Abstract
Leather dyeing is a critical step in leather manufacturing, as it is responsible for providing leather products with an eye-catching visual aspect and adequate quality properties to meet customers' expectations. This step is becoming more and more challenging as the leather industry advances hand in hand with new environmentally friendly policies and regulations to achieve a safer and healthier planet by replacing the highly polluting Cr-based leather tanning technology with greener alternatives. As a result, achieving high-performance dyeing of organic chrome-free leather is one of the bottlenecks for the sustainable development of the leather industry. Herein, we propose a novel strategy to fabricate an isocyanate-based oligomeric dye (IBD) with high coloring capabilities (component content higher than 62.8%) based on toluene 2,4-diisocyanate and reactive red dye 180. This material has been tested for the dyeing of biomass-derived aldehyde (BDA)-tanned leather with excellent outcomes. The experimental results showed that the crust leather dyed with our novel IBD dyeing agent had higher color fastness and better fullness than the leather dyed with conventional anionic (CAD) or reactive red 180 (RRD-180) dyes. These excellent and promising results open new avenues in manufacturing high-performance organic Cr-free leather products and help to ensure the sustainable transition of the leather industry from Cr-based leather tanning to more sustainable alternatives, maintaining the final quality of the leather products.
Graphical abstract

Similar content being viewed by others
1 Introduction
The leather industry is an elderly manufacturing sector producing a broad range of house and living things related to people's livelihoods, e.g., shoes, gloves, bags, belts, sofas, car cushions [1]. The global leather industry has recently sought more environmentally friendly, chrome-free manufacturing technologies due to the ever-increasing public concern, strict legislative control, and ecological awareness of chrome pollution [2]. This new movement toward sustainability has been called eco-friendly leather production. It has led to the development of different chrome (Cr)-free tanning agents, such as the commercial TWT organic tanning agent [3], non-Cr metal tanning agents with novel ligands (e.g., oxidized products from starch, hemicellulose, cellulose, and lignosulfonate, metal–organic frameworks, and POSS-COONa) [4,5,6,7,8,9,10,11,12,13,14,15], nanosilicates-based chrome-free tanning [16], biomass-derived aldehyde tanning agents (BATs) [17,18,19,20,21,22], and other synthetic epoxy tanning agents based on biomass [23,24,25,26]. Compared with the non-Cr metal tanning agents (e.g., zirconium, aluminum, and titanium salts) and the fossil-based organic tanning agents (e.g., glutaraldehyde, modified glutaraldehyde, oxazolidine, organic phosphine), the sustainable BAT has obvious advantages, ascribed to the abundance of raw materials, environmental friendliness, and carbon reduction. Despite these excellent prospects, some drawbacks are linked to using these tanning agents. Notably, these biomass-based organic tanning agents will react with the amino groups of collagen fiber to form a robust covalent crosslinking network in the collagen matrix, decreasing tanned leather’s isoelectric point (IEP) [27]. This will produce the undesirable uptake and fixation of conventional anionic pos-tanning materials (CAPMs), especially in the dyeing process, thus affecting the quality of the resultant leather products [19]. Hence, developing novel dyeing materials for high-performance dyeing of organic Cr-free leather is becoming one of the keys to achieving eco-leather manufacturing.
To overcome the drawbacks derived from the low IEP of leather tanned with BAT agents, bifunctional BATs with in situ dyeing properties have been developed [19, 20]. Although these materials could endow the resultant leather with high color fastness and good dyeing uniformity, the sorts of color are minimal due to the given dye structure of BAT with in situ dyeing properties. Furthermore, some other "X-in-one" post-tanning processes including retanning, filling, dyeing, and fatliquoring have been proposed and investigated. For instance, acrylic acid (AA), diallyl dimethyl ammonium chloride (DMDAAC), dodecyl acrylate (DA), and 1,8-naphthalic anhydride (NA), were employed to prepare an amphoteric polymer (labeled as pADD-DMENA) for successfully achieving the high-performance retanning, fatliquoring, and dyeing of Cr-free organic tanned leather [28]. The pADD-DMENA treated leather had a bright yellow color surface with high uniformity, superior lightfastness, durability, and washability. Although these "X-in-one" strategies provide a time-energy-saving solution to manufacture high-performance BAT-leather, more investigations need to be conducted to develop low-cost and large-scale dyeing materials with excellent application performances. Another option to address the dyeing challenge of BAT-leather is using polymeric dyes, i.e., polymers containing chromophore groups on the main or side chain [29,30,31]. In comparison, polymeric dyes have the advantage of containing adjustable structures so that various colors can be designed and developed by adjusting the coloring molecules, thus improving their versatility. However, the existing polymeric dyes have a low coloring component content, thus causing limitations in their practical applications in leather dyeing due to the low color saturation [32, 33]. Besides, polymeric dyes with overhigh molecular weight may not be favorable to penetrate the leather matrix, thus negatively affecting their dyeing performance.
