Abstract
An environmentally friendly method using real or artificial bovine milk permeate to both depilate and preserve sheepskins has been reported which completely and cleanly removed the wool from the hair follicle and had no detrimental effects on the skin. A proteomic analysis, assessing the relative abundance of proteins in matched permeate-depilated and chemically depilated (sulfide) sheepskins, showed variations in the levels of specific collagen types in the skin's basement membrane and other proteins associated with the follicles. These findings were corroborated by biochemical analyses of matched permeate depilated and raw skin samples, and provide clues to the mechanism of non-invasive and complete depilation. They also support the observation that permeate-depilated skins were smoother than their sulfide-depilated counterparts and resulted in leather with a superior surface.
Graphical abstract

Similar content being viewed by others
1 Introduction
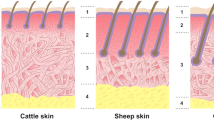
Sheepskin, a by-product of the meat industry, is used to manufacture a light supple leather that is in demand for luxury high-end fashion garments and accessories, as well as soft wool-lined clothing, gloves, and hats. Sheepskin is comprised of water (53%), protein (27.5%), lipids (18.3%), and trace minerals. Like all animal skins, it has three general layers: the epidermis, dermis and hypodermis (Fig. 1). The epidermis, the outer-most protective layer of the skin, contains mainly keratinocytes (keratin-producing cells) and parts of the hair shaft (hair fibre) and is usually destroyed by chemical depilation [1]. At the junction of epidermis and dermis, there is a coarse basement membrane that, after removal of the epidermis, becomes the surface of the leather. This layer contains collagens type IV, VII, XV and XVIII, laminin, nidogen, and perlecan (a basement membrane-specific heparan-sulfate proteoglycan core protein) [2]. The dermis (true skin) makes up 90% of the total skin weight and is rich in collagen fibres (collagens type I and III) that give it strength. Dermal fibroblasts synthesise and secrete the major protein components of skin; collagen, elastin, laminin, and proteoglycans (PGs), that are collectively termed the extracellular matrix (ECM) [3].
Diagram of the skin structure. Modified from Visser (2019) with permission from the author [4]
Collagen, approximately 60–70% of the dry weight of skin, is largely made up of a repeat sequence containing three amino acids glycine (Gly)-X–Y, where X and Y are commonly proline (Pro) and 4-hydroxyproline (Hyp), respectively. Although Hyp was once thought to be unique to collagen, it is also found in elastin and two other intracellular proteins, hypoxia-inducible factor (HIF) and argonaute 2 (Ago2) [5]. Nevertheless, it continues to be used as an indicator to determine the collagen content of skin. Hyp plays an important role in the structural and thermal stability of collagen microfibrils [6] directing their assembly through the formation of non-covalent interactions. These same interactions are also involved in the formation of microfibre bundles or fibres which are further stabilised by crosslinks between neighbouring lysine (Lys) or deaminated hydroxylysine (Hyl) residues and other Lys or Hyl sidechains [7, 8]. Finally, proteoglycans and their sugar components, glycosaminoglycans act as cement to hold the fibres and fibre bundles in place. From the leathermakers’ perspective, the dermis is often further divided into three layers: the grain, the grain-corium junction, and the corium (Fig. 1). The grain layer, the uppermost layer of the dermis, is composed of fine interwoven collagen fibres (mainly types I, III and VI), the entire length of the hair follicle, small blood vessels and capillaries [9,10,11]. The uppermost surface of the grain layer, also termed the enamel, is responsible for the outward appearance of the final leather product which is thought to be due to the presence of the beaded microfilament network formed by collagen VI [12]. The grain-corium junction is the transitional zone between the very fine fibres of the grain and the larger fibres of the corium layer. It is composed of relatively small collagen fibres (I, III, V, VI, XII, XIV, XVI) and carries the other structural components of the skin, such as blood vessels, and for sheep, lipocytes [13]. The corium layer contains thicker and more compact types I and III collagen fibres that form higher order structures varying in diameter. As these bundles form a major part of the leather, their relative orientations are responsible for its strength [14, 15]. The bottom layer of skin, the hypodermis, is also known as the flesh layer, is composed of mainly fat cells and is commonly removed prior to depilation [16].
The search for an environmentally friendly method to depilate animal skins to make leather has been going on for decades without much success. Although enzymes initially showed promise [9, 17,18,19], most damaged the skin, and many required the addition of sulfide to the enzyme mixture to increase depilation efficiency [20,21,22,23,24]. Most of all, they were not cost effective in comparison to the traditional chemical methods. A new process for depilating sheepskins using a milk by-product, permeate has recently been reported that appears to have no detrimental effects on the skin or leather produced from it [25]. This process not only depilates the skins but omits 5 steps in the traditional beamhouse process which together are responsible for one third of the pollution generated by the leather industry [17, 25].
To delve further into possible changes in their molecular structures, matched pieces of skin from the same area of a single animal were depilated with permeate or taken to pickle using sulfide depilation. They were then subjected to quantitative proteomic analyses to compare the relative abundances of proteins present in the skin after each treatment. In addition, to determine the effect of permeate depilation on the levels of biomolecules known to contribute to the physical properties of leather, quantitative biochemical analyses of the concentrations of collagen, glycosaminoglycans and specific crosslinks treated skin were carried out and compared to the levels in the same raw skin samples and levels reported by others in pickled skin. The purpose of this study was to correlate any changes seen in the building blocks of skin with the physical properties of leather made using both permeate and traditional beamhouse methods. The results provide the foundation for the development of a ’green’ leather industry that is cost effective.
2 Materials and methods
2.1 Chemicals and materials
Dihydroxy-lysinonorleucine (DHLNL) was purchased from Santa Cruz Biotechnology (Delaware Ave, CA, USA). Hydroxy-lysinonorleucine (HLNL), histidine-hydroxy-lysinonorleucine (HHL) and histidine-hydroxy-merodesmosine (HHMD) were isolated and purified in our laboratory by Dr. R. Naffa. A complete list of chemicals, materials and their manufacturer are listed in Additional file 1: Table S1.
