for optimizing cooking techniques to preserve and enhance the nutritional and antioxidant properties of bottle gourd, making it a valuable fruit for a healthy diet. Pressure cooking could be the most suitable cooking treatment as far as retention of bioactive compounds like phenolic acids is concerned.
Graphical Abstract

Similar content being viewed by others
Introduction
The continuous generation of free radicals in human body is due to oxidative metabolism (Suleman et al. 2019). The free radicals and reactive oxygen species (ROS) such as hydroxyl ion, and super oxide ion are harmful to the body systems through the degradation of macromolecules such as proteins, fats, deoxyribonucleic acids (DNA) and ribonucleic acid (RNA). Antioxidants are substances that have the potential to defend human body from the activity of ROS and free radicals (Tudorachi et al. 2021). Endogenously formed enzymatic antioxidants superoxide catalase (CAT), dismutase (SOD), and glutathione peroxidase protect against free radicals and reactive oxygen species. However, these antioxidant enzymes do not remain effective in scavenging free radicals during diseased conditions (Chandra et al. 2020). On the other hand, synthetic antioxidants, although able to quench oxidizing entities, are involved in inducing genotoxicity and carcinogenicity (Nath et al. 2017). External natural sources of dietary non-enzymatic antioxidants are needed to enhance immunity. Naturally occurring phenolics, tannins, flavonoids and alkaloids are common antioxidant compounds. The phenolic compounds behave as antioxidant mainly because of their redox properties, which allow them to perform as reducing agent, hydrogen donator and singlet oxygen quencher (Li et al. 2018).
Bottle gourd is a warm-season fruit that exhibits diuretic, hypoglycemic, hypolipidemic, analgesic, hepatoprotective, and immunomodulatory activities (Ahmad et al. 2022), making ita potential source of naturally occurring antioxidants (Kulkarni et al. 2014). Raw fruits and vegetables can be converted into edible form by different cooking methods such as pressure cooking, microwave cooking, frying, etc. These domestic thermal methods may affect the retention and potential of bioactive compounds available in plant foods. Conflicting results have been reported in various studies regarding the effect of various cooking treatments on total phenol content (TPC) and antioxidant activity of the cooked gourd fruits and this effect could be increasing or decreasing in TPC as well as antioxidant activity of the cooked vegetables when compared to the raw counterparts (Yadav et al. 2017b). The total phenolics and antioxidant activity of cashew nut extracts were reduced after microwave heating (Uslu & Ozcan 2019). In another study by Wu et al. (2019), boiling impacted a considerable loss of flavonoids, whereas steaming and microwave cooking induced only a minor loss or even apparent gain of flavonoids in thermally processed broccoli. Ilyasoglu and Burnaz (2015) suggested that steaming treatment was the most effective cooking process to hold antioxidant molecules, followed by the microwaving and boiling treatment for the domestic cooking of fresh and frozen kale.
Antioxidant activities of leaves and fruit of wild bottle gourd have been recorded significantly higher in ethanolic and methanolic extracts when compared to acetone and aqueous extract (Patel et al. 2018). Steaming, microwaving and boiling of bottle gourd fruit reduced total phenolics and flavonoid content (Saikia & Mahanta 2013). Higher free radical scavenging activity was observed in various vegetables after microwave cooking (Sengul et al. 2014). Extraction of phenolic compounds was higher from ohmic heated (blanched) bottle gourd than conventionally blanched tissues (Bhat et al. 2017). An increase or decrease in phenolics due to thermal processing could be due to the destruction, release or transformation of existing phytochemicals (Zhan et al. 2018).
Plant phenolics remain in a complex tissue matrix, which may vary from crop to crop; hence, special provisions or extraction solvents are needed for their high and efficient separation. Therefore, different extracts are likely to differ in their phenolic content and antioxidant activity. The polarity of extraction solvents is influential in deciding the extraction power of phytochemicals and in vitro antioxidant potential of plant-based extracts (Yadav et al. 2016). Methodical research on the effects of domestic cooking treatments on the phenolic profile and antioxidant profile of different types of extracts of bottle gourd are very few and inconsistent too. Therefore, the present study was conducted to identify and quantify polyphenolics compounds and resulting in vitro antioxidant activity of different extracts of bottle gourd cooked with different heat processing treatments.
Materials and methods
Freshly harvested bottle gourd fruit was obtained from the local market in Rohtak (India), and subsequently stored (4 h) under refrigerated conditions for future use. The various chemicals and reagents of analytical grade used in the present study were purchased from Sigma Aldrich Fine Chemicals (USA), Himedia Laboratories (Mumbai, India) and Merck (Darmstadt, Germany).
Experimental design
The main factors in the study design were extraction solvents (methanol, ethanol and butanol) and cooking treatments (pressure cooking, microwave cooking and frying). The study followed a 3 × 3 factorial design, where three different extraction solvents were combined with three different cooking treatments, resulting in nine different combinations (treatment groups). This design allowed the researchers to investigate the individual and interactive effects of the extraction solvents and cooking treatments on the phytochemical content (total phenolic content, flavonoid content, tannin content), and antioxidant activity of the gourd extracts.
Cooking of fruit
Bottle gourd fruit pieces were subjected to various cooking methods. To optimize the cooking conditions, 4–5 preliminary cooking trials were conducted to asses the optimum level of palatability, tenderness and taste. For each treatment a 500 g sample was used. For pressure cooking, fruit pieces (3 × 0.5 × 0.5 cm approx.) were cooked for 5 min in a pressure cooker (134 mm dia, Hawkins, Mumbai, India) with the addition of 100 mL water. In another cooking method, soya refined oil was used for frying (170 °C, 10 min) fruit pieces of approx. 0.25 cm uniform thickness. In third method, microwave cooking of fruit pieces (3 × 0.5 × 0.5 cm approx) was performed at 110 °C for 8 min using a microwave oven (model: 25SC3 IFB, Zhonghshan, China).
Fruit extracts preparation
Macerates of all cooked and raw fruit samples were prepared in a waring blender (model 8011ES) and each sample was mixed with the selected organic solvent i.e. absolute methanol, ethanol and n-butanol separately and kept at 25 °C on an automatic shaker (REMI, RS-24 BL) for 6 days with a shaking at 120 rpm after an interval of every 2 h. Solvent extracts were gravitationally filtered through filter paper (Whatman No.1), centrifuged (9800 g for 5 min.) in a cooling centrifuge (REMI, C-24 BL), vacuum evaporated (45 °C; 97.3 kPa) using a rotary vacuum evaporator (IKA-RV 10) and extracts of all the samples were kept in dark at -20 °C before further analyses (Yadav et al. 2016).