Given this background, developing leather dyes with high coloring capabilities and moderate molecular weight is paramount to overcoming the above-mentioned limits. In this work, a novel strategy was proposed to fabricate an isocyanate-based oligomeric dye (IBD) with a high coloring component. The synthesis of IBD was based on toluene 2,4-diisocyanate (TDI) and reactive red dye 180 with primary amino groups (RRD-180) given the high reactivity between the -NCO and -NH2. The application performance of IBD in the dyeing of organic Cr-free leather tanned by biomass-derived aldehyde (BDA) was further investigated and compared with RRD-180 and conventional anionic dye (CAD) that can give crust leather a popular brown color. The structure of IBD was analyzed using gel permeation chromatography (GPC), Fourier transform infrared spectroscopy (FTIR), and nuclear magnetic resonance (NMR). The penetration of IBD in the leather matrix, the dyeing performance, and the mechanical strength and organoleptic properties of the IBD-dyed crust leather, were also systematically clarified. Given this step forward in eco-friendly leather dyeing, this work opens new avenues toward developing novel, feasible strategies to fabricate industrially applicable oligomeric dyes based on isocyanate for the high-performance dyeing of organic Cr-free leather, which will contribute to the green development of the leather industry.
2 Experimental section
2.1 Materials
Cangzhou Dahua Group Co., Ltd. (Cangzhou, China) supplied industrial-grade TDI. Shanghai Macklin Biochemical Co., Ltd (Shanghai, China) provided RRD-180 (CAS number: 72828–03-6). Industrial-grade dimethylolpropionic acid (DMPA) was purchased from Jiangxi Nancheng Hongdu Chemical Technology Development Co. Ltd. (Fuzhou, China). Jining Huakai Resin Co., Ltd (Jining, China) provided industrial-grade stannous octoate (T-9). Tianjin Hengxing Chemical Reagent Co. Ltd. (Tianjin, China) supplied analytical grade triethylamine (TEA). BDA with a solid content of 40 wt% and an aldehyde content of 12.0 mmol/g (based on the absolute dry weight) was prepared based on the method described by Ding et al. [34]. Xinji Lingjue Leather Co., Ltd (Xinji, China) provided the picked sheepskin pelt. Commercial CAD (Acid Brown 122) was provided by Jiangsu Aosheng Enterprise Development Co., Ltd. (Gaoyou, China). The acrylic resin, amino resin, and fatliquors were industrial grade and provided by Sichuan Dowell Science and Technology Inc. (Chengdu, China). The syntan for the filling was supplied by Zhejiang Shenghui Chemical Co., Ltd. (Quzhou, China).
2.2 Synthesis of IBD
The synthesis procedure of IBD was illustrated in Scheme 1. A certain amount (the mole ratio between TDI and RRD-180 were 0.27:1, 0.60:1, and 0.93:1) of TDI, 11.07 g of RRD-180 and acetone (twice the total mass of TDI and RRD-180) were introduced into a 250 mL flask equipped with a condenser. The reaction was conducted under an N2 atmosphere at 70 °C for 1.0 h, with subsequently heating to 85 °C and stirring for 1.0 h. Then, a certain amount of DMPA (6.0% of the total mass of TDI and RRD-180, for improving the hydrophilicity of the resultant dye) and 0.1% of T-9 were introduced to react for another 2.0 h under 85 °C. Next, the temperature of the reaction system was lowered to room temperature. After that, a certain amount (the mole ratio between DMPA and TEA was 1:1) of TEA was introduced. After stirring for 10 min, a certain amount (the volume ratio between water and acetone was 1:1) of deionized water was introduced. After stirring for 20 min, the reaction product was vacuum-filtered. Finally, the filter cake was ground to obtain powdery IBD after vacuum drying. The as-prepared IBDs were labeled as IBD-1, IBD-2, and IBD-3, respectively.