2.2 Preparation of skin samples and the permeate solution
All sheepskins analysed were collected from a local freezing works (Ovation Ltd, Feilding, NZ). Fresh skins were transported to New Zealand Leather Shoe Research Association (LASRA) and the flesh removed. The skins were then taken back to the laboratory to be sampled. Two pieces of sheepskin approximately 15 × 8 cm were cut from two sides of backbone from the official sampling position (OSP) of three different animal skins. One side was processed with permeate as described in Tu et al. [25], and the other side kept as control (untreated for amino acid, collagen crosslink and glycosaminoglycan analysis; pickled for proteomics analysis). For clarity, the artificial permeate solution (4.5% (w/v) lactose, 0.0035% (w/v) CaCl2, 0.0045% (w/v) NaCl, 0.14% (w/v) KCl, and 0.05% lactic acid (v/v)) will be referred to as permeate solution. A more detailed description of the permeate depilation method can be found in supporting information.
2.3 Amino acid analysis of sheepskins
To estimate the amount of collagen in depilated skins, amino acid analyses of raw and permeate-depilated skins were carried out as described by Naffa et al. [26].
2.4 Collagen crosslink analysis of sheepskins
Three raw shaved (using a standard electric hair clipper) and three permeate-depilated sheepskins were randomly cut into three 3 × 3 cm squares which were finely chopped using a clean scalpel blade on a clean glass plate, then lyophilised. Samples were then reduced using NaBH4 and the crosslinks from each sample separated by CF-11 chromatography as described by Naffa et al. [27]. Crosslinks were fractionated by HPLC using a silica hydride column then quantified using mass spectrometry with additional ion fragmentation (Additional file 1: Tables S2 and S3). Parallel reaction monitoring (PRM) using tandem MS acquisition (Additional file 1: Table S4) with an inclusion list of ions was used to detect and quantitate the relevant ions for each crosslink (Additional file 1: Table S5). Fragment ion spectra produced via high-energy collision-induced dissociation (HCD) were acquired with a resolution setting of 35,000. Data was processed using Xcalibur™ Software v.4.1.31.9 (Thermo Scientific™, USA) then exported, and the data quantified using Excel. FreeStyle™ Software v.1.3.115.19 (Thermo Scientific™, USA) was used for data visualisation.
2.5 Glycosaminoglycan (GAG) analysis of sheepskins
GAGs were extracted from three raw and three permeate depilated sheepskins using the method of Farndale et al. [28] as optimised by Naffa [29]. Extracted GAGs were quantitated using the method described by Naffa [29].
2.6 Proteomic analysis of sheepskins
As the purpose of this experiment was to examine the effect of two different depilation treatments on the sheepskin proteome, only one biological sample was used as the objective of the study was to investigate the differences in the treatments of the skin. A skin sample was taken from the OSP region and divided into two parts. One was taken through to pickle using the conventional process while the other was treated with permeate. Five separate 3 × 3 cm squares were randomly cut from each processed skin piece and finely chopped using a scalpel blade before being lyophilised. Proteins were sequentially extracted from these five separate samples using three different methods. A schematic diagram of the sample preparation workflow is shown in Additional file 1: Fig. S1, and a detailed description of the extraction protocol is provided in the Additional file 1: SI Methods and Table S6. The methods used for in-gel trypsin-digestion are described in the Additional file 1: SI Methods and Table S7. The resulting peptide samples were desalted using an on-line reversed-phase peptide trap before being separated by reversed-phase nano-LC (Thermo Scientific™) using a 50 cm PepMap™ 300 C18 LC column (Thermo Scientific™). Eluted peptides were analysed by mass spectrometry (Q Exactive™ Plus Hybrid Quadrupole-Orbitrap™, with a Nano Flex ion source, Thermo Fisher Scientific, Bremen, Germany) using data-dependent tandem MS acquisition. Details for the chromatographic and mass spectrometry settings are provided in Additional file 1: Tables S8 and S9. Sample concentrations were normalised based on total ion current (TIC) for each MS1 spectrum before data collection. Raw data files were processed using Proteome Discoverer™ v. 2.4 and searched against the Ovis aries (sheep) database. Detailed search parameters are listed in Additional file 1: Table S10. The reverse database search option was used as a filter to satisfy a false discovery rate (FDR) of 1%. Abundance and abundance count data for proteins identified with high confidence by Proteome Discoverer™ were then exported into Excel and renormalised for the calculation of relative abundances. A two-tailed student’s t-test was done to obtain the p-values.
3 Results and discussion
3.1 Amino acid composition of raw and permeate-depilated sheepskins.
This analysis was carried out to determine whether there was any detrimental effect to the collagen content of the skin as a result of the permeate depilation process. It is critical that the collagen content is not compromised as it is the major component of leather and responsible for its organoleptic properties. Because Hyp in skin is found only in collagen and in relatively low abundance in elastin, the collagen content in tissues can be estimated from the assumption that Hyp makes up ∼ 13.5% (w/w) of collagen in mammals [30, 31]. Results showed that although there was no significant change in the relative overall protein content, permeate-depilated sheepskin had a much higher relative Hyp (collagen) content than that found in raw sheepskin due to the removal of other non-collagenous proteins from the skin during processing (Additional file 1: Table S11, Fig. 2). Similar results were found for pickled skin where the relative Hyp content reported for pickled skins increased to ~ 70% cf. 36% in raw skin [29]. For both processes, the quality of the resultant leather was not compromised [25].
