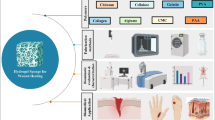
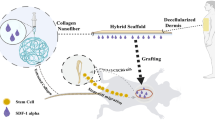
Abstract
The objective of regenerative wound healing dressings is to accelerate skin tissue regeneration and restore normal physiological function at wound sites. Achieving this goal requires biomaterials capable of repairing distinct phases of wound healing in a way that balances material function, degradation, safety, and tissue growth. In this study, we introduced a novel dual-stage wound dressing system comprising methacrylic anhydride-modified recombinant humanized type III collagen (rhCol III-MA) and methacrylic anhydride-modified dopamine (DMA) (RMDM), which was synthesized through free radical polymerization and π-π stacking. Within this system, RMDM was formulated into two forms with identical compositions: hydrogel and sponge, tailored for application across various stages of wound repair. These materials displayed favorable hemocompatibility, biocompatibility, antioxidant properties, and angiogenic potential in vitro. Moreover, the in vivo experiments also demonstrated that sponges could rapidly stop the bleeding of wounds in mouse tail amputation and liver incision models. Notably, the sponge/gel (S/G) system accelerated wound healing compared to individual sponge and gel treatments in a rat full-thickness skin wound model, underscoring the synergistic benefits of combining sponge and gel materials for wound repair at different stages. Therefore, this research provides valuable insights into designing advanced biomaterials that can be tailored to specific stages of wound healing, which may have significant potential for biomedical applications.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Skin wound repair is a complex physiological process involving a cascade of cellular and molecular events toward reinstating tissue integrity and functionality [1,2,3]. Various wound dressings have been devised to facilitate wound healing [4,5,6,7]. While traditional options like gauze and bandages afford physical protection, their tendency to adhere firmly to newly regenerating tissue frequently leads to accidental damage during dressing changes [8]. Furthermore, these dressings lack regenerative capacity. Sponges incorporating chitosan, collagen, or gelatin have gained widespread usage owing to their favorable attributes of biocompatibility, biodegradability, and hemostatic properties [9,10,11]. However, many of these products exhibit limited efficacy in regulating specific phases of wound healing. Recently, hydrogels have emerged as promising alternatives for delivering antibacterial agents, growth factors, or therapeutic stem cells to bolster wound healing [12]. Nevertheless, challenges such as uncontrolled drug release, drug-related adverse effects, intricate fabrication processes, and high costs constrain their widespread adoption [13, 14]. Hence, there is a pressing need for the development of advanced wound dressings in furnishing enduring assistance and fostering healing across the diverse phases of restoration.
Hemorrhage often accompanies traumatic injuries, significantly elevating the risk of prehospital mortality due to complications such as coagulopathy and multiple organ failure. Mitigating blood loss stands as paramount in acute trauma management [15,16,17]. An optimal hemostatic material is characterized by robust wet-tissue adhesion, [18] adequate mechanical strength to withstand blood pressure, enhanced red blood cell aggregation, and expedited wound healing capabilities [19]. Mussels possess remarkable adhesive properties attributed to the catechol side chains of dopamine (DOPA), enabling diverse chemical interactions and crosslinking with various substrates in aquatic environments [20]. However, existing polymeric N-hydroxysuccinimide (NHS) ester- and 3,4-dihydroxyphenylalanine (DOPA)-based bio-glues necessitate intricate bicomponent formulations or laborious multistep processes, such as pre-gelling, polymer infiltration, group activation, and pressure bonding [21]. Moreover, exposure to air can induce structural alterations like oxidation and degradation, [22] leading to diminished adhesion, rendering these bioadhesives impractical for routine clinical application. Drawing inspiration from the efficacy of light-curing resin-based technologies in dentistry for tooth fillings, we hypothesized that photoinitiated crosslinking hydrogel adhesives with precise controllability could offer a promising solution for skin repair [23].
The wound healing process involves debridement, collagen deposition, granulation tissue formation, angiogenesis, and epidermal regeneration [24,25,26,27,28]. Hence, selecting an ideal tissue-inductive biomaterial for wound repair is crucial. Among the various biomaterials investigated, collagen has garnered significant attention owing to its biocompatibility, bioactivity, and ability to mimic the native extracellular matrix (ECM) of the skin [29, 30]. Traditional collagen sources, primarily derived from pig skin and bovine tendons, are associated with risks of viral contamination, impurities, and immunogenicity [31, 32]. With advancements in synthetic biology, recombinant humanized type III collagen (rhCol III) has been synthesized utilizing DNA recombination technology based on the human type III collagen gene sequence [33, 34]. This synthetic offers several advantages, including identical functional regions to human type III collagen, [22, 35] defined conformation, homogeneity, and elimination of zoonotic viral risks [36]. Studies conducted by Wu et al. have revealed that a single-component, drug-free coating scaffold utilizing rhCol III, when implanted into injured vascular tissue, yields multiple reparative effects such as anti-inflammatory properties and accelerated endothelial healing [37]. Similarly, Wang et al. have demonstrated the excellent capability of rhCol III in promoting ECM deposition and facilitating collagen deposition on damaged skin induced by UV-photoaging [38]. Therefore, biomaterials based on rhCol III represent an ideal choice for wound repair applications.
In this study, we aimed to develop a dual-stage wound dressing system comprising rhCol III-MA and DMA (RMDM) through a free radical polymerization reaction and π-π stacking for enhanced skin wound repair (Scheme 1). Initially, we characterized the RMDM materials and investigated their angiogenic capacity in vitro, as well as other biological effects. Specifically, we focused on assessing the bioactivity and therapeutic efficacy of RMDM materials in rat full-thickness skin wounds. Our approach capitalized on the microcellular structure and adhesive properties of RMDM, facilitating the effective hemostasis and immobilization of the biomaterial at the wound site during the initial phases of repair. Furthermore, we intended to sustain hydration and promote cellular infiltration, as well as wound healing during the later stages by switching from sponge to hydrogel as healing progressed. By combining the properties of RMDM sponge with RMDM gel in a dual-stage strategy, a bioactive and robust scaffold for enhanced wound healing was established. Modulating the physical and biochemical properties of the material to align with the dynamic requirements of the wound microenvironment, our aim is to establish a versatile platform for treating various types of skin injuries.
