Abstract
Background
The aim of this study was to report on the validity of the Naviswiss handheld image-free navigation device for accurate intraoperative measurement of THA component positioning, in comparison with the three-dimensional (3D) reconstruction of computed tomography (CT) images as the gold standard.
Methods
A series of patients presenting to a single-surgeon clinic with end-stage hip osteoarthritis received primary hip arthroplasty with the anterolateral muscle-sparing surgical approach in the supine position. Imageless navigation was applied during the procedure with bone-mounted trackers applied to the greater trochanter and ASIS. Patients underwent routine CT scans before and after surgery and these were analyzed by using three-dimensional reconstruction to generate cup orientation, offset and leg length changes, which were compared to the intraoperative measurements provided by the navigation system. Estimates of agreement between the intraoperative and image-derived measurements were assessed with and without correction for bias and declared cases with potential measurement issues.
Results
The mean difference between intraoperative and postoperative CT measurements was within 2° for angular measurements and 2 mm for leg length. Absolute differences for the two indices were between 5° and 4 mm. Mean bias was 1.9°–3.6° underestimation for cup orientation and up to 2 mm overestimation for leg length change, but absolute thresholds of 10° and 10 mm were not exceeded by 95% limits of agreement (LOA), especially after correction for bias. Four cases (12%) were declared intraoperatively for issues with fixation on the greater trochanter. Inclusion of these cases generated acceptable accuracy overall and their omission failed to improve between-case variability in accuracy or LOA for both offset and leg length.
Conclusions
The accuracy of the Naviswiss system applied during primary THA in a supine position and anterolateral surgical approach falls within clinically acceptable recommendations for acetabular cup placement, femoral offset, and length. With refinements to surgical technique to adapt to the navigation hardware, the system could be further improved with regression-based bias correction.
Trial registration
Registered with the Australian New Zealand Clinical Trials Registry (ACTRN12618000317291)
Similar content being viewed by others
Introduction
The number of primary total hip arthroplasties (THA) in Australia has risen 125% since 2003, with the revision burden reported to be at 7.6% at the end of 2021 (Australian Orthopaedic Association National Joint Replacement Registry [1]. Accurate alignment of the implant components and limb length equalization during THA is essential for minimizing the risk of revision surgery [19]. Poor positioning of the acetabular cup can cause dislocation, impingement and instability, while a large leg length discrepancy increases the risk of back pain, gait impairment and overall patient dissatisfaction [5, 16, 22, 29]. Leg length discrepancy has also been cited as a major cause of litigation, accounting for 8%–26% of lawsuits following THA [21].
Robotic-assisted THA can improve the accuracy of implant placement and significantly reduce leg length discrepancies [14]. However, the surgical time is prolonged and there are no significant improvements in the rate of complications and implant survivorship [14, 15, 28]. The financial investment and time required to adopt these systems have also prevented their widespread use [23]. Portable navigation systems have been developed to offer a cost-effective, user-friendly and minimally-invasive solution, and have been demonstrated to improve component positioning [26] even when used with different surgical approaches [4, 9, 12].
The Naviswiss (Naviswiss AG, Brugg, Switzerland) is a portable imageless navigation device equipped with an infrared stereo camera and an inertial measurement unit to facilitate implant positioning intraoperatively. The accuracy of the system has been reported as <3° mean absolute error for cup inclination and anteversion when tested in the supine position via an anterolateral THA approach [11], and <3.5° for cup orientation using a direct anterior THA approach with fluoroscopy [20]. However, clinical data for the system has not been reported in Australia, and there is a dearth of information regarding femoral offset and leg length discrepancy.
As such, a trial protocol was developed to assess the accuracy of the Naviswiss system in measuring acetabular cup inclination, acetabular cup version, femoral offset and leg length discrepancy [6]. The aim of this study was to report on the validity of the Naviswiss handheld image-free navigation device for accurate intraoperative measurement of THA component positioning, in comparison with the three-dimensional (3D) reconstruction of computed tomography (CT) images as the gold standard.