3.2 Collagen crosslink analysis of sheepskins
Intermolecular collagen crosslinks are essential for the integrity of the skin structure contributing to the strength, stability, and quaternary structure of the collagen fibrils [9]. Though present in various ratios, all skin samples were shown to contain hydroxy-lysinonorleucine (HLNL), dihydroxy-lysinonorleucine (DHLNL), histidine-hydroxy-lysinonorleucine (HHL), and histidine-hydroxy-merodesmosine (HHMD) crosslinks (Fig. 3). Overall, the permeate-depilated skin lost approximately 60% its HLNL (immature) crosslinks compared to the concentration found in raw sheepskin (Fig. 3d). There was, however, no significant difference between the ratios of total mature (HHL + HHMD) to total immature (DHLNL + HLNL) crosslinks for the two treatments (Fig. 3b). The results, as expected, varied considerably between biological replicates, as shown by the box and whisker plots (Fig. 3). Interestingly, permeate depilation did not seem to affect the concentration of DHLNL, an immature crosslink, but instead, removed the mature crosslink HHMD almost completely (Fig. 3c, f). Interestingly, permeate depilation did not seem to affect the concentration of DHLNL, an immature crosslink, but instead, removed the mature crosslink HHMD almost completely (Fig. 3c, f). This implies that some permeate-specific degradation of HHMD occurred.
Collagen crosslinks in raw and permeate-depilated sheepskins. All values were normalised to the collagen content based on hydroxyproline concentration in skins. Each result presented was the average of three biological replicates and their respective three extraction technical replicates. A one-way ANOVA test was done to obtain the p-values. *p-value < 0.05
The significantly higher concentration of HHL in the permeate-depilated skin compared to that in raw skin is harder to explain. There are three possibilities: The first is a consistent error in sample preparation. This seems unlikely as the concentration of HHL in raw skin was almost identical to that found by Naffa [29] and Visser [4]. The second, but more unlikely is the presence of an amine oxidase derived from the microbial population, allowing the spontaneous formation of new HHL crosslinks. However, the complex order of events required for the formation of a trivalent crosslink [8] makes this possibility extremely unlikely. Nevertheless, fungi are known to produce amine oxidases [32], and Pichia, a fungus that produces this enzyme was identified in the depilation liquid [25]. It is also possible that because the collagen network of processed skins is opened up compared to that in raw skins, the hydrolysis efficiency and hence crosslink extraction may have been more efficient. However, in this case, the concentrations of the other three crosslinks should also have increased rather than decreased as found. A similar result was reported by Zhang et al. [33], where the levels of HHL in reduced pickled sheepskin were higher than those found in reduced raw sheepskin.
Research showing a direct correlation between collagen crosslinks and skin strength is lacking. Naffa et al. [26] reported that the total concentration of collagen crosslinks is positively correlated to skin strength, i.e., the higher the crosslink concentration, the stronger the skin. Although the collagen crosslink concentrations of pickled sheepskins were not measured in this study, Zhang et al. found that pickled sheepskins had approximately 50% less total collagen crosslinks compared to value in raw skin [33], which is similar to the decrease seen for the permeate-depilated skins (∼ 55%) in this study. Based on these findings, permeate-depilated sheepskins and pickled sheepskins should have similar tear strengths, a fact that was confirmed by the measurement of their tear and tensile strengths [25]. It is therefore clear that there is no detrimental effect to the resultant leather made using this alternative process.
3.3 Glycosaminoglycan analysis of sheepskins
While total carbohydrate concentration could not be measured in permeate processed skin because of the high concentration of lactose in the depilation liquid, it was possible to measure the GAG concentration. The extraction of GAGs from skin samples was optimised by Naffa [29] and showed that raw sheepskin contained approximately 0.9% (w/w) GAGs, which reduced to < 0.05% (w/w) in pickled sheepskins. While this study found similar levels of GAGs in raw sheepskins, those depilated with permeate retained at least 40% (0.4%, w/w) of the GAGs found in raw sheepskins (Additional file 1: Fig. S2). A widely accepted opinion held by leather technologists is that the removal of PGs and their associated GAGs from the skin is closely related to the opening up of collagen fibres during processing [1], improving leather properties such as softness. A reduction in dermatan sulfate, the most common GAG found in skins, to 0.2–0.3% (w/w) was found to be optimal to achieve the desired physical properties in the finished leather product [1]. Research aimed at increasing hair removal efficiency resulted in the introduction of proteases and glycosidases at the depilation stage, and as a result, proteoglycans in skins were almost completely removed [34, 35]. In this study, the increase in GAGs could be related to detection of the core proteins linked to them. Therefore, although the GAG concentration was higher than the ideal, there was a decrease compared to raw skins. Skin proteomic analysis showed that the proteoglycans decorin and lumican, which are modified with sulfated GAG chains were less abundant in permeate-depilated sheepskins than in pickled skins while those in the basement membrane were more abundant in the permeate-depilated skins (Table 2). Jayakumar et al. [34] speculated that the use of proteases and glycosidases to achieve depilation destroyed glycoprotein conjugates in the structures securing the hair root in the skin including the basement membrane. However, it was shown that this treatment also damaged the collagen structure of the skin, especially around the wool follicle. Using permeate, no such damage is seen [25]. Thus, the removal of proteoglycans from the dermis (decorin and lumican), paired with retention of proteoglycans in the basement membrane (versican, perlecan and biglycan) seems to be favourable to leather quality. We therefore speculate that the microbial community that develops in the permeate during incubation secretes enzymes that specifically but gently open the collagen network at the same time as loosening the wool from its follicle, allowing depilation to occur without damaging the collagen fibril network.