2.3 BDA-tanned leather preparation
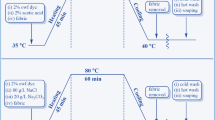
The pickled sheepskin pelt was used to prepare BDA-tanned leather according to the process (Table 1) reported by Ding et al. [33] with a slight modification. Before conducting the tanning process, the mass of the pickled sheepskin pelt was first recorded, and the dosage of chemicals was calculated based on twice the mass of the pickled pelt. The obtained BDA-tanned leather was stored in an airtight tape at 4.0 ºC for further post-tanning treatments.
2.4 IBD dyeing of BDA-tanned leather
The BDA-tanned leather was further shaved to a thickness of 1.0 mm and then treated following the methodology previously reported by Ding et al. [33] for preparing the crust leather (Table 2). It should be noted that the additive amount of chemicals was calculated based on the mass of the shaved BDA-tanned leather. The obtained crust leather was stored in an airtight tape at room temperature for further measurements.
2.5 Characterizations
The molecular weight of IBD was tested using a Rid-20A gel permeation chromatograph (Shimadzu, Japan) with a sample concentration of 20 mg/mL. A Tensor 27 infrared spectrometer (Bruker Inc., Germany) was employed to record the FTIR spectra of TDI, RRD-180, DMPA, and IBD with a scanning range from 600 cm–1 to 4000 cm–1. A Varian 700 MHz NMR spectrometer (Varian, USA) was used to record the 1H NMR spectra of samples using CDCl3 or D2O as a solvent at a concentration of around 1.0 mg/mL. A UV–Vis spectrophotometer (TU-1810, PERSEE, Beijing, China) was employed to record the UV–Vis spectra of RRD-180 and typical IBDs.
A digital leather shrinkage temperature (Ts) instrument (MSW-YD4, Sunshine Electronic Research Institute of Shaanxi University of Science and Technology, China) was used to test the Ts of leather. After post-tanning, the crust leather was naturally dried and air-conditioned at 20 °C and 65% relative humidity for 48 h. After that, a tensile tester (AI-7000S, Gotech, China) was employed to measure the mechanical strengths of curst leathers based on the standard methods (IUP 6, 2000; IUP 8, 2000) [35, 36]. Besides, the standard leather softness tester (GT-303, Gotech, China) was used to test the softness of crust leather (the maximum softness value for blank correction is 7.6 mm) [37], while its fullness was further investigated by analyzing its compression-resilience property following a previously reported method [38]. The dyeing performance of crust leather using IBD was evaluated based on the method described by Ding et al. [33] via testing the chromatic values with a colorimeter (SC-80C, Jingyi Kangguang, China).
2.6 Statistical analysis
The Ts tests were conducted in quadruplicate. Data for each group were presented as the mean ± SD. Statistical analysis was performed with the software GraphPad Prism 9.5.0 (GraphPad Software, USA) according to the method previously reported [39]. The Ts data of BDA-tanned leather or the typical filled-dyed leather were set as a control for analyzing the effects of filling-dyeing and fatliquoring on the leather Ts. Statistical significance was defined at p < 0.05.
3 Results and discussion
3.1 Structure information of IBD
GPC, FTIR, and NMR analyses were employed to study the structural features of IBD. Table 3 lists the molecular weight and distribution of IBD. The results show that the weight average molecular weight of IBD-1 could be up to 1791 when the dosage of TDI was 0.27:1 (mole ratio between TDI and RRD-180), and it slightly increased with further improving the dosage of TDI. This indicated that the polymerization reaction between TDI and RRD-180 proceeded successfully. Given that the molecular weight of IBD just increased slightly with the improvement of the TDI dosage, the IBD-2 with moderate molecular weight was selected for further structure analyses and application experiments.