Methods
Patient selection
The study was embedded within a prospective observational clinical registry, for which ethical approval was obtained from a National Health and Medical Research Council-certified Human Research and Ethics Committee (HREC) (Bellberry; HREC 2017-07-499). The study was also registered with the Australian New Zealand Clinical Trials Registry (ACTRN12618000317291). Adult patients (>18 years) were included in the study if they presented to the participating surgeon with end-stage or rheumatoid arthritis and elected to undergo THA.
Patients who were unable to provide informed consent, or had declined or revoked consent were excluded from the study. Patients were additionally excluded if: they had severe contralateral hip deformity or dysplasia; required a simultaneous bilateral procedure; required an ipsilateral revision procedure; had a short-stem component implanted; were lost to follow-up; use of the navigation system was completely abandoned; received a posterior surgical approach; were revised prior to postoperative imaging being performed; where hardware was unavailable or failed such that intraoperative data could not be retrieved.
Surgical technique
Patients were administered preoperative antibiotic prophylaxis and were placed in the supine position on a radiolucent table to allow intraoperative imaging of cup and stem positions. Once patients were prepped and draped, the iliac crest was identified and a tracker was fixed prior to the registration of anatomical landmarks. An anterolateral muscle-sparing approach was utilized to expose the hip joint and trochanteric region. A screw with a serrated washer was inserted into the lateral trochanter near the caudal attachment of the gluteus medius, and a stalked tracker was inserted to clear the soft tissues. Registration of the hip joint was then performed. The final intraoperative component positions were logged by the navigation system and transferred by electronic form for further analysis. All surgeries were performed by the senior author.
Measurement of component positioning
Patients underwent a postoperative CT at the 6-week follow-up, and the data were retrieved and analyzed. The primary study outcomes were extracted for analysis as previously described [6, 25].
-
Acetabular cup inclination (ACI)—Angle between the acetabular and longitudinal axes when projected onto the functional pelvic plane (FPP)
-
Acetabular cup version (ACV) —Angle between the acetabular axis and the FPP
-
Femoral offset (FO) —Difference between the hip centre of rotation (COR) of the operated joint relative to its starting position at the initial assessment on the coronal plane (medial-lateral) within the pelvic coordinate system
-
Leg length discrepancy (LLD)—Change in the distance between the greater trochanter tag and the hip COR added to the change in the distance between the centre of the acetabulum and the centre of the cup on the transverse plane (superior-inferior)
CT images were obtained pre- and postoperatively in DICOM (Digital Imaging and Communications in Medicine) format, and information relating to the diagnosis, study or surgery was removed for blinded analysis by an independent researcher.
Inclination and version of the acetabular cup were measured on DICOM files using online software (3D Slicer, www.slicer.org) [7] as previously described [6, 25]. FO and LLD were measured through the assessment of anatomical landmarks picked in pre- and postoperative scans. Coordinate systems for the pelvis and femur were determined based on the anatomic landmarks for the postoperative CT assessments. Parameters were expressed relative to the FPP, with the origin placed at the centre of the line connecting the left and right ASIS. The postoperative position of the cup centre was compared with the native hip COR determined from the preoperative CT. FO and LLD were reported as the pre-to-post change of the femoral coordinate frame relative to pelvis FPP coordinates on the coronal (mediolateral) and transverse (inferior-superior) planes respectively.
Data and statistical analysis
Missing data
Missing data were identified predominantly from intraoperative measurements where technical issues precluded retrieval of certain measurements within a case, but not all (exclusion criterion). Due to the low proportion of missing data <10%, case-wise deletion was performed to restrict analysis on each outcome measure.
Reliability
Intraobserver reliability for the image-based measures, including inclination and anteversion relative to the table orientation as well as cup position changes, was assessed using intraclass correlations, with a two-way random effects model on a sample of nine cases. Case identifier and observation (intra-observer) or observer (inter-observer) were considered random effects. The ratings were performed by the same observer on three occasions separated by a minimum of one week, with blinding to previous measures as well as measures by other observers and identified only by case identifier. Standard error of measurement was defined by the root mean square error from the repeated measures analysis of variance [32] with observation or observer as the repeated factor. Standard error of measurement describes the average deviation from one measurement to the next that can be attributed to random observer error.
Interobserver reliability for the image-based measures was derived from the median of the observations from the primary observer as well as singular observations from two additional observers who performed their measurements independently on the same sub-sample (N = 9). Intraclass correlation with two-way mixed-effects models was also calculated, and typical error measurement was calculated in the same manner as for interobserver reliability.