2 Materials and methods
2.1 Materials
Recombinant humanized type III collagen (Mw = 25.564 kDa, the detailed amino acid sequence was provided in Table S1) was procured from Jiangsu Trautec Medical Technology Co., Ltd (Jiangsu, China). Dopamine hydrochloride (DOPA, 99%) was purchased from Best Reagent Corporation (Chengdu, China). α-MEM medium (Hyclone) was purchased by Thermo Fisher Scientific Corporation (USA). Methacrylic anhydride (MA, 99%), as well as phosphinate (LAP, ≥ 98.0%), were both sourced from Aladdin Bio-Chem. The standard gold medal substrate gel was purchased from ABW-bio.
2.2 Synthesis and characterization of rhCol III-MA
RhCol III-MA was synthesized using the previously established method by our research group [35]. Briefly, 1 g of rhCol III was dissolved in 7 mL of Na2CO3 and NaHCO3 buffer solution. Then, 0.1 mL of methyl acrylate (MA) was added to the rhCol III solution (40 °C, 4 h). The resulting solution was dialyzed in ultrapure water at 25 °C for 3 days to obtain rhCol III-MA solution. Finally, rhCol III-MA was freeze-dried. RhCol III-MA was dissolved in D2O, and 1H nuclear magnetic resonance (1H NMR) of rhCol III-MA was recorded at 25 °C (AVANCE III 400 MHz, Bruker). FTIR spectroscopy was conducted using a Thermo Nicolet iS5 spectrometer (Thermo Company, USA), recording transmittance mode spectra from 4000 cm−1 to 400 cm−1.
2.3 Synthesis and characterization of DMA
10 g of sodium borate and 4 g of sodium bicarbonate were dissolved in 100 mL of deionized water. Then, 5 g of dopamine hydrochloride was added and completely dissolved. After that, a mixture of MA and tetrahydrofuran (4.7 mL and 25 mL, respectively) was added, and the reaction was carried out at 25 °C for 12 h (under anaerobic conditions). Subsequently, the DMA solution was extracted with 50 mL of ethyl acetate (pH: 2–3), dried using anhydrous magnesium sulfate, and concentrated to 25 mL. Then, 250 mL of ethyl ether was added and stirred slowly. The mixture was placed in a refrigerator at 4 °C for 12 h, filtered to obtain the precipitate, and vacuum-dried for 24 h. Using DMSO as the solvent, the 1H NMR of DMA was recorded at 25 °C (AVANCE III 400 MHz, Bruker). FTIR spectroscopy was conducted using a Thermo Nicolet iS5 spectrometer (Thermo Company, USA), recording transmittance mode spectra from 4000 cm−1 to 400 cm−1.
2.4 Preparation of RMDM materials
RhCol III-MA, DMA, and LAP were mixed in phosphate-buffered saline (PBS). RMDM materials were formed at 25 °C under UV (365 nm) illumination for 1 min, resulting in gel formation. A portion was directly applied (defined as the gel group), while another portion was freeze-dried to form the sponge group (Table 1).
2.5 Scanning electron microscopy (SEM)
The gel group underwent gradient ethanol dehydration followed by critical point drying (CPD, EMCPD300, Germany). Subsequently, both groups of samples were fractured using liquid nitrogen, coated with a thin layer of gold, and examined for morphology using a field emission scanning electron microscope (FESEM, QUTAN FEG 250, FEI). After subjecting the RMDM material to multiple external force compressions, SEM observation was carried out following the aforementioned steps (Applied a force of 1N, achieving a compressive strain of 0.5, and repeated it 10 times). The diameter of the sponge group was measured using Image J software.
2.6 Swelling test
The swelling test was conducted to determine the equilibrium swelling ratio (ESR). Gel and sponge samples of equal volumes were separately immersed in PBS until reaching swelling equilibrium. Subsequently, the samples were removed, excess water was drained, and the samples were weighed after drying. ESR was calculated using the formula:
where Wx and Wy represent the weights of the samples in the swollen and dried states, respectively. The experiment was repeated three times for accuracy.
2.7 In vitro degradation experiment
For the in vitro degradation experiment, gel and sponge samples of equal volumes were immersed in 10 mL PBS (pH = 7.4) and incubated at 37 °C with agitation at 80 rpm. At predetermined time points, the samples were removed, rinsed with deionized water to remove excess salt, blotted to remove excess water, and weighed. The mass remaining ratio (%) of the samples was defined using the following equation:
where Wt and W0 are the wet weights of the remaining samples at different time points after degradation and the initial wet weight of the samples, respectively.
2.8 Rheological and compression tests
The rheological properties of the wound dressings were tested using a rheometer (DHR-2). During the test, 500 μL of RMDM material was placed between parallel plates with a diameter of 20 mm. Time sweep experiments were conducted at 37 °C under constant conditions of 1 Hz frequency and 1% strain. Additionally, cylindrical samples (approximately 3 mm high × 7 mm diameter) were prepared and subjected to compression testing at the same temperature. Compression tests were performed using a universal mechanical testing machine (Shimadzu, EZLX1kN, Japan) at a speed of 1 mm/min until the samples were crushed, obtaining stress–strain curves. The elastic modulus was the slope of the stress–strain curve (first 5–15% of strain). The compressive strength was the highest point on the stress–strain curve (first 5–15% of strain) [39].
2.9 Adhesion strength test
The adhesion strength of wound dressings was assessed using lap shear tests. Pig skin was cut into strips measuring 20 mm × 8 mm. Twenty microliters of precursor solution were applied to one end of the pig skin, and another piece of pig skin was overlaid, creating a bonding area of approximately 8 mm × 10 mm. They were then exposed to UV light for 3 min, causing gelation between the two pieces of pig skin. The lap shear tests of the UV-cured samples were conducted using a universal mechanical testing machine equipped with a 100 N load at a rate of 1 mm/min. For sponge dressings, two pieces of pig skin were moistened with water, and the sponge samples were placed between them. Placing a substance weighing 100 g in the overlapping region for 60 min, then performing lap-shear tests using a universal testing machine equipped with a 100 N load cell at a rate of 1 mm/min. All tests were performed at least three times.
2.10 Antioxidant experiment
To evaluate the ability of wound dressing materials to scavenge DPPH radicals, gel and sponge materials were prepared as described in Sect. 2.4. Volumes of 60 μL and 120 μL of the prepared gel and sponge materials were taken, and likewise for the control group (Ultra-pure water). Subsequently, the three groups of samples were each immersed in PBS solution for 1 h, followed by immersion in 1 mL of ethanol. Then, 120 μL of 0.5 mM DPPH solution was added to this solution. The mixture was then incubated in the dark for 60 min. Afterward, absorbance at 517 nm was measured. The DPPH radical scavenging rate was calculated using the formula:
where ODc and ODs represent the absorbance of the control group and the sample, respectively.