Estimation of phytochemicals
Determination TPC
TPC in all the extracts were estimated by using Folin–Ciocalteu reagent method as described by Gao et al. (2019), with slight modifications. mThe reaction mixture contained 1.0 mL of diluted fruit extract, 0.5 mL of the Folin – Ciocalteu reagent, 3 mL of 20% sodium carbonate and 10 mL of distilled water. After 2 h of reaction at ambient temperature, the absorbance was measured at 765 nm against the blank using an UV–Vis double beam spectrophotometer (Systronics 2202, India). The same procedure was repeated for the standard solution of gallic acid with prepared concentrations of 0.05, 0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40 mg/mL and the calibration line was construed. The phenolic content was measured in terms of gallic acid equivalent (mg of GAE/100 g dry weight basis) as suggested by the regression equation (Y = 4.262x + 0.043; R2 = 0.997) derived for the standard curve of gallic acid.
Determination of flavonoids
A modified method of da Silva et al. (2015) was selected for the estimation of flavonoids. The extract (1.0 mL) was diluted with equal volume of methanol and added with 0.1 mL of 10% ethanolic AlCl3, 0.1 mL of 1 mol L−1 of potassium acetate and 2.8 mL of distilled water. The mixture was incubated at room temperature for 30 min. and the absorbance was measured using an UV–Visible double beam spectrophotometer (Systronics 2202, India) at 420 nm against the blank. The standard solution of quercetin was prepared with concentrations of 0.01, 0.03, 0.05, 0.07, 0.09, 0.11, 0.13, 0.15 mg/mL and the calibration curve was construed. A regression equation (Y = 14.32x + 0.047; R2 = 0.990) was used to calculate the flavonoid content and the results were expressed in terms of quercetin equivalent (mg of QE/ 100 g dry weight basis).
Estimation of tannin content (Vanillin–HCl method)
A modified vanillin-HCl method (Herald et al. 2014) was used with minor alterations for determination of tannin content. Diluted extract (1.0 mL) added with 5 mL of vanillin reagent (equal volume of 1% methanolic vanillin and 8% methanolic HCl) was incubated (30 °C, 20 min) in a water bath at 30 °C for about 20 min. To correct the background colour, similar samples were prepared with the addition of 5 mL of 4% HCL in methanol. The absorbance was measured at 500 nm. The standard curve was prepared with catechin in the varying concentrations of 0.25, 0.50, 0.75, 1.00, 1.25, 1.50, 1.75, 2.00 mg/mL. The absorbance was converted to catechin equivalent (CE) using regression equation Y = 0.5523x + 0.0273; R2 = 0.998. The results were expressed in terms of mg equivalents of catechin/100 g dry weight basis.
Estimation of antioxidant activity
Ferric thiocyanate (FTC) method
For antioxidant activity, ferric thiocyanate (FTC) method (Shafekh et al. 2012) was followed using water as control and vitamin E and butylated hydroxytoluene (BHT) as standard. The reaction mixture [1 mg of fruit extract in 1 mL of 99.5% ethanol, 1.025 mL of 2.51% linoleic acid in 99.5% ethanol, 2 mL of 0.05 M phosphate buffer (pH 7.0) and 0.975 mL of distilled water] contained in a screw-cap vial was placed in an oven at 40 °C and incubated in the dark. To measure the extent of antioxidant activity, 0.05 mL of the reaction mixture was transferred into a test tube and 4.85 mL of 75% (v/v) aqueous ethanol was added to it, followed by the addition of 0.1 mL of 30% aqueous ammonium thiocyanate and 0.1 mL of 0.02 M ferrous chloride taken in 3.5% hydrochloric acid. Three minutes after the addition of ferrous chloride to the reaction mixture, the absorbance of red colour was measured at 500 nm using an UV–Visible double beam spectrophotometer (Systronics 2202, India). The measurements were taken after an interval of 24 h upto 5 days. The percent inhibition was determined as follows.
Thiobarbituric acid (TBA) method
Thiobarbituric acid (TBA) assay as suggested by Shafekh et al. (2012) was used to estimate antioxidant activity The reaction mixture (0.5 mL) as prepared in FTC method, 1.0 mL of 20% aqueous trichloroacetic acid (TCA) and 1 mL of 0.67% aqueous thiobarbituric acid (TBA) solution were mixed together. The mixture was placed in a boiling water bath for 20 minand centrifuged (Remi-C-24BL) at 885xg for 25 min. Absorbance of the supernatant was measured at 532 nm using an UV–visible double beam spectrophotometer (Systronics 2202, India). Antioxidant activity was recorded based on absorbance of final day of the FTC assay and measured as % inhibition similar to that in FTC method.
Ferric reducing antioxidant power (FRAP) method
The FRAP assay, based on the methodology employed by Spiegel et al. (2020) was used with minor modifications to determine ferric reducing antioxidant power. The FRAP reagent was freshly prepared by mixing 100 mL of acetate buffer (300 mM, pH 3.6), 10 mL TPTZ in 40 mM Hcl), 10 mL FeCl3 0.6 H2O (20 mM) in a ratio of 10:1:1. To perform the assay, 1.8 mL of FRAP reagent, 0.18 mL of distilled water and 60 μL of diluted extract were taken in a test tube and incubated at 37 °C for 4 min. The absorbance was measured at 593 nm, using FRAP working solution as blank. For construction of the calibration curve different concentrations of FeSO4. 7H2O (0.05, 0.10, 0.15, 0.20, 0.25, 0.30 mM) were used. Based on the measured absorbance, the concentration of FeSO4 was measured (mM FeSo4/100 g dw basis) from the regression equation (Y = 0.5783 × 0.0042; R2 = 0.9991) of the standard curve of FeSO4.
Evaluation of the free radical scavenging activity by DPPH assay
Evaluation of free radical scavenging activity was done by using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay as modified protocol of Lalhminghlui and Jagetia (2018). Various dilutions of each extract (0.5 to 3 mg mL−1 at an interval of 0.5 mg mL−1) were prepared with 80% of the extraction solvents. A positive control was made with the mixing of 1 mL of 80% solvent to 2 mL of 0.01 mM DPPH solution (3.9 mg of DPPH in 100 mL of methanol). Approximately 1.0 mL of extract from each dilution was added into test tube containing 2 mL of DPPH solution. The mixture was shaken vigorously and left to stand in the dark for 30 min. The absorbance of the resulting solution was measured using an UV–visible double beam spectrophotometer at 517 nm. The DPPH free radical scavenging potential of all the extracts was estimated by using the standard equation (percent scavenging activity) and outcomes were reported as IC50 value and compared with ascorbic acid as standard.