3.4 Proteomic analysis of sheepskins
To examine the differences in the protein composition of pickled sheepskins and sheepskins depilated using permeate, we subjected the extracted proteins to gel-LC–MS/MS analysis (Additional file 1: Figure S3). This method was chosen because of the superior results obtained using gel pre-fractionation of skins in previous studies, despite its acknowledged limitations [36]. After strict criteria filtering, a total of 189 proteins were identified with high confidence from both of the skin samples (Additional file 1: Table S12). This suggests that subjecting the skin to the beamhouse treatments and the permeate treatment both removed a substantial number of proteins. Mass spectrometry data is available from PRIDE (https://www.ebi.ac.uk/pride/). Username (Email): reviewer_pxd042383@ebi.ac.uk; Password: QOvYVVhI.
Figure 4 shows the volcano plot of permeate-depilated skin versus pickled skin for the 189 proteins found in this analysis. Sheepskin depilated with permeate generally had a higher relative abundance of proteins (permeate-depilated:pickled sheepskin, 52:29), confirming the amino acid analysis findings that the depilation treatment was not as harsh. The 50 most abundant proteins based on relative peptide spectrum matches, (PSMs) were identified and grouped according to their type and biological function (Table 1).
Volcano plot of the differentially abundant proteins found in sheepskins depilated using permeate and pickled sheepskins. The –Log10 p-value is plotted against the Log2 (fold change of permeate depilated sheepskin/pickled sheepskin). Orange dots, proteins that are at least twofold more abundant in permeate depilated sheepskins; Blue dots, proteins that are at least twofold more abundant in pickled sheepskins; Grey dots, proteins that are not significantly different in the two skin samples; Dashed vertical lines highlight fold-changes greater than 2; Dashed horizontal line highlights the cutoff of 0.05 for the p-value
3.4.1 Collagens
Of all the proteins identified, there were 14 different collagen chains representing nine types of collagen (I, II, III, IV, V, VI, VII, XII, XIV) (Table 1). Collagen types I (α1 and α2), and III (α1) are the main fibril-forming collagens of the skin, and are resistant to procedures such as liming, bating and pickling [29].
It was, therefore, no surprise that these were the most abundant collagens identified in skin. Despite the extended time the permeate-depilated skins spent in solution, which would normally result in swelling and loss of collagens, the relative abundances of collagen types I (α1 and α2), and III (α1) remained essentially the same as those in the sulfide depilated skins. The resultant leather produced from both depilation methods should therefore be of similar quality.
Although collagen type II is mainly associated with cartilage, it has been identified at low abundance but with high confidence in other proteomic studies of skin [4, 42, 43]. The results of this study showed that the levels of collagen type II did not significantly change between the two treatments, but it is mentioned because it confirms other results which show the presence of this collagen in sheepskin [4, 37, 38].
Collagen type IV, found mainly in the basement membrane, is part of a highly specialised ECM interface between the epidermis and the dermis, where it interacts with collagen types V, VI, and VII, laminin, nidogen and the basement membrane-specific heparan sulfate proteoglycan core protein, also named perlecan [2, 39,40,41]. Among the six different α IV chains known, the most predominant heterotrimer is formed from two α1 and one α2 chains, which then associate to form lattice like networks that interact with collagen VI [42]. In light of this we cannot explain why only the α2 isoform is present in significantly higher relative abundance in the permeate-depilated skins. It is possible that this isoform is interacting with other molecules retained in the basement membrane such as nidogen, biglycan, perlecan, and collagen VI (in the dermis), which are all also more abundant in permeate processed skin. Nevertheless, the results support the fact that the grain of the leather produced by the permeate process appears to be smoother than that of pickled skin [25].
Collagen type VI (isoforms α1, α2, α3), is the third most abundant collagen identified and isoform 3 is almost twice as abundant in the permeate-depilated skins, similar to the α2 isoform of collagen IV which three times more abundant. Although isoforms 1 and 2 show no significant change in relative abundance, isoform 2 is found at slightly higher abundance. Because isoform 3 controls the formation of the triple helix of collagen VI [43], the results suggest that the collagen VI matrix may be protected during permeate depilation in contrast to sulfide depilation where the skins are exposed to low pH at the pickle stage. In contrast to other collagens, the collagen VI triple helix forms disulfide-bonded tetramers that associate end to end to form an extensive network of beaded micro-filaments that are distributed throughout the dermal matrix and interspersed between the major collagen fibres [41]. It is particularly abundant just below the basement membrane at the epidermal-dermal junction [41, 42], the region of the skin that forms the grain layer in leather. Early proteomic studies identified collagen VI in sheepskins that produced high quality leather [44]. The smooth, even appearance of the leather produced in the two-step tanning process incorporating permeate depilation [25] could therefore be related to the retention of collagen VI, and its interaction with collagen IV and the proteoglycans laminin, perlecan, and lumican [41, 42] to keep the basement membrane intact.
Collagen VII is most abundant in the basement membrane, where it forms antiparallel collagen fibril dimers with each fibril composed of three identical α chains. The dimers ‘loop’ down from the basement membrane and ’hook’ onto collagen fibrils of the upper dermis interacting with principal components of the basement membrane such as laminin and collagen type IV [45], conferring tensile strength to the tissue [41]. In this study it was significantly retained in the permeate-depilated skin compared to pickled skin. The fact that collagens IV, VI and VII are more abundant in permeate-depilated skin, all of which are involved in stabilising the interaction of the basement membrane with the interstitial collagen fibres in the dermis, suggests that permeate depilation keeps the skin structure more intact at the surface, which is supported by the appearance of the depilated skins when examined by scanning electron microscopy (SEM) [25].