2.11 Cell compatibility evaluation
Firstly, rhCol III-MA, DMA, and sponge samples were sterilized under ultraviolet radiation. LAP solution was filtered and sterilized through a 0.22 μm filter (Millipore). Then, sterilized raw materials were dissolved, following the steps described in Sect. 2.4 to prepare gel and sponge samples. The complete growth medium consisted of Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (Gibco), 1.0 × 10^5 U/L penicillin (Hyclone), and 100 mg/L streptomycin (Hyclone). L929 fibroblast cells (L929, Chinese Academy of Sciences Cell Bank) were cultured at a density of 5 × 10^3 cells/mL in 24-well plates and co-cultured with RMDM materials for 1 and 7 days. Cell proliferation and viability were assessed using live/dead staining and the CCK-8 assay.
2.12 Cell migration
To assess cell migration ability, the scratch assay method was employed. Gel and sponge group materials were prepared according to the method described in Sect. 2.4, with three samples for each group. Subsequently, 6 mL of DMEM medium was added separately to each type of material, and they were co-incubated at 37 °C with agitation at 80 rpm for 24 h to obtain two sets of extracts.
A total of 2 × 10^5 L929 cells were cultured in a 24-well plate until reaching 80% confluence, at which point scratches were made. The control group was cultured in a serum-free medium, while the gel and sponge groups were cultured with the corresponding extracts. The migration rate was calculated using the formula:
where Am represented the initial scratch area and Ax represented the scratch area after 24 h.
2.13 Angiogenesis analysis
The angiogenic capability was assessed using human umbilical vein endothelial cells (HUVECs). Gel and sponge group materials were prepared according to the method described in Sect. 2.4, with three samples for each group. Subsequently, 6 mL of DMEM medium was added separately to each type of material, and they were co-incubated at 37 °C with agitation at 80 rpm for 24 h to obtain two sets of extracts. HUVECs (3–4 × 10^4 cells) were seeded in 96-well plates coated with matrix gel (50 µL/well) and cultured. The control group was cultured in a serum-free medium, while the gel and sponge groups were cultured with the corresponding extracts. Cell fluorescence staining was performed upon vascular formation, followed by confocal laser imaging. Image J software was then used for image analysis.
2.14 Hemolysis experiment
Sterile heparin sodium blood collection tubes were used to collect arterial blood from rabbit ears, which were centrifuged for 10 min (2040 rpm) to obtain plasma rich in red blood cells. The plasma was diluted to 5% (v/v), and RMDM materials were uniformly crushed and diluted in PBS buffer to concentrations of 15, 12.5, 10, 7.5, and 5 mg/mL. The diluted liquids were mixed with plasma, incubated at 37 °C for 1 h, and then centrifuged for 10 min (10,000 rpm) to measure the absorbance of the supernatant (A) at 540 nm. 0.1% Triton X-100 was used as a positive control (Am), and PBS as a negative control (An). Commercial gelatin sponge (Gelatin) and RMDM materials were used for comparison. Each sample was tested three times, and the hemolysis rate was calculated using the formula:
2.15 In vitro coagulation experiment
A 0.05 mL (0.1 M) CaCl2 solution was added to 5 mL of rabbit anticoagulant whole blood. After mixing, 40 μL of the mixture was respectively dropped onto the gel group and sponge group. After incubation at 37 °C for 1 min, the blood clots were slowly washed with 10 mL of deionized water. After 30 min, the absorbance of the wash solution was measured at 540 nm and recorded as A. Gelatin was used as a reference. The absorbance of 40 μL CaCl2 solution dropped into 10 mL deionized water was recorded as A0, and the absorbance of 40 μL whole blood dropped into 10 mL deionized water was recorded as Ax. Each group was tested three times. The clotting rate was calculated using the following formula:
2.16 Hemostasis experiment: rat tail bleeding model
After approval by the Ethics Committee of Sichuan University, we conducted experiments using 12 twelve-week-old female SD rats (200–220 g). The rats were randomly divided into 4 groups. After isoflurane anesthesia, their tails were cut to 6 cm in length using sterile surgical scissors. They were then exposed to air for 10 s to ensure normal bleeding. The sponge group and gelatin group covered and lightly pressed the wounds, while the gel group had uncured liquid dripped around the wounds and gelled by UV (365 nm) light exposure. We recorded the amount of bleeding and the time during the hemostasis process. Each experiment was conducted three times, with the untreated group serving as the control group.
2.17 Hemostasis experiment: rat liver bleeding model
After approval by the Ethics Committee of Sichuan University, we conducted experiments using 12 twelve-week-old female SD rats. The rats were randomly divided into 4 groups, and then sterile surgical instruments were used to locate the rats' livers. After clearing the tissue fluid and skin bleeding around the liver, we made a 3–4 mm incision along the edge of the liver using surgical scissors. The sponge group and gelatin group covered the wounds and lightly pressed them, while the gel group had uncured liquid dripped around the wounds and gelled by UV (365 nm) light exposure. We recorded the amount of bleeding and the time during the hemostasis process. Each experiment was conducted three times, with the untreated group serving as the control group.
2.18 Animal experiment
To further evaluate the wound healing-promoting effect of wound dressings, a full-thickness skin defect model was established with approval from the Sichuan University Medical Ethics Committee (20,220,728,001). Eight male SD rats (200-220 g) were randomly divided into control, sponge, gel, and S/G groups, with 4 wounds per rat and a total of 8 wounds per group. After anesthesia with isoflurane, the backs of the rats were shaved, and circular skin defects with a diameter of 10 mm were created using a punch. After modeling, the control group received no RMDM material treatment; instead, 3 M dressing was used for fixation. The sponge group was sponge-based wound dressings applied to cover the wounds. The gel group had 0.2 mL of precursor solution injected into the exposed tissue at the wound site, followed by gelation upon light exposure within 1 min. According to existing research, the hemostatic and inflammatory phases characterized the initial 48 h of wound healing. After three days, inflammation significantly decreased, and granulation tissue began to form on the fourth day, indicating the onset of the proliferation and remodeling phases. Therefore, we chose day 3 as the critical time point for replacing the sponge group materials with gel group materials (S/G group) [4, 40]. All wounds were dressed and secured using 3 M sterile dressings, with wound dressings being changed every 2 days. Wound healing was observed immediately after wound formation and treatments, as well as at the 7th and 14th day post-treatment. The wound healing percentage at each time point was analyzed using Image J software.