Agreement and bias correction
The calculation of agreement and bias assessment used the method described in Scholes et al. [25], and is summarized here for clarity. Mean deviation (delta) for each measure was calculated and summary statistics were generated using a bootstrapping approach and repeated with delta converted to absolute values. Bland-Altman plots were generated and limits of agreement were calculated by using a previously published formula [3, 8]. Bias was assessed using linear regression on each measure with adjustments for age, body mass index (BMI) and sex. Bias-corrected estimates of each measure were generated from regression model predictions. Alternative correction for offset and leg length change was performed by dropping values where an intraoperative declaration was made and the summary agreement analysis repeated as described above (Supplementary 1). All statistical analyses were performed using Stata (v17.1, StataCorp, College Station, TX, USA), with alpha set at 5% to indicate significant effects where appropriate.
Results
Patient characteristics
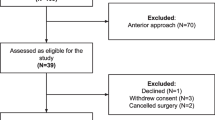
A consecutive sample of 54 primary cases was assessed for eligibility, with 34 included for analysis (Fig. 1). The analysis cohort comprised 42% females, had a mean age of 60.8 years (IQR 50.3–70) and a mean BMI of 29.6 (IQR 27.3–32.4) at surgery.
STROBE diagram [31] of patient inclusion into the study analysis
Imaging reliability
The imaging analysis demonstrated adequate reliability for both within and between observers for the measures of interest (Tables 1 and 2), with intra-observer standard error of measurement being <1° for cup anteversion and inclination and <1.1 mm for femoral offset and leg length changes (Table 3).
Agreement-intraoperative to imaging
Thresholds and declared observations
Intraoperative declarations were made for four patients, with loss of fixation of the tracker on the greater trochanter (n = 4). A number of patients were observed to exceed the specified measurement thresholds and had no intraoperative declarations (Table 4). Two patients included in the analysis had an intraoperative declaration recorded. However, their measurements did not exceed the threshold boundaries.
The mean delta (bias) between the intraoperative measurements and the postoperative imaging are summarized in (Table 5). The 95% limits of agreement for uncorrected data exceeded 10 degrees for inclination and version, and 10 mm for offset and LLD respectively (Table 5, Fig. 2). The linear fit of the average to the delta indicated that the bias between the navigation and the CT measurements was not constant across magnitude for inclination, version and offset (Fig. 2, Table 6). Overall, 91.2% of cases (95%CI 76.3–98.1) were within 10° of the image-measured measurements for both inclination and version (Fig. 3).
Factors associated with agreement and bias correction
The regression results indicated a significant magnitude-dependent bias for inclination (P = 0.014) and offset (P = 0.002) (Supplementary 2). Bias correction applied to the intraoperative measures removed overall bias and shrank the between-case variation (SD) of delta by 1%–20%, and by 22%–41% for absolute values (Table 7). Bias correction also shrank the mean absolute delta by 5%–35% relative to the uncorrected values. Conversely, by omitting declared observations for offset and leg length (Table 8), mean absolute error was not reduced and between-case variability increased by 5%–7%.
Discussion
The aim of this study was to report on the validity of an imageless navigation system (Naviswiss) for intraoperative measurement of THA component, in comparison with the three-dimensional (3D) reconstruction of computed tomography (CT) images as gold standard. The results identified reasonable accuracy for the metrics of interest with opportunities for further development identified.