The FTIR spectra (Fig. 1a) show that the characteristic peak of the –NCO group from TDI appeared at 2256 cm−1 [40], which disappeared after reacting with RRD-180 and DMPA. Meanwhile, the peak assigned to − NH2 (3456 cm−1) also decreased substantially. Besides, a new peak appeared at 1685 cm−1, which might be attributed to the vibrations of carbonyls of the urethane methylene from TDI and the carboxyl groups from DMPA (1689 cm−1 in Fig. 1a) [41, 42]. Figure 1b illustrates that IBD inherited the typical groups (1.2 ppm for C − CH3; 4.4 ppm for -OCH3) from DMPA, the typical H from primary/secondary amino groups, and the aromatic nucleus from RRD-180 (7.5 ~ 8.5 ppm) [32, 43]. Besides, the characteristic peak at 2.4 ppm is due to the protons in the methyl group (Ph-CH3) of TDI [43], which was passed on to IBD (2.2 ppm in Fig. 1b). Notably, the intensity of C − OH from DMPA and the intensity of primary/secondary amino groups from RRD-180 decreased remarkably after reacting with TDI. In addition, a new peak appeared at 3.13 ppm, which might be assigned to the urethane methylene [44]. During the synthesis process of IBD, the -NCO of TDI first reacted with the -NH2 of RRD-180 to form linear polyurea intermediates with terminal -NCO [45]. After introducing DMPA, the -OH of DMPA further reacted with the terminal -NCO of linear polyurea intermediates [46], thus blocking TDI to react with unconsumed − NH2. Based on the above-mentioned results and the reaction mechanism, the structure of IBD was proposed in Fig. 2. These results confirmed that the RRD-180 and DMPA had been bonded with TDI, which increased the molecular weight of IBD and potentially improved its hydrophilicity.
3.2 UV–Vis absorption characteristics of IBD
The UV–Vis spectra of RRD-180 and IBD were recorded to analyze the color properties of IBD. Figure 3 reveals that the characteristic absorption peaks of IBD were similar to those of RRD-180, suggesting the successful incorporation of RRD-180 molecules into the molecular chain of IBD. Importantly, the final IBD product inherited the original color properties of RRD-180, which might be due to the high content of this latter in the as-prepared IBD. This property might endow the resultant IBD-dyed crust leather with a similar color to that of crust leather dyed by RRD-180, thus endowing IBD with an application efficiency comparable to RRD-180 or conventional leather dyes.
3.3 Application performances of IBD
3.3.1 Effect of IBD on the uptake of post-tanning materials
Table 4 shows the TOC (Total Organic Carbon) of the post-tanning wastewater from different dyeing systems. For the filling and dyeing wastewater, the TOC concentration of wastewater followed the order of IBD group > RRD-180 group > CAD group. These results might be accounted for by the reaction between RRD-180/IBD and other anionic fillers, as well as the relatively high molecular weight of IBD. In detail, RRD-180 and IBD have cationic amino groups, which may interact with the anionic groups from acrylic fillers to increase the molecular weight of the resultant interaction products. This will limit the penetration of these fillers into the interior of the leather matrix, thus showing lower compatibility with anionic fillers than CAD. IBD had a higher molecular weight than RRD-180 (369.4 g/mol) and CAD (798.6 g/mol), so its osmotic inhibition effect on anionic fillers may be stronger than RRD-180. After filling and dyeing, the bath was drained, and a fresh bath was used to conduct the fatliquoring process. As shown in Table 4, the TOC concentration of fatliquoring wastewater from the CAD group was comparable to that of fatliquoring wastewater from the IBD group, the latter being much lower than that of fatliquoring wastewater from the RRD-180 group. This might be explained by the fact that CAD and IBD with higher molecular weight had better bonding forces in the leather substrate. Besides, the weak cationic property of IBD and the anionic property of CAD were of more benefit to the penetration of fatliquors than the RRD-180 with a stronger cationic property. Given these results, it is recommended to redesign the application process of IBD to maximize its performance benefits based on the molecular weight and charge property.
3.3.2 Effect of IBD on the Ts of leather
Figure 4 shows the effect of IBD on the Ts of leather during the post-tanning of BDA-tanned leather. After filling and dyeing treatments, the Ts of the three kinds of treated leathers were 82.8 ºC for RRD-180-treated leather, 82.9 ºC for RRD-180-treated leather, and 82.7 ºC for RRD-180-treated leather, respectively, which were comparable to the Ts of BDA-tanned leather (82.3 ºC). This indicated that there was no obvious effect of filling and dyeing on the shrinkage temperature of BDA-tanned leather (Fig. 4a). Furthermore, after being fatliquored at a high temperature (50 ºC), the Ts of fatliquored leathers decreased obviously but were still higher than 80 ºC (Fig. 4b). This was due to the cleavage of the covalent Schiff-base structure of BDA-tanned leather under the acidic fixation conditions at the end of fatliquoring (pH was 3.6 ~ 3.8). Among the three kinds of crust leathers, the Ts decline of RRD-180-crust leather was the highest, followed by the moderate CAD-crust leather and the lowest IBD-crust leather (Fig. 4c). This might be ascribed to a more robust load of IBD onto the BDA-tanned leather matrix via filling into the interspaces between collagen fibers through electrostatic force and covalent bonding [33]. These results suggested that IBD had a relatively higher stabilizing effect on the collagen fiber network of the leather matrix.