HPTLC analysis of phenolic acids and flavonoids compounds
The high performance thin layer chromatography (HPTLC) analysis was executed for the identification and quantification of phenolic acids and flavonoids. Standards used for phenolic acids were gallic, benzoic, p-coumaric, tannic, ellagic, vanillic, chlorogenic acids (Himedia, Mumbai, India), vannilic acid and caffeic acid (Sigma-Aldrich, St. Louis, MO). Flavonoid standards used in the study were quercetin and rutin (Himedia), kaempferol, myricetin, catechin, leutolin and apigenin (Sigma-Aldrich). Analyses were performed on a HPTLC system provided with silica gel 60 F254 plates (20 × 10 cm) (Merck, Darmstardt, Germany) using a HPTLC- CAMAG (Muttenz, Arlesheim, Switzerland) regulated with WinCATS software (CAMAG). The HPTLC grade methanol was used for preparation of standard stock solution (0.1 mg mL−1) as well as sample (100 mg mL−1), which were filtered further through 0.2μ syringe filter. A micro-syringe (100μL) was used to apply the sample (8 μL) using an automatic Linomat-5 system (CAMAG). The standard stock solutions of each selected standard compounds were made in methanol with a concentration ranged from 2–8 μL. The plates were loaded with sample and placed and developed to 80% of the plate height in a chamber (CAMAG) using the mobile phases: solvent system I comprised of chloroform, hexane, methanol and formic acid in the ratio of 6.4: 3.9: 2.0: 0.5 for the identification of quercetin, caffeic acid, gallic acid, apigenin, chlorogenic acid and kampferol; solvent system II comprised of chloroform, hexane, methanol and formic acid in the ratio of 4:1:1:1 for detection of leutolin, ellagic acid, p-coumaric acid, catechin and myricetin and solvent system III comprising of acetonitrile, methanol and water in the ratio of 4.5:1.0:0.5 for detection of vanillic acid, benzoic acid, ferulic acid and cinnamic acid and respectively. The densitometric evaluation was performed at a wavelength of 254 nm with TLC scanner 3 (CAMAG). The TLC visualizer documentation system (CAMAG) was used for documentation of plate image and all these documentation were quantified with winCAT software. Identification of peak table, peak display and peak densitograms was performed and a calibration curve prepared from peak area and concentration of standard compounds was used to determine concentration of each compound.
Statistical analysis
Two-way ANOVA followed by Tukey’s HSD post hoc comparison test at p < 0.05 was performed to determined main and interaction effects of extraction solvents and cooking treatments on their antioxidant activity. The statistical analysis was done by using statistical package for the social sciences (SPSS) version 21. For statistical analysis of the data, 3 independent replications were obtained for each measurement and results expressed as mean ± standard deviation (SD).
Results and discussion
Quantitative estimation of total phenol contents
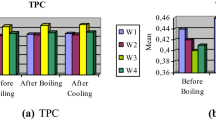
TPC of different extracts of uncooked and cooked samples of bottle gourd fruit is given in Table 1. Irrespective of the cooking methods, TPC of methanolic extract (ME) was maximum followed by ethanolic extract (EE) and butanolic extract (BE). The lower TPC in BE could be ascribed to the less polar and high viscous nature of butanol when compared with ethanol and methanol organic solvent, thus rendering it a less efficient extraction solvent (Hussain et al. 2022). The various cooking treatments impacted TPC significantly (p = 0.000) as TPC increased significantly after pressure cooking (23.8%) and frying (13.3%), however it was observed to decrease by 26.2% after microwave cooking in respect to their uncooked (464.8 mg GAE/100 g) samples. Similarly, earlier investigations have also reported the gain in TPC after frying and pressure cooking in sponge gourd (Yadav et al. 2017b), broccoli (Ng et al. 2011) and green paprika (Chuah et al. 2008). This increase in phenolic content could be attributed to the escape of bound phenolic compounds on account of breakdown of the cell walls and the formation of dissociated by-products from the conjugated phenolic forms (Kim et al. 2020). Sometimes individual phenolic compound content may rise because of the heat-induced releasing of the bound phenolics with the structural breakdown of supra molecular, which have the potential to react with the Folin–Ciocalteu (FC) reagent (Calinoiu & Vodnar 2019). Whereas, the reduction in TPC after microwave cooking may be because of the rapid and direct heating mechanism by electro-magnetic waves with in comparison to other thermal treatments (Yadav et al. 2017a; Zin et al. 2020). Moreover, some phenolic compounds might be particularly sensitive to microwave radiation and may undergo chemical transformations or degradation in response to the electromagnetic waves, affecting a reduction in phenolic content as well as antioxidant activity in vegetables (Hayatet al. 2010). Previously, it has been reported a higher loss of phenolic content in microwave cooking of various fruits and vegetables (Perla et al. 2012). A significant interaction effect (p = 0.000) of extracting solvents and cooking treatments indicated that the combination of these factors had a substantial impact on the phenolic content of the different extracts. This suggests that the choice of extracting solvents and cooking methods influenced the quantity of phenolic compounds present in the extracts.