The mean absolute deviation of acetabular inclination (3.3, 95%CI 2.1–4.5) between the navigation system and the CT-based analysis was comparable to the deviation reported by Hasegawa et al. in .2022 (2.8, 2.3–3.3) in the supine position, and by Scholes et al. [25] in the lateral decubitus position (3.6, 2.6–4.7), but it was higher than the pooled deviation (2.6, 2.4–2.8) for previous studies in the supine patient position using CT-based, imageless and accelerometry systems (Supplementary 3). In contrast, anteversion mean absolute deviation (4.3, 3.3–5.2) was greater than that reported by Hasegawa (2.8, 2.3–3.3) but comparable to pooled deviation for previous studies (3.6, 3.4–3.8). Overall, the mean bias was 1.9°–3.6° underestimation for cup orientation and up to 4 mm overestimation for leg length change, with 95% LOA at or below 11° for orientation and 6-11 mm for offset/leg length change. Absolute thresholds of 10° and 10 mm were established a priori in the study protocol [6] and were exceeded by 95% LOA, but this was reduced by bias correction to <10. Between-patient variation in published guidelines for cup orientation varies between 5 and 12° for inclination and up to 18° for version [10]. In general, less than 10 mm of LLD is considered acceptable after THA [18]. In addition, a simulation study [27] reported impingement and loss of motion range with a 4 mm medialization/lateralization of the cup, although this amount of change was not justified in their methods. The bias-corrected LOA in the present study suggests that 95% of the patient sample would fall within these tolerances for cup orientation, but not for offset. The findings are comparable to those reported by Scholes et al. in [25], who performed the same analysis with patients in the lateral decubitus position. A better understanding of the factors contributing to error in patients, the extremes of anthropometry and morphology, irrespective of surgical approach or patient position, may provide an important direction for further research.
In any validation study, a desire to explain deviations from true agreement is a natural progression of the analysis. In this study, a biphasic pattern of magnitude-dependent bias was observed for inclination (FPP), version and leg length change, and was also noted by Scholes et al. in [25]. The navigation system tended to overestimate smaller average measurements and underestimate larger averages (Fig. 2). While bias correction was able to re-centre the sample around zero and reduce between-patient variation, further work is needed to validate regression-based bias correction algorithms to mitigate the magnitude-dependency (slope) [25]. In addition, further work may be required to improve tracker fixation for offset and leg length with 4 cases (11.8%) declared intraoperatively for issues with fixation on the greater trochanter. In contrast to the study by Scholes et al. [25], the inclusion of these cases generated acceptable accuracy overall, while their omission worsened the between-case variability in accuracy and increased the LOA for both offset and leg length. A previous study [11], mentioned the potential vulnerability of the system to pin fixation on the iliac crest.
In the context of the magnitude-dependent bias of the system, the most realistic explanation for deviations from true agreement is a combination of imaging measurement error (all measures), dampening of the intraoperative measurements from a combination of soft tissue coverage and draping across key anatomical landmarks, as well as soft-tissue interference due to the proximity of the surgical incision (offset, leg length). Three-dimensional CT-based measurements are considered the gold standard when determining acetabular cup position and leg length postoperatively [2, 13, 24]. The present study, however, demonstrated lower reliability of the inter-observer imaging analysis with up to 1° for cup anteversion to 1.1 mm for LLD. In CT-based texture analysis, it has been demonstrated that the robustness, repeatability and reproducibility of measurements are sensitive to the scanner and scanning parameters [30]. The impact of radiological environment and context on postoperative measurements in THA has not been widely explored, but cannot be disregarded when comparing the results of different investigators and investigations. In addition, the selection of manual landmarks on CT datasets, while considered reliable, can still result in THA acetabular abduction and anteversion angle between-case variance of up to 2.5° [17]. In this study, the combination of scans performed on different scanners (patient access and convenience) and intra/inter-observer error may have contributed to the typical error of the imaging measurements. For example, the typical error for offset measurement was equal to 29% of the navigation absolute error. The present results, as well as others that have relied on CT/radiographic validation, should be interpreted with these findings in mind.
While the use of imageless navigation in the supine patient position is not as vulnerable to position changes as the lateral decubitus position [25], many of the issues raised by Scholes et al. [25] also apply to validation attempts in the supine position. Certainly, the mean BMI of the present sample is comparable to their study, while general approaches to draping and soft tissue distribution across key landmarks are almost identical. The replication of magnitude-dependent bias, as well as improved overall accuracy through bias correction in both studies suggests that intraoperative accuracy could be further improved by targeting potential measurement dampening. Anecdotally, these issues are exacerbated with increase in patient size and the amount of soft tissues distributed around the surgical field. A potential for interference between the soft tissue and the tracker fixed to the greater trochanter may be somewhat specific to the present study, however, due to the location of the anterolateral surgical incision. The incision created a segment of tissue flap that had a propensity to physically interact with the trochanter tracker without mitigation strategies in place. When higher BMI patients co-existed with a greater propensity for soft tissue distribution around this area, the tissue flap could cause unpredictable deviations in measurement through the course of the procedure. Of note were the attempts at mitigation that were implemented over the course of the trial by adjusting the incision location to reduce the amount of loose tissues, as well as taking additional steps to secure the flap and prevent abutment against the tracker itself. Further work is required to quantify the efficacy of these strategies.