Two of the fibril-associated collagens with interrupted helices, collagen types XII, and XIV (known as FACIT collagens), that associate with the major structural collagen (type I) in skin [41, 46, 47] were also found in significantly higher relative abundances in permeate-depilated skin. Collagen XII, a homotrimer, is thought to modify the interactions between collagen I fibrils and the surrounding matrix and is commonly found in the dermis around hair follicles [46, 47]. It is present at threefold higher relative abundance in permeate-depilated skin compared to pickled skin. Because incubation of raw sheepskin in permeate resulted in complete removal of the wool fibre without damage to the hair follicle, it is perhaps not surprising that more collagen type XII was extracted from these skins. Of all the collagens showing differential abundances in skins processed using the two different methods, collagen type XIV showed the largest difference, being nearly fivefold more abundant in permeate-depilated skin. Collagen XIV is an important component of the fibre network in skin as it regulates fibrillogenesis and is responsible for the biomechanical function of skin. Both collagens XII and XIV are involved in the maintenance of skin integrity [48, 49]. The fact that a higher proportion of these is retained in permeate-depilated skins strongly suggests that the original collagen matrix of the skin is more preserved using this reported method of depilation, which includes pre-tanning and may be related to the improved appearance shown for leather produced from this novel two-step process.
3.4.2 Keratins and trichohyalin
Keratins are important structural proteins found primarily in the epidermis, the hair follicle, and the ECM (Fig. 5). Most of the keratins identified in the 50 most abundant proteins were epithelial keratins, i.e., cytoskeletal keratins expressed in the epidermal cells (Table 1). However, a wool keratin, K33a, was found to be 72-fold more abundant in permeate-depilated sheepskins. This type of keratin is the structural component of the wool fibre [50]. As the depilation process involved soaking whole sheepskin (i.e., with wool attached) in permeate for three to four days it is not surprising that the wool structure itself was affected. The fact that it had a silky texture compared to wool removed using the sulfide method suggests some modification to its structure occurred during the process that could have released some type I microfibrillar low sulfur keratin into the solution, which was then trapped on the skins. The process also possibly dissolved keratins 25–28 and 71–75 that are specifically found in the root sheaths of the hair follicle [51], as more than half of them were found to have a lower abundance in permeate-depilated skin compared to pickled skin (K25, K27, K71, K72, K75) (Table 1). In particular, keratins 27, 71 and 72 were found to be approximately 1.5 to fourfold less abundant in samples depilated with permeate compared to those depilated with sulfide and pickled (p-value < 0.05; Table 1). Some keratins are specifically expressed in the inner root sheath (IRS), a channel formed to protect the growing hair fibre (Fig. 5). The lower abundance of these keratins found in the permeate-depilated skin could potentially explain its relatively easy depilation, with these keratins being removed by microbial enzymes (most likely a mix of proteases and glycosidases) secreted into the permeate solution loosening the wool fibre. Trichohyalin (THH) is highly expressed in the IRS of the hair follicle, where it serves a biomechanical role by forming crosslinks with keratin intermediate filaments (KIFs) and other THHs, to form the rigid structure of the IRS [52, 53]. Though present in low abundance, THH was approximately twofold less abundant in the sheepskin depilated with permeate compared to that in pickled skin (p-value < 0.05; Additional file 1: Table S12), suggesting a greater breakdown of the sheath structure.
3.4.3 ECM proteins
Proteoglycans are glycoprotein conjugates comprised of a core protein with glycosaminoglycan moieties, such as chondroitin sulfate, dermatan sulfate, and heparin sulfate. They are important collagen interfibrillar proteins distributed throughout the skin and the hair follicle and are known to contribute to the skin and hair structure by interacting with collagen fibrils and other ECM proteins [45]. The major collagen-interacting PGs in skin, such as versican, basement membrane-specific heparan sulfate proteoglycan core protein (perlecan), and some small leucine-rich proteoglycans (SLRPs), including decorin, biglycan, lumican, mimecan, and asporin were identified in this study and those present in significantly different abundances to those in pickled skin are highlighted in blue in Table 2. Decorin and lumican, identified as proteoglycans interacting with collagens IV, VI and VII, in the basement membrane appear to be present in lower abundances in permeate-depilated skin compared to pickled skin, a result hard to correlate with the increase in the relative abundances of those same collagens in this study. Decorin, which was named for its decoration of collagen fibrils, is the most abundant PG found in the skin ECM and mediates collagen fibrillogenesis [54]. As it can also interact with collagen types I and III [55, 56], the major collagens in the dermis or corium, it is possible that the main losses of decorin are coming from this region of skin rather than the basement membrane. It has been shown that the collagen fibre ’opening-up process’ is related to the loss of decorin interactions with collagen fibrils, although the mechanism is not fully elucidated, and that the beamhouse processes reduce the abundance of decorin in skins [57]. Previous research has shown that the presence of residual amounts of decorin has a detrimental effect on the grain quality of the final leather product, i.e., higher decorin content results in inflexible leather [58]. Thus, the reduction in decorin in the permeate-depilated skins could result in a better leather product, although that remains to be seen. Lumican, a keratan sulfate PG, co-localises with fibrillar collagens and decorin in the ECM and like decorin is involved in collagen fibrillogenesis [59]. Similarly, its relative concentration in permeate-depilated skin was approximately half that found in pickled sheepskins. These findings indicate that the treatment with permeate potentially opens up the collagen fibre network of the dermis by removing the major ’glue’ proteoglycans, decorin and lumican. Asporin, biglycan, versican, mimecan and the basement membrane-specific heparan sulfate proteoglycan (perlecan) are, in contrast, more abundant in permeate-depilated skins. Biglycan is highly expressed in the basement membrane and the connective tissue sheath along the hair follicle [60]. While the removal of biglycan may promote hair/wool root loosening, its retention is in line with the observed increase in the relative abundance of basement membrane collagens supporting the probability that permeate depilation does not damage this membrane and hence the integrity of the grain structure. Mimecan, also called osteoglycan, regulates fibrillogenesis of collagen in the ECM by binding directly to collagens, and is responsible for the elasticity and tensile strength of skin [61]. The relative abundance of mimecan was increased 2.5-fold in permeate-depilated sheepskin but the significance of this result is difficult to explain, as mimecan interacts with the collagen fibrils in the dermis and would therefore be expected to follow the same trend as decorin and lumican. Versican, a large proteoglycan belonging to the hyalectan family, is abundant at the dermal papilla and proximal part of the connective tissue sheath (i.e. around the hair follicles) [45, 62]. It co-localises with elastic fibres (e.g. elastin and fibrillin) in the dermis, and interacts with hyaluronan in the ECM [45, 62]. Perlecan is specifically expressed in the basement membrane and interacts with collagen IV and laminin to stabilise the basement membrane and dermal papilla [45]. Both versican and perlecan are found around the hair follicle, and thus their higher concentration (74-fold and two-fold, respectively) in permeate-depilated sheepskin indicates the structure around the hair/wool follicle may suffer less damage after permeate depilation than in treatment with sulfide. As a result, the skin structure, and especially that of the basement membrane should be better preserved.