Quantitative estimation of flavonoid contents
Flavonoids are the dominant bioactive molecules in fruits and vegetables and these are mainly dominated by glycosidic flavonoids (Panche et al. 2016). The average flavonoid concentration of various extracts of raw bottle gourd was 66.3 mg QE/100 g (dwb), which under the influence of different cooking treatments ranged from 63.2 to 158.1 mg QE/100 g (dwb) (Table 1). These results indicated significant (p = 0.000) but highly inconsistent varying influence of cooking methods on flavonoid content as the influence profile was from a decrease of 4.6% in microwave cooked sample to an increase of 138.4% in fried sample. The previous studies have also described that thermal treatments raised the level of free flavonoids and this increase was ascribed to the reason that heat destruction of the cellular matrix surrounding the flavonoid compounds rendered the pectin and cellulose-bound flavonoids more extractable into the solvents (Saikia & Mahanta 2013). The loss of flavonoids after microwave cooking may be because of the rapid and direct heating mechanism of electromagnetic waves compared to other studied cooking treatments (Zin et al. 2020). The results revealed that some phenolic compounds were more sensitive to microwave radiation and underwent degradation, leading to a decrease in their concentration. The flavonoids are highly unstable compounds susceptible to destruction with electromagnetic radiation which may be due to the increase in the formation of hydroxyl compound (Biesaga 2011). Regardless of the cooking method, the average flavonoid content of different extracts followed the order of EE (ethanolic extract) > ME (methanolic extract) > BE (butanolic extract). This variation could be due to the different hydrogen bonding and dipole–dipole interactions of flavonoids with extraction solvents. The nature and strength of such kind of interactions with ethanol might have contributed to the higher extraction efficiency in comparison to methanol and butanol (Do et al. 2014).Similar findings on flavonoid content concerning extraction solvents were reported in Carica papaya (Asghar et al. 2016). The interactive effect of extraction solvents and thermal treatments was also significant (p = 0.000) and in light of this interactive effect, the recovery of total flavonoids was in the order of EE (fried sample) > ME (fried sample) > EE (pressure cooked sample). This means that the combination of different extraction solvents and thermal treatments had a profound impact on the recovery of total flavonoids from the samples. These findings highlight the importance of both the choice of extraction solvent and the thermal treatment applied, as they significantly influenced the amount of total flavonoids recovered from the samples.
Condensed tannin contents
The average tannin content after cooking treatments ranged from 11.0 to 19.5 mg CE/100 g, whereas in uncooked samples it was measured to be 13.5 mg CE/100 g (Table 1). The results indicated an inconsistent effect of different thermal treatments on the condensed tannin content. Similar to total phenol and flavonoid content, the average tannin content was also found to decrease by 18.5% during microwave cooking as compared to the raw counterpart. This decrease in tannin content could be due to rapid and intense heating of fruit by electromagnetic waves, this quick heating might have led to a higher temperature around the food surface, which induced reactions like hydrolysis, oxidation, polymerization and thermal decomposition (Ahmed 2021). Previous studies have also reported a heavy loss of tannin content during microwave cooking of vegetables (Maqbool et al. 2021). In contrast, an increase in tannin content was observed in pressure cooking (11.85%) and frying (44.4%) when compared to their raw counterpart. This increase in tannin content after heat treatment could be ascribed to softening or disrupting plant cell walls and the breakdown of the complex phenolics into simpler ones (Nagarani et al. 2014). Irrespective of thermal treatment, the average value of tannin content was maximum in EE and minimum in ME. The differences in activities of ME, EE and BE can be attributed to the varying affinities of these solvents for specific bioactive compounds (Yadav et al. 2017b). The high tannin content in the ME and BE in comparison to their uncooked samples was indicative of the significant interaction effect (p = 0.000) between extraction solvent and heat processing treatment. However, the recovery of tannins in the EE of the cooked samples was more inconsistent. This indicated that extracting efficiency of ethanol in respect to tannins from the cooked samples varied significantly and was less reliable than the ME and BE. The results highlights the importance of considering the appropriate extraction method for tannins, especially when dealing with cooked samples.
Percent inhibition as measured by ferric thiocyanate (FTC) and thiobarbituric acid (TBA) methods
The antioxidant potential of ME, EE and BE of various samples evaluated in terms of their capacity to inhibit peroxidation as observed by FTC and TBA methods is given in Table 2. The various extracts of the cooked samples exhibited a significant difference (p < 0.05) in their extent of percent inhibition, which was again indicative of the marked influence of the extracting solvent in extracting out the phytochemicals. After an incubation period of 96 h, the various extracts of bottle gourd inhibited peroxidation of linoleic acid to a varying extent of 26–87.7%. In the same context, Deore et al. (2009) reported the percent inhibition of bottle gourd extract ranging from 73.4 to 93.9%. Nevertheless, this extent of percent inhibition was measured significantly (p < 0.05) lower in comparison to standard BHT (butylated hydroxytoluene) (88.8%). Overall, ME showed significantly higher antioxidant level when compared to BE and EE. Similarly, in another study more antioxidant activity of methanolic extract compared to chloroform extract was reported in bitter gourd (Rezaeizadeh et al. 2013).
In general, pressure-cooked and microwave-cooked fruit were found to be poor in inhibiting peroxidation compared to their uncookedcounterpart. The interaction effect (p = 0.000) suggested that inhibitory activity of the ME and EE of pressure cooked sample was significantly higher (p < 0.05), whereas it was measured to be 19.1% lower in BE when compared to their respective extracts of uncooked samples. In general, it was observed that various extracts of microwave cooked samples exhibited increased inhibitory activity, whereas extracts mainly, BE and ME showed reduced peroxidation inhibitory activity. Sultana et al. (2008) also conveyed a similar reduction in percent inhibition in various fruits and vegetables after microwave cooking. The increased percent inhibition was estimated in all types of fried fruit extracts compared to their raw samples. This was probably due to the retention of various phenols and flavonoid compounds in fried samples. The findings of the study by Aryal et al. (2019) suggested that the potential antioxidant activity of various agricultural crops against linoleic acid peroxidation was attributed to their phenolic content.
The peroxides gradually decomposed to malondialdehyde, the measurement of whose concentration forms the basis of the TBA test. Irrespective of cooking type, the extent of inhibition of the decomposition of peroxides was in order of ME (76.4%) > BE (70.7%) > EE (55.1%). On the other hand, the percent inhibition by the thermally cooked samples regardless of the extraction solvent was inconsistent and it was observed higher after pressure cooking and frying, while measured lower after microwave cooking of the samples. The significant interaction effect suggested maximum percent inhibition by the EE of the fried sample followed by BE and ME. Further, compared to standard vitamin E (78.3%) and BHT (90.9%), most of the extracts showed lower antioxidant activity.