Wound healing percentage = (1-area of unhealed wound / original wound area) × 100%.
2.19 In vivo wound healing evaluation
Histological staining was used to assess wound tissue repair. Rats were euthanized on days 7 and 14 post-injury, and skin tissues were fixed in 4% paraformaldehyde for three days, embedded in paraffin, and sectioned for H&E and Sirius Red staining.
2.20 Statistical analysis
Data are expressed as mean ± standard deviation (SD) of at least three independent tests. Analysis of variance (ANOVA) and T-tests were used for analytical evaluations. Data analysis was performed using SPSS 22.0 software.
3 Results and discussion
3.1 Preparation and characterization of RMDM materials
To fabricate an RMDM material, we synthesized two polymers, rhCol III-MA and DMA, as illustrated in Fig. 1A and B, respectively. Proton nuclear magnetic resonance (1H-NMR) spectroscopy was employed to characterize rhCol III-MA and DMA and ascertain successful synthesis. As depicted in Fig. 1C and D, the 1H-NMR spectra of rhCol III-MA and DMA exhibited prominent resonance peaks at 5–6 ppm, indicative of methacrylate functionalities, with degrees of substitution approximately at 67% and 28%, respectively. The DOPA showed characteristic peaks at 1618 cm−1 (amide I), 1502 cm−1 (amide II), and 1284 cm−1 (amide III). After successful grafting, peaks at 3364 cm−1 and 3189 cm−1 were attributed to amino and phenolic hydroxyl group stretching vibrations, while 1652 cm−1 and 1588 cm−1 corresponded to amide (-N–C = O) and C = C bond stretching vibrations, respectively (Fig. S1A). The FTIR spectrum revealed distinctive signals, including the C = O stretching vibrations of Amide I at 1627 cm−1, N–H deformation vibrations of Amide II at 1542 cm−1, and C = C-H in-plane bending vibrations at 1053 cm−1. This confirmed the occurrence of successfully introducing the C = C double bond (Fig. S1B). These findings collectively signify the effective synthesis of rhCol III-MA and DMA. As depicted in Fig. 1E, upon exposure to ultraviolet irradiation, the material transitioned from a flowable solution to an immobile gel state, primarily attributed to rhCol III-MA undergoing free radical polymerization and DMA engaging in π-π stacking, culminating in the formation of a hydrogel. Scanning electron microscopy analysis and macrostructural evaluation (Figs. 1F and S2) demonstrated that the gel group displayed a surface devoid of discernible pore distribution. In contrast, the sponge group's surface and cross-section revealed a micro-porous architecture, with pore dimensions predominantly ranging from 16.19 ± 0.6618 μm. No noticeable collapse or deformation was observed in the pore diameter of RMDM material after multiple external forces were applied, indicating that the material possessed appropriate mechanical properties and demonstrated a certain ability to resist tissue extrusion in clinical applications (Fig. 1G). Notably, the sponge group, subjected to freeze-drying treatment, exhibited a well-defined porous structure compared to the gel group. The enhanced porous architecture of the sponge may potentially facilitate blood coagulation in wound regions relative to gel materials, in addition to promoting the absorption of wound exudates and facilitating nutrient exchange.
A The synthesis route of rhCol III-MA. B The synthesis route of DMA. C 1H-NMR (D2O) spectra of rhCol III-MA. D 1H-NMR (DMSO) spectra of DMA. E RMDM materials gelling process diagram and microstructure diagram. F SEM observation of RMDM materials. G SEM observation of RMDM materials after multiple external forces. N = 3, *** P < 0.001
The investigation of swelling and degradation properties holds significant importance for evaluating hydrogels, given their implications for stability both in vitro and within biological tissues. As depicted in Fig. 2A, the equilibrium swelling ratio of the distinct material forms was assessed. It was observed that the sponge group exhibited a remarkable capacity to imbibe liquid, equivalent to approximately 10 times its weight, whereas the gel group displayed a considerably lower capacity, absorbing liquid equivalent to approximately 0.5 times its weight. This discrepancy underscored the superior swelling performance of the sponge group, likely enhancing its efficacy in wound exudate absorption.
Figure 2B presented the in vitro degradation kinetics of the two material groups. Initially, both the sponge and gel groups maintained relatively high solid content over the first 3 days, followed by complete degradation occurring by the 6th to 7th day. Notably, the degradation rate of the sponge group surpassed that of the gel group within the initial 3-day period, thereafter converging to similar degradation rates. These findings underscored the excellent biodegradability of the proposed wound dressing material.
Rheometry and Dynamic Mechanical Analysis (DMA) were employed for a comprehensive evaluation to discern the variances in mechanical properties between the two material groups. As depicted in Fig. 2C, the hydrogel exhibited a higher storage modulus (G') than the loss modulus (G''), indicative of its tendency towards the characteristics of an elastic solid. Notably, the sponge material demonstrated superior mechanical attributes, featuring a higher elastic modulus (2.36 MPa) and compressive strength (574 kPa) compared to the gel group, which registered an elastic modulus of 17.6 kPa and a compressive strength of 163 kPa. These findings underscored the appropriate mechanical performance of the RMDM materials, showcasing their capacity to withstand external pressures, thereby mitigating the risk of further wound damage and minimizing the potential for wound exposure attributable to material abrasion.
Optimal adhesion is paramount for wound dressings to securely adhere to the skin surface, thereby mitigating detachment risks under external forces and subsequently reducing the likelihood of wound infection. As illustrated in Fig. 2E, the tissue adhesion strength of the wound dressings was evaluated, revealing a significantly higher adhesion strength for the gel group (8.305 ± 2.070 kPa) compared to the sponge group (2.462 ± 0.4261 kPa). The interface bonding between materials and tissues primarily stemmed from forming multiple hydrogen bonds between moieties, such as catechol hydroxyl groups within the material and amine or hydroxyl groups on the tissue surface, thereby augmenting tissue adhesion [42, 43]. Additionally, the diminished adhesive strength observed in the sponge group may be attributed to structural alterations induced by freeze-drying. The porous nature of sponge-based materials reduced the contact area between the dressing and tissue surface, thereby diminishing the engagement of DOPA hydroxyl groups and consequently reducing adhesive strength. Conversely, gel-based formulations typically exhibited heightened adhesive capabilities due to their structural characteristics, facilitating the uniform distribution of water molecules and other constituents. This enabled a larger contact area between DOPA hydroxyl groups and tissue, thereby enhancing adhesion to the tissue surface.