Shrinkage temperature (Ts) of the filled-dyed leather (a) and the fatliquored leather (b). Results were expressed as the mean ± SD (n = 4). (*, **, ***) represented significant difference in comparison with the control group (BDA-tanned leather) or the typical filled-dyed leather (RRD-180 group) at p < 0.05, 0.01, 0.001, respectively; ns represented no significant difference in comparison with the control group (BDA-tanned leather) at p < 0.05
3.3.3 Coloring performance
The penetration of IBD in the BDA-tanned leather matrix is illustrated in Fig. 5. The experimental results demonstrate that the oligomeric IBD could almost fully penetrate the inner of the leather matrix, showing comparable penetrability with RRD-180 and CAD. Besides, it can be observed that the color of IBD-dyed leather was similar to that of RRD-180-dyed leather due to the high coloring component content of IBD. Furthermore, the chromatic values ("L", "a", "b") of different post-tanned leathers after natural drying and softening are presented in Table 5. It can be noted that the "L"-value of IBD-crust leather was slightly higher than that of RRD-180-crust leather, and the "a"-value of IBD-crust leather was slightly lower than that of RRD-180-crust leather, suggesting that the IBD-crust leather was a little brighter than RRD-180-crust leather. This was ascribed to the difference in the absolute coloring component content between IBD and RRD-180.
The dyeing uniformity and the color fastness of crust leathers are shown in Fig. 6. These results illustrate that the average total color difference (ΔE) of RRD-180-crust leather and IBD-crust leather compared to standard white (L = 95.26, a = -0.64, b = 2.02) was comparable (Table 5), indicating that they had a similar color in the same color system due to the high coloring component content of IBD. However, it is worth noting that the STDEV (standard deviation) value of the ΔE value of RRD-180-crust leather was 1.68, the STDEV value of the ΔE values of CAD-crust leather was 1.51, and the STDEV value of the ΔE values of IBD-crust leather was 0.82. A lower STDEV value of the ΔE values represents a higher dyeing uniformity [47]. Therefore, IBD-crust leather had the highest dyeing uniformity, followed by CAD-crust leather, with RRD-180-crust leather having the lowest. As shown in Fig. 4b, the three kinds of crust leather had favorable dry-rubbing fastness, while RRD-180-crust leather and IBD-crust leather had higher wet-rubbing fastness than CAD-crust leather. These results suggest that the as-prepared IBD can endow the BDA-tanned crust leather with better coloring performance than CAD and RRD-180.
3.3.4 Mechanical strength and organoleptic properties
As is well acknowledged, the mechanical strengths and organoleptic properties of crust leather are closely related to its practical final applications. Therefore, these properties were further tested to evaluate the acceptability of IBD as a leather dye. Figure 7 shows that the tensile strength of crust leather prepared by IBD dyeing (denoted as IBD-crust leather, similarly from now on) was up to 28.98 N/mm2, which was higher than that of RRD-180/CAD-crust leathers. Besides, IBD-crust leather had a favorable tear strength (91.20 N/mm), which was much higher than that of RRD-180-crust leather (66.19 N/mm) and slightly lower than that of CAD-crust leather (96.66 N/mm). Owing to the difference in the uptake of fatliquor (Table 4), CAD-crust leather had higher elongations than RRD-180/IBD-crust leathers, indicating that IBD-crust leather was tighter than CAD-crust leather. Overall, these results suggested that IBD-crust leather had favorable mechanical strength.
Figure 8 shows the softness and the compression-resilience performance of crust leathers. The three crust leathers had comparable and favorable softness, which was higher than 6.00 mm. Additionally, the IBD-crust leather had higher compressed and resilient thickness than RRD-180-crust leather and CAD crust leather. Generally, the higher the compressed and resilient thicknesses of crust leather are, the better the fullness of the produced leather [48]. Therefore, IBD-crust leather exhibited better fullness than RRD-180-crust leather and CAD-crust leather. This might be due to the higher molecular weight of IBD that could provide more favorable support for the collagen fiber network. These results indicate that IBD-crust leather exhibited satisfactory overall performances, which were better than those of the crust leathers prepared from RRD-180 and CAD dyeing systems. Thus, IBD can be regarded as a favorable and promising leather dye for the high-performance dyeing of organic Cr-free leather, thus contributing toward the sustainable development of the leather industry.