Other proteins found in higher relative abundance in permeate-depilated skin include dermatopontin, fibronectin and microfibril-associated glycoprotein 4 (Table 2). Dermatopontin, a structural ECM protein, is almost tenfold more abundant in the sheepskin depilated with permeate. It interacts with decorin and accelerates collagen fibril formation, influencing the arrangement of the collagen fibrils within the ECM [63]. Fibronectin, a high molecular weight glycoprotein in the ECM, binds to collagens and heparan sulfate proteoglycans, and is essential in the organisation and stability of ECM matrix [64]. Microfibril-associated glycoprotein 4 also interacts with ECM fibres such elastin and collagen. Although present in relatively low relative abundance, these proteins are all involved in the formation and maintenance of the collagen architecture of skin in its natural state. As raw sheepskin is inherently strong, the presence of these proteins should be beneficial to the product resulting from the process, i.e., the natural structure of the skin should be maintained to a greater extent in leather made using this novel two-step process than it is in the traditional beamhouse process. As reported in an earlier paper [25], this was indeed the case.
Overall, these results show that on a macro level, there is little difference in the protein building blocks of skins processed using either permeate or sulfide/lime/delime/bate/pickle. There are however differences in the detail that appear to be an advantage to the permeate depilation method. The most important of these appears to be the increased integrity of the basement membrane in the permeate processed skin that may have implications for the appearance of leather made using this process. The higher relative abundance of collagens IV and VII in the permeate-depilated skin, responsible for the structural integrity of the basement membrane; collagen VI that forms a structural network of beaded micro-filaments at the junction of the basement membrane and dermis; and perlecan and versican proteoglycans found at the basement membrane and connective tissue sheath, indicate that the treatment of permeate is milder than sulfide, and preserves these structural proteins. The lower abundance of decorin and lumican, however, shows the removal of these ’glue’ proteins in the dermis, results in the ’opening-up’ of the collagen fibre network, and the loss of interfibrillar proteins resulting in the lower number of proteins identified in this proteomic study. Finally, the lower relative abundance of keratins 27, 71 and 72 in the inner root sheath, probably reflects the reason the hair fibre is loosened in the hair follicle, allowing it to be easily removed.
4 Conclusions
Analysis of specific molecular components of sheepskins prepared for tanning using two different methods, one traditional (the beamhouse process) and one using permeate, a by-product of the milk industry has shown why the leather produced by the latter process on a laboratory scale produces an equivalent, if not better product. Although some collagen was lost, the collagens making up the basement membrane were better preserved than they were in skins prepared using chemical depilation. Unexpectedly, more proteoglycans remained in the skins depilated with permeate compared to those processed using sulfide. However, the proteomic results showed they were mainly those associated with the basement membrane, rather than decorin, which is primarily associated with links between collagen fibres, and is associated with fibrillogenesis and the mechanical properties of collagen [65]. Contrary to current opinion, the increased concentration of GAGs did not appear to affect the quality of the resultant leather. Crosslinks between collagen fibrils and fibre play an important role in skin architecture and the leather that is produced from it. Although there was no significant difference between the ratios of immature to mature crosslinks for skins processed using the two different methods, there were differences in the concentrations of individual crosslinks. While this did not seem to affect the quality of the processed skin or the resultant leather, we are unable to explain why it occurred. Unfortunately, we were unable to identify the specific enzymes that allowed the wool to be cleanly removed from the skin without damage, but it is clear that the microbes in the depilation liquid during the process were responsible for their generation. A metagenomic study of this population and its changes has been carried out and will be published at a later date. This study has shown that sheepskin can be successfully depilated using a natural product, avoiding the use of sulfide and strong alkaline solutions. The next step towards a cleaner process will be to trial it on a larger scale.
Availability of data and materials
Mass spectrometry raw data is available from PRIDE.
Username (Email): reviewer_pxd042383@ebi.ac.uk.
Password: QOvYVVhI.
References
Alexander K. Influence of proteoglycan removal on opening-up in the beamhouse. J Am Leather Chem Assoc. 1986;81:85–102.
Breitkreutz D, Koxholt I, Thiemann K, Nischt R. Skin basement membrane: the foundation of epidermal integrity–BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed Res Int. 2013;2013: 179784. https://doi.org/10.1155/2013/179784.
Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(24):4195–200. https://doi.org/10.1242/jcs.023820.
Visser DR. Unravelling the molecular contributions to collagen higher order structure. Master thesis, Massey University, Manawatu, New Zealand (2019)
Kaelin WG Jr. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–28.
Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58.
Junqueira L, Montes G. Biology of collagen–-proteoglycan interaction. Arch Histol Jpn. 1983;46(5):589–629.
Gaar J, Naffa R, Brimble M. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Org Chem Front. 2020;7(18):2789–814.
Bailey DG. Leather. In: Encyclopedia of polymer science and technology, 4th ed. Wiley (2011). https://doi.org/10.1002/0471440264.pst184
Kabashima K, Honda T, Ginhoux F, Egawa G. The Immunological Anatomy of the Skin. Nat Rev Immunol. 2019;19(1):19–30.