Early skin injuries often trigger the secretion of reactive oxygen species (ROS), which can impede the wound healing process. Materials possessing antioxidant properties play a crucial role in ROS reduction, thereby facilitating wound repair. Figure 2F presented the outcomes of antioxidant assessments conducted on the wound dressings. When the total material volume was 60 μL, the DPPH scavenging rates for the gel and sponge groups were recorded at 52.3 ± 1.415% and 46.90 ± 2.512%, respectively. Upon increasing the total material volume to 120 μL, the DPPH scavenging rates for the gel and sponge groups rose to 89.8 ± 1.076% and 89.2 ± 0.4787%, respectively, indicating an escalation in DPPH scavenging rates with increased dressing volume. Furthermore, the findings suggested a positive correlation between material quantity and DPPH scavenging capacity at the wound site. Dressings with a volume of 120 μL may potentially offer enhanced therapeutic efficacy. Consequently, the wound dressings in our investigation exhibited commendable antioxidative attributes, facilitating the removal of excess ROS generated during the inflammatory phase and contributing to anti-inflammatory effects, ultimately expediting wound healing.
3.2 Cell viability, migration and tube formation of RMDM materials
Considering the resemblance of RMDM materials to the extracellular matrix (ECM) in both structure and biological function, an initial assessment of their biological activity was conducted at the cellular level. The cytotoxicity of RMDM materials was evaluated by coculturing L929 cells with the materials. As depicted in Fig. 3A, L929 cells exhibited widespread distribution at the well bottom, displaying viability indicated by the abundance of green fluorescence dots throughout the fluorescence images by live/dead assay. Quantitative assessment of cell viability using cholecystokinin-8 (CCK-8) assay, Fig. 3B revealed a gradual increase in optical density (OD) values from day 1 to day 7, indicative of continuous cell proliferation. These findings collectively affirmed the non-toxic nature of RMDM materials toward L929 cells.
A Representative fluorescence images for L929 cells cultured over the leach liquor of RMDM materials after incubation for 1 and 7 days. Scale bar, 100 μm. B Relative viability of L929 cells inoculated on the leach liquor of RMDM materials. C The migration of L929 cells in the culture plate under the leach liquor of RMDM materials. Scale bar, 200 μm. D Quantification of migration ratio for L929 cells. E Tube formation of HUVECs cultured over the leach liquor of RMDM materials (The area within the pink dashed lines represents the area of the neovascular). F Quantification of neovascular area. Scale bar, 100 μm. Data were shown as means ± SDs (n = 3). *P < 0.05. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, ns P > 0.05
Te regeneration process of the epidermis and dermis crucially hinges upon the proliferation and migration of fibroblasts [44]. Subsequently, a scratch assay was conducted to assess the capacity of RMDM materials to facilitate the migration of L929 cells. As depicted in Fig. 3 (C and D), the migration ratios of the gel, sponge, and control groups were determined as 41.08 ± 7.327%, 29.17 ± 1.308%, 5.730 ± 1.365%, respectively. These findings suggested a robust capability of RMDM materials to promote cell migration compared to cells seeded solely on the plate surface. Therefore, RMDM materials hold promise in accelerating wound closure and fostering the development of epithelium and granulation tissue, leading to an improved quality of regenerated tissue.
Angiogenesis stands as a pivotal process in wound healing, supplying essential nutrients and oxygen vital for cellular and tissue regeneration [41]. Lumen formation, or tubulogenesis, represents a critical stage in angiogenesis. To assess the tubulogenic potential of Human Umbilical Vein Endothelial Cells (HUVECs) treated with RMDM materials, we conducted a tubulogenesis assay. HUVECs exposed to leach liquor from RMDM materials were seeded onto Matrigel to evaluate their impact on tube formation. As depicted in Fig. 4E–F, cells exposed to the gel group (47.70 ± 2.243%) exhibited the highest number of tubule junctions, nodes, and meshes compared to those exposed to the culture medium (3.73 ± 0.9346%) and sponge (43.57 ± 1.604%). Notably, the gel group displayed a greater area of blood vessel formation than the sponge group, potentially attributed to the higher swelling rate of the sponge material. Consequently, the sponge material absorbed more nutrients from the culture medium, reducing components in the remaining cell culture medium and subsequently leading to a slightly diminished blood vessel area compared to the gel group. These findings underscored the favorable cytocompatibility of RMDM materials and their capacity to facilitate cellular proliferation, migration, and blood vessel formation in vitro, thereby holding promise for tissue repair in compromised tissues.
3.3 The biosafety assessment of RMDM materials
As wound adhesives and dressings would contact blood and tissues, in vitro and in vivo biocompatibility must be determined [45]. The hemolysis experiments were first conducted to assess the hemocompatibility of the RMDM materials, in which Triton X-100 and PBS were used as the positive and negative controls, respectively. As depicted in Fig. 4A, the positive control group (Triton X-100) displayed pronounced hemolytic effects, evident by its bright red appearance, while the negative control group (PBS) remained transparent, indicating minimal red blood cell lysis. Notably, the liquid appearance of RMDM materials and commercial gelatin sponges closely resembled that of the PBS group, signifying favorable blood compatibility. Hemolysis rates for both experimental groups were below 1.6% (Fig. 4B), meeting the international standard for biomaterials (< 5%), [46] thus affirming the commendable blood compatibility of both RMDM materials and the gelatin group.
Additionally, the in vivo biocompatibility of RMDM materials was assessed by implanting them into the skin defects of SD rats. Evaluation of tissue morphology in internal organs served to discern any potential abnormalities induced by the RMDM materials, aiding in assessing its impact on tissue structure, function, and toxicity in vivo. As depicted in Fig. 4C, no organic lesions were observed in rat organs. Myocardial cells exhibited a red hue, with cell nuclei displaying a blue-purple coloration and collagen fibers appearing pink. Hepatocytes presented distinctive polygonal arrangements, with clear cytoplasmic and nuclear staining, and well-defined hepatic lobule structures. In the spleen, red pulp displayed abundant red blood cells, appearing deep red, while white pulp contained lymphocytes and plasma cells, presenting a light blue hue. Epithelial cells and lung alveolar tissue exhibited pink, with air-filled alveoli displaying a light purple tint. Renal tubules and collecting ducts appeared pink, while renal glomeruli exhibited a blue-purple hue. These observations collectively suggested that RMDM materials exerted no toxicity, demonstrated relative safety, and did not induce organ lesions.