Ferric reducing antioxidant power (FRAP) assay
As assessed by the FRAP assay (Table 2), the antioxidant activity of bottle gourd fruit was significantly influenced by the cooking methods (p < 0.05). Regardless of the extraction solvent, the increased ferric reducing power was measured after all the cooking treatments. Further, the antioxidant activity in terms of FRAP assay values of different extracts irrespective of cooking treatments ranged from 1645.0 to 2620.9 μM FeSO4/100 g in order of EE > ME > BE. In another study on bottle gourd, a similar level of FRAP value was reported by Saikia and Mahanta (2013). The previous studies have suggested that the flavonoids were very much responsible for the increased ferric reducing antioxidant power (Armesto et al. 2019). Similar to these findings, the gain in reducing power after various thermal treatments has also been reported in earlier investigation (Ng et al. 2011). This enhanced ferric reducing power after the cooking treatments could be ascribed to the transformation/formation of rutin (flavonoid), which is considered enriched with hydroxyl groups, resulting in more antioxidant activity (Yadav et al. 2017a). Further, the maillard reaction induced generation of secondary metabolites with high redox potential and thermal breakdown of complex bound phenolics (Dini et al. 2013) could also be accounted for the enhanced reducing activity of the heat processed samples as observed in the present study. The significant interaction effect between cooking treatments and extraction solvents (p = 0.000) recommended that various extracts of pressure cooked fruit exhibited the higher Fe3+ reducing power in order of ME (85.1%) > EE (35.2%) > BE (31.3%). The microwave cooking and frying impacted the reducing power inconsistently; ME and EE of such samples exhibited higher FRAP value, whereas it was calculated to be lower in BE compared to their respective uncooked samples.
Free radical scavenging activity by DPPH assay
The scavenging activity of the extracts of raw as well as cooked samples of bottle gourd against DPPH free radicals was measured concerning the scavenging activity of ascorbic acid taken at a concentration of 30 μg/mL (92.0% activity). The results as given in Table 3 revealed that irrespective of the cooking methods, the extraction solvents significantly (p = 0.000) influenced the DPPH scavenging activity in order of ME > BE > EE. Previous studies of gourd fruits have also reported the better extractability of antioxidant compounds with methanol than other solvents (Atique et al. 2018). This could probably be ascribed to better solvation of phytochemicals because of the effective interaction (hydrogen bonds) between the polar solvents (methanol) and polar sites of antioxidant molecules. The effect of different heat processing treatments on the radical scavenging action of different extracts was inconsistent. The average percent scavenging activity of 55.1% of raw fruit samples decreased by 34.0% and 24.7% in pressure cooked and fried fruit samples respectively, whereas it increased by 20.8% in microwave cooked samples. Although TPC, flavonoids and tannins content decreased in microwave cooking, but this increase in free radical scavenging activity could possibly be ascribed to the maillard browning under the influence of microwave activation leading to the formation of various flavour compounds and melanoidins some of which might have antioxidant activity (Suri et al. 2020). It has been reported that microwave cooking of bottle gourd increased the antioxidant activity (Saikia & Mahanta 2013) and it has been attributed to the thermal deactivation of oxidative enzymes and the liberation of potent radical scavenging antioxidants by microwave heating (Mushtaq et al. 2015). Additionally, an increased antioxidant activity might be due to the generation of new compounds under the maillard reaction (Liu et al. 2018). In contrast, a decrease in antioxidant capacity was observed after pressure cooking and frying and this decrease in radical scavenging property might be due to the thermal degradation of antioxidant molecules (Baljeet et al. 2016). Yadav et al. (2017a) also observed a similar reduced radical scavenging activity after thermal treatment of other gourd fruits.
There is an inverse relationship between IC50 and antioxidant activity. The IC50 value, an indicator of the free radical scavenging activity of different extracts of raw and cooked samples of bottle gourd fruit is given in Table 3. Irrespective of the method of cooking, the average IC50 value was measured to be the lowest in ME (28.8 μg/mL) followed by BE (32.6 μg/mL) and EE (39.4 μg/mL), suggesting that the methanolic extracts exhibited the highest antioxidant activity.
Correlation studies
The correlation results (Table 4) showed that the inhibitory activity as investigated by the TBA method was ascribed to the flavonoids present in bottle gourd, which could have inhibited the malonaldehyde formation. Further, a significant positive correlation (r = 0.999, p < 0.01) between TBA and FTC values was suggestive of the fact that a gain in peroxide level induced the generation of malonaldehyde compounds (Siddique et al. 2012). These findings of the current study are consistent with the already reported positive correlations between antioxidant potential and phenolic compound concentration (Yadav et al. 2017b). The outcome also suggested that phenols and flavonoids are responsible for the reduction of ferrous ions into ferric ions due to the strong and positive correlations of the FRAP value with phenols content (r = 0.892, p < 0.01) and flavonoid content (r = 0.809, p < 0.01). Earlier studies have also suggested that phenols and flavonoids are the mainstay of the antioxidant activity as calculated by the FRAP method (Loizzo et al. 2012). Moreover, it was also evident from the correlation studies that the free radical scavenging activity of heat-processed fruit samples was positively correlated with flavonoid content (r = 0.743, p < 0.01) and tannin content (r = 0.852, p < 0.01). However, it was interesting to note that although the flavonoid content of EE was the highest, the free radical scavenging activity (calculated by DPPH) was the lowest. This odd observation indicated that flavonoids' efficacy as antioxidants was not only due to their available concentration but also because of the degree of polymerization and interactive compatibility of their diverse chemical structures (Yadav et al. 2017b). Additionally, in the study no significant correlation was measured between DPPH free radical scavenging activity and TPC, which indicated that it was not only the TPC, but also the type of phenolic acid which could have affected the antioxidant activity (Rao et al. 2018). The position and number of hydroxyl groups (OH) in the phenolic compounds may significantly influence free radical scavenging activity (Platzer et al. 2022). Further, it has been proven that the not only phenolic compounds are responsible for the antioxidant activity, but it could be due to the presence of other compounds like vitamin C, β-carotene, α-tocopherol, selenium etc. (Song et al. 2010).