The present study established that the system of interest was capable of providing comparable accuracy to similar systems in a broad range of patient populations, while identifying potential avenues of further development to achieve superior accuracy. Nevertheless, the findings and interpretations should be considered in light of the study’s limitations. Firstly, as discussed, variability in the imaging situation from one patient to another may have contributed to some of the errors observed in the imaging analysis. Clinical imaging in our region is referral-based, for the patient to organize a booking with a provider based on convenience and usually geographic location relative to their residence. While every attempt to standardize the imaging protocol was made with usual providers, some patients undertook their imaging studies outside this network. Despite the mitigation attempts, there is still the potential for variable imaging parameters due to different machines being used. Secondly, comparisons with the related literature remain problematic, and according to Scholes et al. [25], there are a range of different analytical techniques that have been used to describe the validity of the system intraoperatively, across a broad range of populations. However, the most problematic is the metric(s) used to report accuracy, which tend to use a summary of average deviation. Considering the findings of the present study, the assumption that error does not vary based on measurement magnitude clearly does not hold, although it is impossible to determine if this phenomenon has been observed elsewhere, as no other studies have employed Bland-Altman plots or similar techniques to describe this relationship. This makes an average deviation inappropriate for the description of average error and future work should report use of regression-based metrics. Thirdly, while this study did not represent the learning curve for the operating surgeon, the early part of the present series was characterized by the continued development of the system with the manufacturer, with a limited number of cases having to be abandoned entirely due to technical faults (e.g., system lockup, dropped tracker tags). This may have inserted a low level of selection bias into the series and maybe a consideration for clinical interpretation for those less experienced with imageless navigation in THA. Lastly, the current series was deliberately restricted to primary, relatively uncomplicated THA, which might limit its generalizability to more extreme morphological presentations (revision, tumor) where navigation may be of specific benefit. Further work is required to extend the validity of the system to this broader case mix.
Conclusion
The navigation system assessed in a primary THA cohort for end-stage hip osteoarthritis provided acceptable validity within clinical recommendations for cup orientation, femoral offset and leg length in the supine patient position. With additional attention to the proposed mechanisms for error identified in the present analysis, the application of the imageless system of interest to pathological anatomy in the context of challenging anthropometry, varying patient position and surgical approach, could become the standard for improving surgical outcomes in primary THA.
Availability of data and materials
Upon reasonable request to the authors, only de-identified data (i.e., data with sensitive and personal information removed), will be provided as per the ANDS Publishing and Sharing Sensitive Data Guide.
References
Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). (n.d.). Hip, Knee & Shoulder Arthroplasty 2022 Annual Report. AOA. https://aoanjrr.sahmri.com/documents/10180/732916/AOA+2022+AR+Digital/f63ed890-36d0-c4b3-2e0b-7b63e2071b16
Bayraktar V, Weber M, von Kunow F, Zeman F, Craiovan B, Renkawitz T, Grifka J, Woerner M. Accuracy of measuring acetabular cup position after total hip arthroplasty: comparison between a radiographic planning software and three-dimensional computed tomography. Int Orthop. 2017;41(4):731–8.
Bland JM, Martin Bland J, Altman DG. Measuring agreement in method comparison studies. In Statistical Methods Med Res. 1999;8(2):135–60. https://doi.org/10.1177/096228029900800204.
Bradley MP, Benson JR, Muir JM. Accuracy of acetabular component positioning using computer-assisted navigation in direct anterior total hip arthroplasty. Cureus. 2019;11(4):e4478.
Domb BG, Redmond JM, Louis SS, Alden KJ, Daley RJ, LaReau JM, Petrakos AE, Gui C, Suarez-Ahedo C. Accuracy of component positioning in 1980 total hip arthroplasties: a comparative analysis by surgical technique and mode of guidance. J Arthroplasty. 2015;30(12):2208–18.