3.4 In vitro blood coagulation and in vivo hemostatic performance of the RMDM materials
Further investigation into the blood coagulation property of RMDM materials was conducted through in vitro coagulation experiments, aiming to assess the interaction between biomaterials and blood and their potential to induce coagulation reactions. As indicated by the gross observation of the in vitro coagulation test (Fig. 5A), blood was exposed to three different materials, further analyzing the performance of the RMDM materials. As illustrated in Fig. 5B, the sponge group exhibited a clotting rate of 20.56 ± 0.6245% within one minute, surpassing the clotting rates of the gel and gelatin groups, recorded at 16.63 ± 0.6429% and 14.19 ± 1.026%, respectively. This observation suggested the sponge's effective promotion of blood coagulation, attributable to its larger microporous size compared to the gel. This structural attribute facilitated the adsorption and transportation of red blood cells (RBCs) and platelets, with RBCs and platelets typically measuring 2–5 µm in size. Consequently, smaller pore sizes are prone to blockage by blood clots, impeding further adsorption [47, 48]. These findings underscored the superior in vitro blood coagulation performance of the RMDM sponge, characterized by its larger microporous size.
A Gross view of material interaction with blood. B In vitro coagulation rate in all groups. C Flow chart and general view of rat tail amputation experiment. D Blood loss in each group. E Hemostasis time of each group. F Flow chart and gross view of experimental liver bleeding in rats. G Blood loss in each group. H Hemostasis time of each group. N = 3, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, ns P > 0.05
Considering the favorable adhesion and promising in vitro blood coagulation performance, it is hypothesized that the RMDM sponge exhibits notable hemostatic capabilities. To validate this speculation, rat liver hemorrhage and incision models were utilized to assess the in vivo hemostatic performance (Fig. 5C-H). In the rat tail amputation experiment (Fig. 5D), the control group (without any material treatment) exhibited a bleeding volume of 211.6 ± 10.76 mg, while the commercial gelatin sponge (gelatin) group recorded 80.13 ± 16.22 mg, the gel group registered 94.5 ± 17.09 mg, and the sponge group demonstrated the least bleeding volume at 32.97 ± 9.768 mg. Notably, following using the three materials for hemostasis, a significant reduction in bleeding volume was observed. Particularly, the sponge group exhibited the most effective hemostatic outcome. Moreover, the time required for hemostasis was notably the shortest for the sponge group (3.33 ± 0.577 s) compared to the control group (13 ± 2.646 s), the gelatin group (9.33 ± 1.528 s), and the gel group (6.33 ± 0.577 s) (Fig. 5E).
As depicted in Fig. 5F-H, in the rat liver incision experiment, observations revealed varying bleeding volumes and hemostasis times across experimental groups. The control group exhibited a bleeding volume of 484.2 ± 12.72 mg with a hemostasis time of 5.3 ± 1.52 s, while the gelatin group displayed a bleeding volume of 143 ± 10.52 mg with a hemostasis time of 3.33 ± 0.577 s. Similarly, the gel group recorded a bleeding volume of 102.9 ± 7.966 mg with a hemostasis time of 2.67 ± 0.577 s, and notably, the sponge group demonstrated the lowest bleeding volume of 69.93 ± 7.702 mg with the shortest hemostasis time of 0.67 ± 0.577 s. This outcome underscored the influence of the sponge's large microporous sizes and superior blood coagulation properties on its hemostatic efficacy. These findings also indicated that microporous size played a predominant role in hemostasis. Consequently, upon application to bleeding wound sites, sponges effectively sealed the wounds through adhesion, forming physical barriers to staunch bleeding. Particularly noteworthy was the large microporous structure of the sponge, characterized by positively charged amines and catechols at interfaces, facilitating the adsorption of red blood cells (RBCs) and platelets. This accelerated blood coagulation without obstruction, owing to various physical, electrostatic, and hydrogen bonding interactions [49].
3.5 In vivo wound healing performance of the RMDM materials
Motivated by the promising hemostatic performance, we assessed the wound healing properties of the RMDM materials using a full-thickness rat-skin defect model. The schematic depiction of the full-thickness skin wound modeling and treatments was presented in Fig. 6A. Representative photographs of wounds and the percentage of wound healing on day 0, 7, and 14 were illustrated in Fig. 6B and C. Notably, a gradual closure of the wound site was observed over time. Particularly striking was the pronounced pro-healing effect exhibited by the S/G group on the 7th day, evidenced by a significant reduction in the wound area, achieving a healing area of 78 ± 4.189% compared to 58 ± 5.991% in the control group. Additionally, the gel group demonstrated approximately 75 ± 1.984% healing area, while the sponge group exhibited around 71 ± 1.564% healing area on the 7th day. It was noteworthy that was the superior performance of the S/G and gel groups compared to the sponge and control groups, with wound closures of 87.34 ± 9.803% and 78.18 ± 15.68% on day 14, further affirming the synergistic promotion of different stages for sponge and gel (Fig. 6C).
A Schematic diagram of the experiment schedule and grouping. B Representative images of the wounds treated with RMDM gel, RMDM sponge and S/G on the 0th, 7th, and 14th day. C Wound size change from day 7 to day 14 of post wounding. Wound size at each time point was normalized to day 0. D Hematoxylin and eosin staining of the wounded skin at days 7 and 14. A significant difference analysis was conducted between the S/G group and the remaining groups. N = 3, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, ns P > 0.05
Furthermore, histological analysis of the healing wound tissues through H&E staining (Fig. 6D) revealed enhanced granulation tissue formation across all experimental groups, indicative of accelerated healing. By the 14th day, the S/G group demonstrated the highest presence of newly formed hair follicles, approaching natural tissue composition, thereby reflecting superior tissue regeneration. In summary, the findings underscored the efficacy of specific repair interventions at different stages of wound healing for enhanced wound closure outcomes.