4 Conclusions
In this work, a novel isocyanate-based polymeric dye (IBD) with excellent coloring properties (component content higher than 62.8%) was successfully prepared and tested in the dyeing of biomass-derived aldehyde-(BDA)-tanned leather. A plethora of different characterization techniques were used to demonstrate that IBD and BDA significantly interacted during the dyeing process, with such a development resulting from the reaction between the isocyanate groups in IBD and the amino groups in BDA. Besides, dimethylolpropionic acid (DMPA) was also loaded onto the molecular chain of IBD, endowing it with hydrophilicity. The properties of the BDA-tanned leather dyed with our IBD dyeing agent were compared to those produced employing commercial dyeing agents, including conventional anionic dye (CAD) and reactive red dye 180 (RRD-180). The tear strength, elongation at a specific load, and softness of the IBD-crust leather were comparable with those of CAD-crust leather. However, the IBD-crust leather had better dyeing uniformity, color fastness, higher tensile strength, and fullness than the CAD- and RRD-180-crust leathers due to the effective interactions taking place between IBD and BDA. These promising results demonstrate that advanced multifunctional polymeric dyes can be produced from IBD, with these novel dyeing agents showing appropriate properties to dye organically tanned chrome-free leather, maintaining the favorable mechanical and organoleptic properties of the tanned leather. These favorable features are paramount to the eco-friendly manufacturing of high-performance chrome-free leather products, making it possible for the leather industry and sustainable developments to go hand in hand.
Availability of data and materials
All data from this study are presented in the paper.
References
Liang F, Wang T, Fan H, Xiang J, Chen Y. A leather coating with self-healing characteristics. J Leather Sci Eng. 2020;2:5.
Oruko RO, Selvarajan R, Ogola HJO, Edokpayi JN, Odiyo JO. Contemporary and future direction of chromium tanning and management in sub Saharan Africa tanneries. Process Saf Environ Prot. 2020;133:369–86.
Ding W, Wang Y, Zhou J, Shi B. Effect of structure features of polysaccharides on properties of dialdehyde polysaccharide tanning agent. Carbohydr Polym. 2018;201:549–56.
Wang Z, Wang YN, Yu Y, Shi B. Tanning performance of a novel chrome-free complex tanning agent: penetration and distribution. J Am Leather Chem Assoc. 2021;116:277–83.
Yu Y, Wang Y, Ding W, Zhou J, Shi B. Preparation of highly-oxidized starch using hydrogen peroxide and its application as a novel ligand for zirconium tanning of leather. Carbohydr Polym. 2017;174:823–9.
Yu Y, Wang H, Wang Y, Zhou J, Shi B. Chrome-free synergistic tanning system based on biomass-derived hydroxycarboxylic acid–zirconium complexes. J Clean Prod. 2022;336: 130428.
Gao M, Jiang Z, Ding W, Shi B. Selective degradation of hemicellulose into oligosaccharides assisted by ZrOCl2 and their potential application as a tanning agent. Green Chem. 2022;24:375–83.
Jiang Z, Gao M, Remón J, Ding W, Hu C, Shi B. On the development of chrome-free tanning agents: an advanced Trojan horse strategy using ‘Al–Zr-oligosaccharides’ produced by the depolymerization and oxidation of biomass. Green Chem. 2021;23:2640–51.
Jiang Z, Gao M, Ding W, Huang C, Hu C, Shi B, et al. Selective degradation and oxidation of hemicellulose in corncob to oligosaccharides: From biomass into masking agent for sustainable leather tanning. J Hazard Mater. 2021;413: 125425.
Jiang Z, Ding W, Xu S, Remón J, Shi B, Hu C, et al. A ‘Trojan horse strategy’ for the development of a renewable leather tanning agent produced via an AlCl3-catalyzed cellulose depolymerization. Green Chem. 2020;22:316–21.
Jiang Z, Xu S, Ding W, Gao M, Fan J, Hu C, et al. Advanced masking agent for leather tanning from stepwise degradation and oxidation of cellulose. Green Chem. 2021;23:4044–50.
Yu Y, Wang Y, Ding W, Zhou J, Shi B. Effect of catalyst on structure of hydrogen peroxide oxidized starch and its performance as a ligand in zirconium tanning of leather. Fine Chem. 2018;35:1928–34.
Xue P, Yu Y, Wang H, Cao Y, Shi B, Wang Y. Oxidized sodium lignosulfonate: a biobased chrome-free tanning agent for sustainable eco-leather manufacture. Ind Crops Prod. 2024;208: 117916.