Maidment CA. Investigating the molecular building blocks of loose and tight cattle hide. Master thesis, Massey University, Manawatu, New Zealand (2019)
Edmonds RL, Deb Choudhury S, Haverkamp RG, Birtles M, Allsop TF, Norris GE. Using proteomics, immunohistology, and atomic force microscopy to characterize surface damage to lambskins observed after enzymatic dewooling. J Agric Food Chem. 2008;56(17):7934–41.
Addy V, Covington A, Langridge D, Watts A. Microscopy methods to study fat cells. Part 1: characterisation of ovine cutaneous lipids using microscopy. J Soc Leather Technol Chem. 2001;85(1):6–15.
Haines BM, Barlow JR. The anatomy of leather. J Mater Sci. 1975;10(3):525–38.
Sizeland KH, Basil-Jones MM, Edmonds RL, Cooper SM, Kirby N, Hawley A, Haverkamp RG. Collagen orientation and leather strength for selected mammals. J Agric Food Chem. 2013;61(4):887–92.
Nazer DW, Siebel MA. Reducing the environmental impact of the unhairing-liming process in the leather tanning industry. J Clean Prod. 2006;14(1):65–74.
Kamini N, Hemachander C, Mala JGS, Puvanakrishnan R. Microbial enzyme technology as an alternative to conventional chemicals in leather industry. Curr Sci. 1999;77:80–6.
Dettmer A, Ayub M, Gutterres M. Hide unhairing and characterization of commercial enzymes used in leather manufacture. Braz J Chem Eng. 2011;28(3):373–80.
de Sousa FM. Advances in understanding of enzymatic unhairing of bovine hides. J Am Leather Chem Assoc. 2014;109(8):268–76.
Kandasamy N, Velmurugan P, Sundarvel A, Raghava RJ, Bangaru C, Palanisamy T. Eco-benign enzymatic dehairing of goatskins utilizing a protease from a Pseudomonas fluorescens species isolated from fish visceral waste. J Clean Prod. 2012;25:27–33.
Lopéz LMI, Viana CA, Errasti ME, Garro ML, Martegani JE, Mazzilli GA, Freitas CDT, Araújo ÍMS, da Silva RO, Ramos MV. Latex peptidases of Calotropis procera for dehairing of leather as an alternative to environmentally toxic sodium sulfide treatment. Bioprocess Biosyst Eng. 2017;40(9):1391–8.
Sivasubramanian S, Manohar BM, Rajaram A, Puvanakrishnan R. Ecofriendly lime and sulfide free enzymatic dehairing of skins and hides using a bacterial alkaline protease. Chemosphere. 2008;70(6):1015–24.
Thanikaivelan P, Rao JR, Nair BU, Ramasami T. Recent trends in leather making: processes, problems, and pathways. Crit Rev Environ Sci Technol. 2005;35(1):37–79.
Wahyuntari B, Hendrawati H. Properties of an extracellular protease of Bacillus megaterium DSM 319 as depilating aid of hides. Microbiol Indones. 2012;6(2):77–82.
Tu Y-H, Ahn M, Rakonjac J, Holmes G, Norris G. The use of natural products in the leather industry: depilation without damage. Clean Eng Technol. 2022;8: 100464. https://doi.org/10.1016/j.clet.2022.100464.
Naffa R, Maidment C, Holmes G, Norris G. Insights into the molecular composition of the skins and hides used in leather manufacture. J Am Leather Chem Assoc. 2019;114(1):29–37.
Naffa R, Holmes G, Ahn M, Harding D, Norris G. Liquid chromatography-electrospray ionization mass spectrometry for the simultaneous quantitation of collagen and elastin crosslinks. J Chromatogr A. 2016;1478:60–7.
Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–8.
Naffa RM. Understanding the molecular basis of the strength differences in skins used in leather manufacture. Doctoral thesis, Massey University, Manawatu, New Zealand (2017)
Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950;184(1):299–306.
Neuman RE, Logan MA. The determination of collagen and elastin in tissues. J Biol Chem. 1950;186(2):549–56.
Duff AP, Cohen AE, Ellis PJ, Kuchar JA, Langley DB, Shepard EM, Dooley DM, Freeman HC, Guss JM. The crystal structure of Pichia pastoris lysyl oxidase. Biochemistry. 2003;42(51):15148–57.
Zhang Y, Naffa R, Garvey CJ, Maidment CA, Prabakar S. Quantitative and structural analysis of isotopically labelled natural crosslinks in type I skin collagen using LC-HRMS and SANS. J Leather Sci Eng. 2019;1(1):10.
Jayakumar G, Sathish M, Aravindhan R, Raghava-Rao J. Studies on the use of bi-functional enzyme for leather making. J Am Leather Chem Assoc. 2016;111(12):455–60.
de Souza FR, Gutterres M. Application of enzymes in leather processing: a comparison between chemical and coenzymatic processes. Braz J Chem Eng. 2012;29(3):473–82.
Goldman AR, Beer LA, Tang H-Y, Hembach P, Zayas-Bazan D, Speicher DW. Proteome analysis using Gel-LC-MS/MS. Curr Protoc Prot Sci. 2019;96: e93. https://doi.org/10.1002/cpps.93.
Maidment C, Ahn M, Naffa R, Loo T, Norris G. Comparative analysis of the proteomic profile of cattle hides that produce loose and tight leather using in-gel tryptic digestion followed by LC-MS/MS. J Am Leather Chem Assoc. 2020;115(11):399–408.
Mikesh LM, Aramadhaka LR, Moskaluk C, Zigrino P, Mauch C, Fox JW. Proteomic anatomy of human skin. J Proteomics. 2013;84:190–200.
Burgeson RE, Lunstrum GP, Rokosova B, Rimberg CS, Rosenbaum LM, Keene DR. The structure and function of type VII collagen. Ann NY Acad Sci. 1990;580:32–43.