Collagen, a key constituent of skin tissue, serves as a critical indicator of tissue repair efficacy, with its deposition directly reflecting the extent and effectiveness of the repair process [50]. Sirius Red staining, employed to assess Col I (in orange or bright red) and Col III (in green) under polarized light microscopy [51]. Over the treatment period, collagen deposition in all groups exhibited greater uniformity compared to the control group (Fig. 7A), with the S/G group approaching natural rat skin tissue characteristics by day 14, consistent with H&E staining results. Analysis of newly formed Col I and Col III in the tissue indicated that the S/G group exhibited significantly higher levels of type I collagen at days 7 and 14 compared to the other experimental groups, with the control group exhibiting the lowest levels (Fig. 7B). These findings highlighted the significant role of the S/G dressing in accelerating tissue regeneration and wound healing processes. This acceleration could be attributed to the porous structure of the sponge wound dressing, facilitating early-stage wound hemostasis, absorption of wound exudates, and nutrient exchange. Subsequent transition to the gel wound dressing in later stages of wound healing provided an advantageous closed healing microenvironment, owing to the hydrogel's superior tissue adhesion, antioxidant properties, and biocompatibility. Collectively, these attributes contributed to promoting wound healing and achieving excellent wound repair outcomes.
A Sirius red staining of the wounded skin on day 7 and day 14. B Analysis of type I collagen proportion in each group. C Analysis of type III collagen proportion in each group. A significant difference analysis was conducted between the S/G group and the remaining groups. N = 3, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, ns P > 0.05
4 Conclusion
A novel dual-stage photo-crosslinked wound dressing system, composed of rhCol III-MA and DMA (RMDM), has been developed for wound repair during different stages. The RMDM materials exhibited superior mechanical properties, swelling properties, degradation properties and tissue adhesion abilities. Furthermore, the RMDM materials demonstrated exceptional hemocompatibility, biocompatibility, antioxidant activity, angiogenic potential, and ability to stimulate fibroblast migration in vitro. Utilizing a free radical polymerization reaction and π-π stacking, sponges with larger pore sizes (16 µm) achieved superior hemostatic efficacy, characterized by the lowest blood loss of ≈ 32.97 mg, underscoring the significance of microporous dimensions in controlling in vivo hemostasis. Notably, when applied sequentially with gel following initial sponge application, this combined approach accelerated wound healing and enhanced collagen deposition compared to individual sponge and gel treatments in a full-thickness skin injury model. Hence, this study highlighted the efficacy of combining sponge and gel components as a rational strategy for designing advanced hemostatic and healing dressings, with promising applications in biomedicine. In the future, we anticipate that more potential applications, such as hemostasis and cartilage defect repair, will befeasible based on this biomimetic hydrogel or patch and its derived products.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665–706.
Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019;21(1):18–24.
Uberoi A, Bartow-McKenney C, Zheng Q, Flowers L, Campbell A, Knight SAB, et al. Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell Host Microbe. 2021;29(8):1235–48.
Liang Y, He J, Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021;15(8):12687–722.
Dong Z, Liu Q, Han X, Zhang X, Wang X, Hu C, et al. Electrospun nanofibrous membranes of recombinant human collagen type III promote cutaneous wound healing. J Mater Chem B. 2023;11(27):6346–60.
Wang M, Li T, Tian J, Zhang L, Wang Y, Li S, et al. Engineering single-component antibacterial anti-inflammatory polyitaconate-based hydrogel for promoting methicillin-resistant staphylococcus aureus-infected wound healing and skin regeneration. ACS Nano. 2024;18(1):395–409.
Shen J, Fu S, Liu X, Tian S, Liu D, Liu H. Fabrication of low-temperature fast gelation β-cyclodextrin-based hydrogel-loaded medicine for wound dressings. Biomacromolecules. 2024;25(1):55–66.
Dart A, Bhave M, Kingshott P. Antimicrobial peptide-based electrospun fibers for wound healing applications. Macromol. Biosci. 2019;19(9):1800488.
He Y, Wang J, Si Y, Wang X, Deng H, Sheng Z, et al. A novel gene recombinant collagen hemostatic sponge with excellent biocompatibility and hemostatic effect. Int J Biol Macromol. 2021;178:296–305.
Liu Z, Xu Y, Su H, Jing X, Wang D, Li S, et al. Chitosan-based hemostatic sponges as new generation hemostatic materials for uncontrolled bleeding emergency: modification, composition, and applications. Carbohydr Polym. 2023;311:120780.
Lv F, Cong X, Tang W, Han Y, Tang Y, Liu Y, et al. Novel hemostatic agents based on gelatin-microbial transglutaminase mix. Sci China Life Sci. 2017;60(4):397–403.
Chen Z, Lv Z, Zhuang Y, Saiding Q, Yang W, Xiong W, et al. Mechanical signal-tailored hydrogel microspheres recruit and train stem cells for precise differentiation. Adv Mater. 2023;35(40):2300180.
Kim YS, Mikos AG. Emerging strategies in reprogramming and enhancing the fate of mesenchymal stem cells for bone and cartilage tissue engineering. J Control Release. 2021;330:565–74.
Ishihara K, Kaneyasu M, Fukazawa K, Zhang R, Teramura Y. Induction of mesenchymal stem cell differentiation by co-culturing with mature cells in double-layered 2-methacryloyloxyethyl phosphorylcholine polymer hydrogel matrices. J Mater Chem B. 2022;10(14):2561–9.
Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: the past, present, and future. J Thromb Haemost. 2019;17(6):852–62.
Cannon JW. Hemorrhagic shock. N Engl J Med. 2018;378(4):370–9.
Rossaint R, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Gordini G, et al. Key issues in advanced bleeding care in trauma. Shock (Augusta Ga). 2006;26(4):322–31.
Hong Y, Zhou F, Hua Y, Zhang X, Ni C, Pan D, et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat Commun. 2019;10(1):2060.
Behrens AM, Sikorski MJ, Li T, Wu ZJ, Griffith BP, Kofinas P. Blood-aggregating hydrogel particles for use as a hemostatic agent. Acta Biomater. 2014;10(2):701–8.
Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–30.
Rodriguez-Emmenegger C, Preuss CM, Yameen B, Pop-Georgievski O, Bachmann M, Mueller JO, et al. Controlled cell adhesion on poly(dopamine) interfaces photopatterned with non-fouling brushes. Adv Mater. 2013;25(42):6123–7.