Ektas N, Scholes C, Ruiz AM, Ireland J. Validity of intraoperative imageless navigation (Naviswiss) for component positioning accuracy in primary total hip arthroplasty: protocol for a prospective observational cohort study in a single-surgeon practice. BMJ Open. 2020;10(10):e037126.
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magnetic Resonance Imaging. 2012;30(9):1323–41.
Giavarina D. Understanding Bland Altman analysis. Biochem Med. 2015;25(2):141–51.
Grosso P, Snider M, Muir JM. A smart tool for intraoperative leg length targeting in total hip arthroplasty: a retrospective cohort study. Open Orthop J. 2016;10(1):490–9.
Harrison CL, Thomson AI, Cutts S, Rowe PJ, Riches PE. Research synthesis of recommended acetabular cup orientations for total hip arthroplasty. J Arthroplasty. 2014;29(2):377–82.
Hasegawa M, Naito Y, Tone S, Sudo A. Accuracy of a novel accelerometer-based navigation (Naviswiss) for total hip arthroplasty in the supine position. BMC Musculoskeletal Disorders. 2022;23(1):537.
Hayashi S, Hashimoto S, Takayama K, Matsumoto T, Kamenaga T, Fujishiro T, Hiranaka T, Niikura T, Kuroda R. Evaluation of the accuracy of acetabular cup orientation using the accelerometer-based portable navigation system. J Orthop Sci. 2019;25(4):612–7.
Kjellberg M, Al-Amiry B, Englund E, Sjödén GO, Sayed-Noor AS. Measurement of leg length discrepancy after total hip arthroplasty. The reliability of a plain radiographic method compared to CT-scanogram. Skeletal Radiol. 2012;41(2):187–91.
Kumar V, Patel S, Baburaj V, Rajnish R. K., & Aggarwal S. Does robotic-assisted surgery improve outcomes of total hip arthroplasty compared to manual technique? a systematic review and meta-analysis. Postgraduate Med J. 2021. https://doi.org/10.1136/postgradmedj-2021-141135
Kunze KN, Bovonratwet P, Polce EM, Paul K, Sculco PK. Comparison of surgical time, short-term adverse events, and implant placement accuracy between manual, robotic-assisted, and computer-navigated total hip arthroplasty: a network meta-analysis of randomized controlled trials. J Am Acad Orthop Surg Glob Res Rev. 2022;6(4):e21.00200. https://doi.org/10.5435/JAAOSGlobal-D-21-00200.
Liebs TR, Nasser L, Herzberg W, Rüther W, Hassenpflug J. The influence of femoral offset on health-related quality of life after total hip replacement. Bone Joint J. 2014;96-B(1):36–42.
Lubovsky O, Peleg E, Joskowicz L, Liebergall M, Khoury A. Acetabular orientation variability and symmetry based on CT scans of adults. Int J Comput Assist Radiol Surg. 2010;5(5):449–54.
McWilliams AB, Grainger AJ, O’Connor PJ, Redmond AC, Stewart TD, Stone MH. A review of symptomatic leg length inequality following total hip arthroplasty. Hip Int. 2013;23(1):6–14.
Migliorini F, Cuozzo F, Oliva F, Eschweiler J, Hildebrand F, Maffulli N. Imageless navigation for primary total hip arthroplasty: a meta-analysis study. J Orthop Traumatol. 2022;23(1):21.
Ong CB, Chiu Y.-F, Premkumar A, & Gonzalez Della Valle A. Use of a novel imageless navigation system reduced fluoroscopy exposure and improved acetabular positioning in anterior approach total hip arthroplasty: a case-control study. Archives of Orthopaedic and Traumatic Surgery. Archiv Fur Orthopadische Und Unfall-Chirurgie. 2022. https://doi.org/10.1007/s00402-022-04520-3.
Pai S. Medico-legal issues related to hip and knee arthroplasty: a literature review including the Indian Scenario. Indian J Orthop. 2021;55(5):1286–94.
Renkawitz T, Weber T, Dullien S, Woerner M, Dendorfer S, Grifka J, Weber M. Leg length and offset differences above 5mm after total hip arthroplasty are associated with altered gait kinematics. Gait Posture. 2016;49:196–201.
Renner L, Janz V, Perka C, Wassilew GI. What do we get from navigation in primary THA? EFORT Open Rev. 2017;1(5):205–10.