Identification and quantification of phenolic acids and flavonoids by HPTLC
Different phenolic compounds exhibit varying extent of antioxidant activity (Rao et al. 2018). Further, the kind and polarity of the extraction solvents also exert a lot of impact on the separation of target phytochemical from the biomass matrix (Sulaiman & Ooi 2013). Considering the reasonably higher TPC and antioxidant potential (FTC, TBA and DPPH method) of ME in comparison to EE and BE, ME of various raw and cooked samples were subjected to identification and quantification of different phenolic acids and flavonoids. The outcomes of HPTLC are depicted in Table 5 and Figs. 1, 2 and 3. Gallic acid was observed to be the most ubiquitously present phenolic acid in bottle gourd. However, a drastic loss of gallic acid was observed in the thermally processed samples with no identification in microwave-cooked samples. Following the almost same trend, vanillic acid and p-coumaric acid, which were identified only in the raw counterpart, did not retain in the cooked samples. Interestingly, the ellagic acid and rutin, unidentified in the raw sample, were available in all the thermally processed samples. The occurrence of novel phenolic compounds in the cooked samples could possibly be ascribed to the availability of a precursor generated under the influence of non-enzymatic inter-conversion among the selected phenolic molecules (Vega-Galvez et al. 2011). This formation of rutin (flavonoid) which is considered to be more reactive due to the attachment of higher number of -OH groups in its structure, could be responsible for the increased antioxidant activity (as measured by FRAP) of the ME of cooked samples, as the free radical scavenging capacity is known to rise with the increasing number of phenolic -OH groups (Platzer et al. 2022). The presence of rutin in the cooked samples of bottle gourd may deliver beneficial health roles as rutin has been investigated for its anti-inflammatory, antioxidant and protective effects in contradiction to hepatotoxicity (Rahmani et al. 2023). Flavonoids such as myricetin and quercetin were observed in reduced concentrations only in pressure-cooked samples, whereas; catechin (28.31 μg/g dry weight of sample) was detected only in the fried fruit sample. The generation of catechin in the sample subjected to frying could possibly be because of breakdown and depolarization of condensed tannins releasing the basal structural compound (catechin). The HPTLC results illustrated that subjecting the bottle gourd to thermal processing treatments could result in the destruction/transformation of the existing phenolic compounds. The studied cooking treatments for bottle gourd were most detrimental to vanillic acid and p-coumaric acid, which were not recognized in all the cooked samples. The destruction of polyphenolic compounds may also occur because of hydrolysis, dimerization, epimerization, oxidative and polymerization reaction (Liu et al. 2021). The availability of various polyphenolics like vanillic acid, myricetin, quercetin etc. was reported in various portions of gourd fruits (Irshad et al. 2010).
Conclusions
The present study indicated that the cooking methods and organic solvent used for extraction significantly influenced the recovery of various polyphenolic compounds available in bottle gourd fruit. Overall, methanol was the most active solvent in extracting polyphenolic compounds. Similarly, concerning the antioxidant activity (measured by FTC, TBA and DPPH methods) ME has great significance. The outcome specified that thermal treatments had both positive as well as negative influences on antioxidant activity and concentration of various phytochemicals. Overall, pressure cooking and frying emerged as most effective domestic cooking treatments for retaining phenolics and antioxidant activity. Correlation studies showed positive associations between antioxidant activity and phenolic compound content, but the relationship was not linear, indicating that other factors might also have contributed to the observed antioxidant activity. Furthermore, the depletion, transformation and formation of new antioxidant compounds were confirmed from the HPTLC profiles. Overall, this study highlighted the importance of both the choice of extraction solvent and the cooking method in determining the phenolic content and antioxidant activity of bottle gourd fruit extracts. These findings provide valuable insights for optimizing cooking methods to retain and enhance the nutritional and antioxidant properties of this fruit.
Availability of data and materials
All data supporting this study are included in this manuscript. Further detailsare available upon request from the corresponding author.
Abbreviations
- FTC:
-
Ferric thiocyanate
- TBA:
-
Thiobarbituric acid
- FRAP:
-
Ferric reducing antioxidant power
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ROS:
-
Reactive oxygen species
- CAT:
-
Catalase
- SOD:
-
Dismutase
- HPTLC:
-
High performance thin layer chromatography
- TPC:
-
Total Phenol content
- ME:
-
Methanolic extract
- EE:
-
Ethanolic extract
- BE:
-
Butanolic extract
- GAE:
-
Galic acid equivalent
- QE:
-
Quercetin equivalent
References
Ahmad, M. D., Ahmad, I., El-Chaghaby, G., & Rashad, S. (2022). Nutritional and medicinal potential of bottle gourd (Lageneria siceraria): A mini review. Egyptian Journal of Botany, 62(1), 1–10.
Ahmed, J. (2021). Emerging technologies for pulse processing. In B. K. Tiwari, A. Gowen, & B. McKenna (Eds.), Pulse foods (pp. 265–293). Academic Press.
Armesto, J., Gomez-Limia, L., Carballo, J., & Martinez, S. (2019). Effects of different cooking methods on the antioxidant capacity and flavonoid, organic acid and mineral contents of Galega Kale (Brassica oleracea var. acephala cv. Galega). International Journal of Food Sciences and Nutrition, 70(2), 136–149.
Aryal, S., Baniya, M. K., Danekhu, K., Kunwar, P., Gurung, R., & Koirala, N. (2019). Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants, 8(4), 96.
Asghar, N., Naqvi, S. A. R., Hussain, Z., Rasool, N., Khan, Z. A., Shahzad, S. A., & Jaafar, H. Z. (2016). Compositional difference in antioxidant and antibacterial activity of all parts of the Carica papaya using different solvents. Chemistry Central Journal, 10(1), 1–11.
Atique, I., Ahmed, D., Maqsood, M., & Malik, W. (2018). Solvents for extraction of antidiabetic, iron chelating, and antioxidative properties from Bottle Gourd fruit. International Journal of Vegetable Science, 24(3), 212–226.
Baljeet, S. Y., Roshanlal, Y., & Ritika, B. Y. (2016). Effect of cooking methods and extraction solvents on the antioxidant activity of summer squash (Cucurbita pepo) vegetable extracts. International Food Research Journal, 23(4), 1531–1540.
Bhat, S., Saini, C. S., & Sharma, H. K. (2017). Changes in total phenolic content and color of bottle gourd (Lagenaria siceraria) juice upon conventional and ohmic blanching. Food Science and Biotechnology, 26, 29–36.
Biesaga, M. (2011). Influence of extraction methods on stability of flavonoids. Journal of Chromatography, 1218(18), 2505–2512.
Calinoiu, L. F., & Vodnar, D. C. (2019). Thermal processing for the release of phenolic compounds from wheat and oat bran. Biomolecules, 10(1), 21.
Chandra, P., Sharma, R. K., & Arora, D. S. (2020). Antioxidant compounds from microbial sources: A review. Food Research International, 129, 108849.
Chuah, A. M., Lee, Y. C., Yamaguchi, T., Takamura, H., Yin, L. J., & Matoba, T. (2008). Effect of cooking on the antioxidant properties of coloured peppers. Food Chemistry, 111(1), 20–28.
da Silva, L. A. L., Pezzini, B. R., & Soares, L. (2015). Spectrophotometric determination of the total flavonoid content in Ocimum basilicum L.(Lamiaceae) leaves. Pharmacognosy Magazine, 11(41), 96–101.