Gao D, Li N, Li X, Zhang A, Lyu B, Ma J. A green tanning method based on POSS-COONa and zirconium: achieving cleaner leather production. Prog Org Coat. 2023;183: 107718.
Chen J, Ma J, Fan Q, Zhang W. An eco-friendly metal-less tanning process: Zr-based metal-organic frameworks as novel chrome-free tanning agent. J Clean Prod. 2023;382: 135263.
Shi J, Sheng L, Salmi O, Masi M, Puig R. Life cycle assessment insights into nanosilicates-based chrome-free tanning processing towards eco-friendly leather manufacture. J Clean Prod. 2024;434: 139892.
Ding W, Yi Y, Wang Y, Zhou J, Shi B. Preparation of a highly effective organic tanning agent with wide molecular weight distribution from bio-renewable sodium alginate. ChemistrySelect. 2018;3:12330–5.
Ding W, Wu Y. Sustainable dialdehyde polysaccharides as versatile building blocks for fabricating functional materials: an overview. Carbohydr Polym. 2020;248: 116801.
Ding W, Remón J, Jiang Z. Biomass-derived aldehyde tanning agents with in situ dyeing properties: a ‘Two Birds with One Stone’ strategy for engineering chrome-free and dye-free colored leather. Green Chem. 2022;24:3750–8.
Ding W, Liu H, Li S, Remón J, Pang X, Ding Z. Providing natural organic pigments with excellent tanning capabilities: a novel, “one-pot” tanning-dyeing integration strategy for sustainable leather manufacturing. ACS Sustain Chem Eng. 2022;10:17346–54.
Ding W, Yi Y, Wang Y, Zhou J, Shi B. Peroxide-periodate co-modification of carboxymethylcellulose to prepare polysaccharide-based tanning agent with high solid content. Carbohydr Polym. 2019;224: 115169.
Jiang Z, Ding W, Fan J, Liao Y, Remón J, Shi B. Biomass derived oligosaccharides for potential leather tanning. Collagen Leather. 2023;5:7.
Wang X, Su R, Hao D, Dang X. Sustainable utilization of corn starch resources: a novel soluble starch-based functional chrome-free tanning agent for the eco-leather production. Ind Crops Prod. 2022;187: 115534.
Hao D, Wang X, Liu X, Su R, Duan Z, Dang X. Chrome-free tanning agent based on epoxy-modified dialdehyde starch towards sustainable leather making. Green Chem. 2021;23:9693–703.
Hao D, Wang X, Yue O, Liang S, Bai Z, Yang J, et al. A “wrench-like” green amphoteric organic chrome-free tanning agent provides long-term and effective antibacterial protection for leather. J Clean Prod. 2023;404: 136917.
Liang S, Wang X, Hao D, Yang J, Dang X. Facile synthesis of a new eco-friendly epoxy-modified oligomeric chitosan-based chrome-free tanning agent towards sustainable processing of functional leather. Process Saf Environ Prot. 2023;172:753–63.
Xiao Y, Wang C, Zhou J, Wu J, Lin W. Modular design of vegetable polyphenols enables covalent bonding with collagen for eco-leather. Ind Crops Prod. 2023;204: 117394.
Wei C, Wang X-C, Sun S, Lu Q, Zou X, Xie L, et al. A “three-in-one” strategy based on an on-demand multifunctional fluorescent amphoteric polymer for ecological leather manufacturing: a disruptive wet-finishing technique. Green Chem. 2023;25:5956–67.
Guo S, Liu N, Ding W, Pang X, Ding Z, Chen Y. Graphene oxide modified waterborne polyurethane-based dye with high color-fastness performance. J Appl Polym Sci. 2020;e50390.
Ciardelli F, Ruggeri G, Pucci A. Dye-containing polymers: methods for preparation of mechanochromic materials. Chem Soc Rev. 2013;42:857–70.
Zhu J, Li J, Cai W, Luo Y. Synthesis and application of a phosphorus-containing waterborne polyurethane based polymeric dye with excellent flame retardancy. Prog Org Coat. 2020;140: 105525.
Ding W, Zhang Y, Li S, Remón J, Wang K, Bao L, et al. Novel biomass-based polymeric dyes: preparation and performance assessment in the dyeing of biomass-derived aldehyde-tanned leather. Polymers. 2023;15:2300.