Chanut-Delalande H, Bonod-Bidaud C, Cogne S, Malbouyres M, Ramirez F, Fichard A, Ruggiero F. Development of a functional skin matrix requires deposition of collagen V heterotrimers. Mol Cell Biol. 2004;24(13):6049–57.
Theocharis AD, Manou D, Karamanos NK. The extracellular matrix as a multitasking player in disease. FEBS J. 2019;286(15):2830–69.
Cescon M, Gattazzo F, Chen P, Bonaldo P. Collagen VI at a glance. J Cell Sci. 2015;128(19):3525–31.
Lamandé SR, Sigalas E, Pan TC, Chu ML, Dziadek M, Timpl R, Bateman JF. The Role of the Alpha3(VI) Chain in Collagen VI Assembly. Expression of an Alpha3(VI) Chain Lacking N-Terminal Modules N10-N7 Restores Collagen VI Assembly, Secretion, and Matrix Deposition in an Alpha3(VI)-Deficient Cell Line. J Biol Chem. 1998;273(13):7423–30.
Choudhury SD, Allsop T, Passman A, Norris GE. Use of a proteomics approach to identify favourable conditions for production of good quality lambskin leather. Anal Bioanal Chem. 2006;384(3):723–35.
Theocharidis G, Connelly JT. Minor collagens of the skin with not so minor functions. J Anat. 2019;235(2):418–29.
Agarwal P, Zwolanek D, Keene DR, Schulz JN, Blumbach K, Heinegård D, Zaucke F, Paulsson M, Krieg T, Koch M, Eckes B. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J Biol Chem. 2012;287(27):22549–59.
Berthod F, Germain L, Guignard R, Lethias C, Garrone R, Damour O, van der Rest M, Auger FA. Differential expression of collagens XII and XIV in human skin and in reconstructed skin. J Investig Dermatol. 1997;108(5):737–42.
Reichenberger E, Baur S, Sukotjo C, Olsen BR, Karimbux NY, Nishimura I. Collagen XII mutation disrupts matrix structure of periodontal ligament and skin. J Dent Res. 2000;79(12):1962–8. https://doi.org/10.1177/00220345000790120701.
Ansorge HL, Meng X, Zhang G, Veit G, Sun M, Klement JF, Beason DP, Soslowsky LJ, Koch M, Birk DE. Type XIV collagen regulates fibrillogenesis: premature collagen fibril growth and tissue dysfunction in null mice. J Biol Chem. 2009;284(13):8427–38.
Wilson BW, Edwards KJ, Sleigh MJ, Byrne CR, Ward KA. Complete sequence of a type-I microfibrillar wool keratin gene. Gene. 1988;73(1):21–31.
Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129(6):705–33.
Rothnagel JA, Rogers GE. Trichohyalin, an intermediate filament-associated protein of the hair follicle. J Cell Biol. 1986;102(4):1419–29.
Steinert PM, Parry DA, Marekov LN. Trichohyalin mechanically strengthens the hair follicle: multiple cross-bridging roles in the inner root sheath. J Biol Chem. 2003;278(42):41409–19.
Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19(4–5):249–55.
Raspanti M, Viola M, Forlino A, Tenni R, Gruppi C, Tira ME. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J Struct Biol. 2008;164(1):134–9.
Rühland C, Schönherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007;274(16):4246–55.
Shakilanishi S, Shanthi C. Specificity studies on proteases for dehairing in leather processing using decorin as model conjugated protein. Int J Biol Macromol. 2017;103:1069–76.
Kronick P, Iandola S. Persistence of minority macromolecules of hide through the beamhouse. III. Persistence of decorin. J Am Leather Chem Assoc. 1998;93:148–55.
Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141(5):1277–86.
Malgouries S, Thibaut S, Bernard BA. Proteoglycan expression patterns in human hair follicle. Br J Dermatol. 2008;158(2):234–342. https://doi.org/10.1111/j.1365-2133.2007.08339.x.
Kampmann A, Fernández B, Deindl E, Kubin T, Pipp F, Eitenmüller I, Hoefer IE, Schaper W, Zimmermann R. The proteoglycan osteoglycin/mimecan is correlated with arteriogenesis. Mol Cell Biochem. 2009;322(1–2):15–23.
Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15(7):483–94.
Takeda U, Utani A, Wu J, Adachi E, Koseki H, Taniguchi M, Matsumoto T, Ohashi T, Sato M, Shinkai H. Targeted disruption of dermatopontin causes abnormal collagen fibrillogenesis. J Investig Dermatol. 2002;119(3):678–83.
Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care (New Rochelle). 2016;5(3):119–36.
Pins GD, Christiansen DL, Patel R, Silve FH. Self-assembly of collagen fibres. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J. 1997;73:2164–72.
Acknowledgements
The authors would like to thank Dr. Rafea Naffa for the isolation of collagen crosslink standards and the advice given for the analysis of collagen crosslink and amino acid analysis, and LASRA for supplying the sheepskins. We would like to express our appreciation to Miss Christine Tan for her involvement in illustrating the graphical abstract and Fig. 1.
Funding
Massey University Agricultural and Life Sciences Trust (Project Number RM3000028979) and New Zealand Leather and Shoe Research Association (LASRA) through the Ministry of Business, Innovation and Employment (MBIE) Grant No. LSRX1801.
Author information
Authors and Affiliations
Contributions
GEN designed and monitored the research and edited the manuscript. MLP monitored the experiments and edited the manuscript. Y-HT conducted all the experimental work, analysed the results, carried out the proteomic analysis and wrote the manuscript. TSL collected mass spectrometry data.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary information of materials and methods, and results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tu, YH., Loo, T.S., Patchett, M.L. et al. Using proteomics to compare the molecular structures of sulfide and permeate-depilated sheepskins. Collagen & Leather 6, 4 (2024). https://doi.org/10.1186/s42825-023-00147-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-023-00147-1