Deore, S. L., Khadabadi, S. S., Patel, Q. R., Deshmukh, S. P., Jaju, M. S., Junghare, N. R., & Jain, R. G. (2009). In vitro antioxidant activity and quantitative estimation of phenolic content of Lagenaria siceraria. Rasayan Journal of Chemistry, 2(1), 129–132.
Dini, I., Tenore, G. C., & Dini, A. (2013). Effect of industrial and domestic processing on antioxidant properties of pumpkin pulp. LWT Food Science and Technology, 53(1), 382–385.
Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E., Ismadji, S., & Ju, Y. H. (2014). Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. Journal of Food and Drug Analysis, 22(3), 296–302.
Gao, M. R., Xu, Q. D., He, Q., Sun, Q., & Zeng, W. C. (2019). A theoretical and experimental study: The influence of different standards on the determination of total phenol content in the Folin-Ciocalteu assay. Journal of Food Measurement and Characterization, 13, 1349–1356.
Hayat, K., Zhang, X., Chen, H., Xia, S., Jia, C., & Zhong, F. (2010). Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Separation and Purification Technology, 73(3), 371–376.
Herald, T. J., Gadgil, P., Perumal, R., Bean, S. R., & Wilson, J. D. (2014). High-throughput micro-plate HCl–vanillin assay for screening tannin content in sorghum grain. Journal of the Science of Food and Agriculture, 94(10), 2133–2136.
Hussain, S., Rehman, A. U., Obied, H. K., Luckett, D. J., & Blanchard, C. L. (2022). Extraction, chemical characterization, in vitro antioxidant, and antidiabetic activity of Canola (Brassica napus L.) meal. Separations, 9(2), 38.
Ilyasoglu, H., & Burnaz, N. A. (2015). Effect of domestic cooking methods on antioxidant capacity of fresh and frozen kale. International Journal of Food Properties, 18(6), 1298–1305.
Irshad, M., Ahmad, I., Mehdi, S. J., Goel, H. C., & Rizvi, M. M. A. (2010). Antioxidant capacity and phenolic content of the aqueous extract of commonly consumed cucurbits. International Journal of Food Proprties, 17(1), 179–186.
Kim, M. Y., Yoon, N., Lee, Y. J., Woo, K. S., Kim, H. Y., Lee, J., & Jeong, H. S. (2020). Influence of thermal processing on free and bound forms of phenolics and antioxidant capacity of rice hull (Oryza sativa L.). Preventive Nutrition and Food Science, 25(3), 310–318.
Kulkarni, S. D., Sinha, B. N., & Kumar, K. J. (2014). Modified release and antioxidant stable Lagenaria siceraria extract microspheres using co-precipitated starch. International Journal of Biological Macromolecules, 66, 40–45.
Lalhminghlui, K., & Jagetia, G. C. (2018). Evaluation of the free-radical scavenging and antioxidant activities of Chilauni, Schima wallichii Korth in vitro. Future Science OA, 4(2), FSO272.
Li, Q. Q., Wang, K., Marcucci, M. C., Sawaya, A. C. H. F., Hu, L., Xue, X. F., & Hu, F. L. (2018). Nutrient-rich bee pollen: A treasure trove of active natural metabolites. Journal of Functional Foods, 49, 472–484.
Liu, P., Lu, X., Li, N., Zheng, Z., & Qiao, X. (2018). Characterization, variables, and antioxidant activity of the Maillard reaction in a fructose–histidine model system. Molecules, 24(1), 56.
Liu, X., Le Bourvellec, C., Guyot, S., & Renard, C. M. (2021). Reactivity of flavanols: Their fate in physical food processing and recent advances in their analysis by depolymerization. Comprehensive Reviews in Food Science and Food Safety, 20(5), 4841–4880.
Loizzo, M. R., Tundis, R., Bonesi, M., Menichini, F., Mastellone, V., Avallone, L., & Menichini, F. (2012). Radical scavenging, antioxidant and metal chelating activities of Annona cherimolaMill.(cherimoya) peel and pulp in relation to their total phenolic and total flavonoid contents. Journal of Food Composition and Analysis, 25(2), 179–184.
Maqbool, N., Sofi, S. A., Makroo, H. A., Mir, S. A., Majid, D., & Dar, B. N. (2021). Cooking methods affect eating quality, bio-functional components, antinutritional compounds and sensory attributes of selected vegetables. Italian Journal of Food Science, 33(SP1), 150–162.
Mushtaq, M., Sultana, B., Anwar, F., Adnan, A., & Rizvi, S. S. (2015). Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. The Journal of Supercritical Fluids, 104, 122–131.
Nagarani, G., Abirami, A., Nikitha, P., & Siddhuraju, P. (2014). Effect of hydrothermal processing on total polyphenolics and antioxidant potential of underutilized leafy vegetables, Boerhaavia diffusa and Portulaca oleracea. Asian Pacific Journal of Tropical Biomedicine, 4(1), S468–S477.
Nath, D., Banerjee, P., Shaw, M., & Mukhopadhyay, M. K. (2017). Bottle gourd (Lagenaria siceraria). Fruit and vegetable phytochemicals: Chemistry and human health (2nd ed.) (pp. 909–920).
Ng, Z. X., Chai, J. W., & Kuppusamy, U. R. (2011). Customized cooking method improves total antioxidant activity in selected vegetables. International Journal of Food Sciences and Nutrition, 62(2), 158–163.
Panche, A. N., Diwan, A. D., & Chandra, S. R. (2016). Flavonoids: An overview. Journal of Nutritional Science, 5, e47.
Patel, S. B., Attar, U. A., & Ghane, S. G. (2018). Antioxidant potential of wild Lagenaria siceraria (Molina) Standl. Thai Journal of Pharmaceutical Sciences, 42(2), 90–96.
Perla, V., Holm, D. G., & Jayanty, S. S. (2012). Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT-Food Science and Technology, 45(2), 161–171.
Platzer, M., Kiese, S., Tybussek, T., Herfellner, T., Schneider, F., Schweiggert-Weisz, U., & Eisner, P. (2022). Radical scavenging mechanisms of phenolic compounds: A quantitative structure-property relationship (QSPR) study. Frontiers in Nutrition, 9, 882458.
Rahmani, S., Naraki, K., Roohbakhsh, A., Hayes, A. W., & Karimi, G. (2023). The protective effects of rutin on the liver, kidneys, and heart by counteracting organ toxicity caused by synthetic and natural compounds. Food Science & Nutrition, 11(1), 39–56.