Zhang X, Zhan X, Cheng H, Dong Z, Hu C, Liu C, et al. Cartilage-like protein-polysaccharide hybrid hydrogel for enhancing chondrogenic differentiation of bone marrow mesenchymal stem cells. Collagen Leather. 2024;6(1):3.
Bayarsaikhan E, Lim JH, Shin SH, Park KH, Park YB, Lee JH, et al. Effects of postcuring temperature on the mechanical properties and biocompatibility of three-dimensional printed dental resin material. Polymers. 2021;13(8):1180.
Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526–37.
Galiano RD, Michaels V, Joseph, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12(4):485–92.
Fidoamore A, Cristiano L, Antonosante A, d’Angelo M, Di Giacomo E, Astarita C et al. Glioblastoma stem cells Microenvironment: the paracrine roles of the Niche in Drug and Radioresistance. Stem Cells Int. 2016;2016:6809105.
Henn D, Chen K, Fehlmann T, Trotsyuk AA, Sivaraj D, Maan ZN, et al. Xenogeneic skin transplantation promotes angiogenesis and tissue regeneration through activated Trem2(+) macrophages. Sci. Adv. 2021;7(49):eabi4528.
Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125(4):917–28.
Lotz C, Schmid FF, Oechsle E, Monaghan MG, Walles H, Groeber-Becker F. Cross-linked collagen hydrogel matrix resisting contraction to facilitate full-thickness skin equivalents. ACS Appl Mater Interfaces. 2017;9(24):20417–25.
Sorushanova A, Delgado LM, Wu Z, Shologu N, Kshirsagar A, Raghunath R, et al. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv Mater. 2019;31(1):1801651.
Meyer M. Processing of collagen based biomaterials and the resulting materials properties. Biomed Eng Online. 2019;18(1):24.
Sionkowska A. Collagen blended with natural polymers: recent advances and trends. Prog Polym Sci. 2021;122:101452.
Ben C, Liu X, Shen T, Song Y, Li H, Pan B, et al. A recombinant human collagen hydrogel for the treatment of partial-thickness burns: a prospective, self-controlled clinical study. Burns. 2021;47(3):634–42.
Tytgat L, Dobos A, Markovic M, Van Damme L, Van Hoorick J, Bray F, et al. High-resolution 3d bioprinting of photo-cross-linkable recombinant collagen to serve tissue engineering applications. Biomacromolecules. 2020;21(10):3997–4007.
Xiong L, Zhou C, Tong L, Han X, Zou Y, Dong Z, et al. Injectable hydrogels of recombinant human collagen type III and chitosan with antibacterial and antioxidative activities for wound healing. J Mater Chem B. 2023;11(18):4131–42.
Yang Y, Campbell Ritchie A, Everitt NM. Recombinant human collagen/chitosan-based soft hydrogels as biomaterials for soft tissue engineering. Mater Sci Eng C-Mater Biol Appl. 2021;121:111846.
Wu H, Yang L, Luo R, Li L, Zheng T, Huang K, et al. A drug-free cardiovascular stent functionalized with tailored collagen supports in-situ healing of vascular tissues. Nat Commun. 2024;15(1):735.
Wang J, Qiu H, Xu Y, Gao Y, Tan P, Zhao R, et al. The biological effect of recombinant humanized collagen on damaged skin induced by UV-photoaging: an in vivo study. Bioact Mater. 2022;11:154–65.
Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15(3):326–34.
Clark RAF. Fibrin and wound healing. Ann N Y Acad Sci. 2001;936:355–67.
Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453(7193):314–21.
Xue B, Gu J, Li L, Yu W, Yin S, Qin M, et al. Hydrogel tapes for fault-tolerant strong wet adhesion. Nat Commun. 2021;12(1):7156.
Fan P, Dong Q, Yang J, Chen Y, Yang H, Gu S, et al. Flexible dual-functionalized hyaluronic acid hydrogel adhesives formed in situ for rapid hemostasis. Carbohydr Polym. 2023;313:120854.
Wojtowicz AM, Oliveira S, Carlson MW, Zawadzka A, Rousseau CF, Baksh DJWR et al. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014;22(2):246-55.
Wang Z, Zhang Y, Yin Y, Liu J, Li P, Zhao Y, et al. High-strength and injectable supramolecular hydrogel self-assembled by monomeric nucleoside for tooth-extraction wound healing. Adv Mater. 2022;34(13):2108300.
Liang Y, Li Z, Huang Y, Yu R, Guo B. Dual-dynamic-bond cross-linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS Nano. 2021;15(4):7078–93.
Du X, Liu Y, Yan H, Rafique M, Li S, Shan X, et al. Anti-infective and pro-coagulant chitosan-based hydrogel tissue adhesive for sutureless wound closure. Biomacromolecules. 2020;21(3):1243–53.
Wang X-X, Liu Q, Sui J-X, Ramakrishna S, Yu M, Zhou Y, et al. Recent advances in hemostasis at the nanoscale. Adv Healthc Mater. 2019;8(23):1900823.
Wang R, Li J, Chen W, Xu T, Yun S, Xu Z, et al. A biomimetic mussel-inspired ε-poly-l-lysine hydrogel with robust tissue-anchor and anti-infection capacity. Adv Funct Mater. 2017;27(8):1604894.
Sharma S, Rai VK, Narang RK, Markandeywar TS. Collagen-based formulations for wound healing: a literature review. Life Sci. 2022;290:120096.
Guan Y, Niu H, Liu Z, Dang Y, Shen J, Zayed M, et al. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv. 2021;7(35):eabj0153.
Acknowledgements
This work was supported by National Key R&D Program of China (Grant No. 2022YFC2401800); National Natural Science Foundation of China (Grant No. 32301116) and Sichuan Science and Technology Program (Grant No. 2023NSFSC0996).
Author information
Authors and Affiliations
Contributions
The manuscript was collectively authored by all contributors, and all authors have provided their consent for the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The animal experiments were approved by the Medical Ethics Committee of Sichuan University (20220728001). The animal experiment guidance from the ethical committee and the guide for the care and use of laboratory animals of Sichuan University were followed during the whole experiment course.
Competing interests
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Zhan, X., Hu, C. et al. Light-controlled crosslinked multifunctional "Band-Aids" as dual-stage wound dressing for dynamic wound closure. Collagen & Leather 6, 23 (2024). https://doi.org/10.1186/s42825-024-00167-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-024-00167-5