Ding W, Guo S, Liu H, Pang X, Ding Z. Synthesis of an amino-terminated waterborne polyurethane-based polymeric dye for high-performance dyeing of biomass-derived aldehyde-tanned chrome-free leather. Mater Today Chem. 2021;21: 100508.
Ding W. Bridging-induced densification strategy based on biomass-derived aldehyde tanning integrated with terminal Al(III) crosslinking towards high-performance chrome-free leather production. J Environ Manag. 2022;307: 114554.
IUP 6. Measurement of tensile strength and percentage elongation. J Soc Leather Technol Chem. 2000;84:317–21.
IUP 8. Measurement of tear load-double edge tear. J Soc Leather Technol Chem. 2000;84:327–9.
IUP 36. Measurement of leather softness. J Soc Leath Tech Ch. 2000;84:377–9.
Peng W, Zhang X, Chen S. The principle and method of testing leather fullness and softness. J Soc Leather Technol Chem. 2006;90:117–22.
Ding W, Pang X, Ding Z, Tsang DCW, Jiang Z, Shi B. Constructing a robust chrome-free leather tanned by biomass-derived polyaldehyde via crosslinking with chitosan derivatives. J Hazard Mater. 2020;396:Article 122771.
Koprululu A, Onen A, Serhatli IE, Guner FS. Synthesis of triglyceride-based urethane macromers and their use in copolymerization. Prog Org Coat. 2008;63:365–71.
He H-W, Zhang B, Yan X, Dong R-H, Jia X-S, Yu G-F, et al. Solvent-free thermocuring electrospinning to fabricate ultrathin polyurethane fibers with high conductivity by in situ polymerization of polyaniline. RSC Adv. 2016;6:106945–50.
Song Q, Chen H, Zhou S, Zhao K, Wang B, Hu P. Thermo- and pH-sensitive shape memory polyurethane containing carboxyl groups. Polym Chem. 2016;7:1739–46.
Issam AM, Ismail J. New aromatic poly(azomethine urethane)s containing o-tolidine moiety in the polymer backbone. Des Monomers Polym. 2006;9:237–46.
Ruiz L, Aghmiz A, Masdeu-Bultó AM, Lligadas G, Ronda JC, Galià M, et al. Upgrading castor oil: from heptanal to non-isocyanate poly(amide-hydroxyurethane)s. Polymer. 2017;124:226–34.
Han H, Li S, Zhu X, Jiang X, Kong XZ. One step preparation of porous polyurea by reaction of toluene diisocyanate with water and its characterization. RSC Adv. 2014;4:33520–9.
Muzaffar S, Bhatti IA, Zuber M, Bhatti HN, Shahid M. Synthesis and characterization of aqueous chitosan-polyurethanes dispersion for textile applications with multipurpose performance profile. Fibers Polym. 2018;19:587–98.
Ding W, Remón J, Gao M, Li S, Liu H, Jiang Z, et al. A novel synergistic covalence and complexation bridging strategy based on multi-functional biomass-derived aldehydes and Al(III) for engineering high-quality eco-leather. Sci Total Environ. 2023;862: 160713.
Ding W, Wang Y, Sun J, Bao L, Pang X. Dialdehyde sodium alginate bonded dicyandiamide for formaldehyde-free leather production with enhanced properties. Carbohydr Polym. 2022;295: 119838.
Acknowledgements
Javier Remón thanks MCIN/AEI/https://doi.org/10.13039/501100011033 and the European Union «NextGenerationEU»/PRTR» for the Ramón y Cajal Fellowship (RYC2021-033368-I) awarded, and the Aragón Government (Research Group Reference T22_23R) for providing frame support.
Funding
The authors were grateful to the financial support provided by the National Natural Science Foundation of China (22108297), the Science and Technology Innovation Key Project of Sinolight Corporation (ZQ2021YY05), and the National Key Research and Development Program (2020YFE0203800).
Author information
Authors and Affiliations
Contributions
WD: conceptualization, methodology, investigation, data analysis, visualization, funding acquisition, writing—original draft; SG and HTL: investigation, data analysis; XYP and ZWD: project administration, resources; JR: formal analysis, reviewing, editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ding, W., Guo, S., Liu, H. et al. Manufacturing of isocyanate-based oligomeric dyes with high coloring capabilities: synthesis and application in the dyeing of organic chrome-free leather. Collagen & Leather 6, 8 (2024). https://doi.org/10.1186/s42825-024-00153-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-024-00153-x