Sariali E, Mueller M, Klouche S. A higher reliability with a computed tomography scan-based three dimensional technique than with a two dimensional measurement for lower limb discrepancy in total hip arthroplasty planning. Int Orthop. 2021;45(12):3129–37.
Scholes C, Fatima M, Schwagli T,Liu D. Imageless navigation system (Naviswiss) provides accurate component position in total hip arthroplasty with lateral decubitus position for end-stage hip osteoarthritis: A prospective cohort study with CT-validation. In medRxiv. 2023:2023.06.05.23289691. https://doi.org/10.1101/2023.06.05.23289691.
Shigemura T, Baba Y, Murata Y, Yamamoto Y, Shiratani Y, Hamano H, Wada Y. Is a portable accelerometer-based navigation system useful in total hip arthroplasty?: a systematic review and meta-analysis. Orthop Traumatol Surg Res. 2021;107(1):102742.
Shoji T, Yamasaki T, Izumi S, Kenji M, Sawa M, Yasunaga Y, Adachi N. The effect of cup medialization and lateralization on hip range of motion in total hip arthroplasty. Clin Biomechanics. 2018;57:121–8.
Singh V, Realyvasquez J, Simcox T, Rozell JC, Schwarzkopf R, Davidovitch RI. Robotics versus navigation versus conventional total hip arthroplasty: does the use of technology yield superior outcomes? J Arthroplasty. 2021;36(8):2801–7.
Tanaka R, Shigematsu M, Motooka T, Mawatari M, Hotokebuchi T. Factors influencing the improvement of gait ability after total hip arthroplasty. J Arthroplasty. 2010;25(6):982–5.
Varghese BA, Hwang D, Cen SY, Levy J, Liu D, Lau C, Rivas M, Desai B, Goodenough DJ, Duddalwar VA. Reliability of CT-based texture features: Phantom study. J Appl Clin Med Phys. 2019;20(8):155–63.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Medicine. 2007;4(10): e296.
Weir JP, Vincent WJ. Statistics in Kinesiology (5th ed.). Human Kinetics. 2020
Acknowledgements
The authors wish to acknowledge the important efforts of the staff of Sydney Bone and Joint Clinic, as well as Active Surgical (Ashley Pienaar and Francois Pienaar) in data collection for the study. In addition, the efforts of Milad Ebrahimi and Nalan Ektas (EBM Analytics) in assisting with data collection, data checking and analysis, and Manaal Fatima and Meredith Harrison-Brown (EBM Analytics) in assisting with the manuscript preparation are acknowledged.
Funding
This work was sponsored by Naviswiss AG.
Author information
Authors and Affiliations
Contributions
C.S. was responsible for the study design, statistical analysis, and interpretation of data for the study. T.S. performed the image analysis. J.I. was responsible for clinical data collection and providing stewardship to the study. All authors were involved in either drafting the manuscript or providing critical review with technical or clinical input and agreement for final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was embedded within a prospective observational clinical registry, for which ethical approval was obtained from a National Health and Medical Research Council certified human research and ethics committee (HREC) (Bellberry; HREC 2017-07-499). The study was also registered with the Australian New Zealand Clinical Trials Registry (ACTRN12618000317291).
Consent for publication
Not applicable
Competing interests
C.S. is employed by EBM Analytics, which was contracted by Naviwsiss AG to assist with the completion of a protocol manuscript and data analysis for this and another study of the same device. C.S. is a shareholder in EBM Analytics. J.I. is a shareholder in Naviswiss AG. T.S.’s employer, Medivation, was contracted by Naviswiss AG to assist with the completion of the current study. T.S. additionally declares stock in Naviswiss AG.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary material 1. Code
Additional file 2:
Supplementary material 2. Regression model results summary
Additional file 3:
Supplementary material 3. Summary of validation findings
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scholes, C., Schwagli, T. & Ireland, J. CT validation of intraoperative imageless navigation (Naviswiss) for component positioning accuracy in primary total hip arthroplasty in supine patient position: a prospective observational cohort study in a single-surgeon practice. Arthroplasty 5, 63 (2023). https://doi.org/10.1186/s42836-023-00217-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42836-023-00217-z