Rao, S., Santhakumar, A. B., Chinkwo, K. A., Wu, G., Johnson, S. K., & Blanchard, C. L. (2018). Characterization of phenolic compounds and antioxidant activity in sorghum grains. Journal of Cereal Science, 84, 103–111.
Rezaeizadeh, A., Zuki, A. B. Z., Abdollahi, M., Goh, Y. M., Noordin, M. M., Hamid, M., & Azmi, T. I. (2013). Determination of antioxidant activity in methanolic and chloroformic extracts of Momordica charantia. African Journal of Biotechnology, 10(24), 4932–4940.
Saikia, S., & Mahanta, C. L. (2013). Effect of steaming, boiling, and microwave cooking on the total phenolics, flavonoids and antioxidant properties of different vegetables of Assam. International Journal of Food and Nutrition, 2(3), 47–53.
Sengul, M., Yildiz, H., & Kavaz, A. (2014). The effect of cooking on total polyphenolic content and antioxidant activity of selected vegetables. International Journal of Food Properties, 17(3), 481–490.
Shafekh, E. S., Khalili, M. A. R., Catherine, C. C. W., Syakiroh, S. Z., Habibah, U. A., Norhayati, A. H., & Zubaidi, A. A. (2012). Total phenolic content and in vitro antioxidant activity of Vigna sinensis. International Food Research Journal, 19(4), 1393–1400.
Siddique, Y. H., Ara, G., & Afzal, M. (2012). Estimation of lipid peroxidation induced by hydrogen peroxide in cultured human lymphocytes. Dose-Response, 10(1), 1–10.
Song, W., Derito, C. M., Liu, M. K., He, X., Dong, M., & Liu, R. H. (2010). Cellular antioxidant activity of common vegetables. Journal of Agricultural and Food Chemistry, 58(11), 6621–6629.
Spiegel, M., Kapusta, K., Kołodziejczyk, W., Saloni, J., Zbikowska, B., Hill, G. A., & Sroka, Z. (2020). Antioxidant activity of selected phenolic acids–ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules, 25(13), 3088.
Sulaiman, S. F., & Ooi, K. L. (2013). Antioxidant and α-glucosidase inhibitory activities of cucurbit fruit vegetables and identification of active and major constituents from phenolic-rich extracts of Lagenaria siceraria and Sechium edule. Journal of Agricultural and Food Chemistry, 61(42), 10080–10090.
Suleman, M., Khan, A., Baqi, A., Kakar, M. S., & Ayub, M. (2019). Antioxidants, its role in preventing free radicals and infectious diseases in human body. Pure and Applied Biology, 8(1), 380–388.
Sultana, B., Anwar, F., & Iqbal, S. (2008). Effect of different cooking methods on the antioxidant activity of some vegetables from Pakistan. International Journal of Food Science and Technology, 43(3), 560–567.
Suri, K., Singh, B., Kaur, A., Yadav, M.P., & Singh, N. (2020). Influence of microwave roasting on chemical composition, oxidative stability and fatty acid composition of flax seed (Linum usitatissimum L.) oil. Food Chemistry, 326, 126974.
Tudorachi, N. B., Totu, E. E., Fifere, A., Ardeleanu, V., Mocanu, V., Mircea, C., & Carauşu, E. M. (2021). The implication of reactive oxygen species and antioxidants in knee osteoarthritis. Antioxidants, 10(6), 985.
Uslu, N., & Ozcan, M. M. (2019). Effect of microwave heating on phenolic compounds and fatty acid composition of cashew (Anacardium occidentale) nut and oil. Journal of the Saudi Society of Agricultural Sciences, 18(3), 344–347.
Vega-Galvez, A., Uribe, E., Perez, M., Tabilo-Munizaga, G., Vergara, J., Garcia-Segovia, P., Lara, E., & Scala, K. D. (2011). Effect of high hydrostatic pressure pretreatment on drying kinetics, antioxidant activity, firmness and microstructure of aloe vera (Aloe barbadensis Miller) gel. LWT – Food Science and Technology, 44(2), 384–391.
Wu, X., Zhao, Y., Haytowitz, D. B., Chen, P., & Pehrsson, P. R. (2019). Effects of domestic cooking on flavonoids in broccoli and calculation of retention factors. Heliyon, 5(3), e01310.
Yadav, B. S., Yadav, R., Yadav, R. B., & Garg, M. (2016). Antioxidant activity of various extracts of selected gourd vegetables. Journal of Food Science and Technology, 53, 1823–1833.
Yadav, R., Yadav, B. S., & Yadav, R. B. (2017a). Effect of heat processing treatments and extraction solvents on the phenolic content and antioxidant activity of Momordica charantia fruit. Journal of Food Processing and Preservation, 41(4), e13037.
Yadav, R., Yadav, B. S., & Yadav, R. B. (2017b). Phenolic profile and antioxidant activity of thermally processed sponge gourd (Luffa cylindrica) as studied by using high performance thin layer chromatography (HPTLC). International Journal of Food Properties, 20(9), 2096–2112.
Zhan, L., Pang, L., Ma, Y., & Zhang, C. (2018). Thermal processing affecting phytochemical contents and total antioxidant capacity in broccoli (Brassica oleracea L.). Journal of Food Processing and Preservation, 42(3), e13548.
Zin, M. M., Anucha, C. B., & Banvolgyi, S. (2020). Recovery of phytochemicals via electromagnetic irradiation (microwave-assisted-extraction): Betalain and phenolic compounds in perspective. Foods, 9(7), 918.
Acknowledgements
The Department of Food Technology and Pharmaceutical Sciences of Maharishi Dayanand University, Rohtak is acknowledged for allowing us to use their facility for all the analytical work.
Funding
This research is supported by the University Grant Commission (UGC) of India.
Author information
Authors and Affiliations
Contributions
RLY: designed experiments, collected data, analysed data and drafted manuscript, BSY: conceived theresearch idea, designed the study, supervised research work, and reviewed the manuscript, RY: reviewed andedited the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing interest associated with this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yadav, R., Yadav, B.S. & Yadav, R. Influence of various cooking treatments and extraction solvents on bioactive compounds and antioxidant capacities of bottle gourd (Lagenaria siceraria) fruit in India. Food Prod Process and Nutr 6, 19 (2024). https://doi.org/10.1186/s43014-023-00189-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43014-023-00189-2