Abstract
This study aimed to identify and classify the type of plants used for tanning historical leathers using cost-effective Fourier transform infrared (FTIR) spectroscopy. The investigation was carried out on five plants (oak bark, sumac, valonia, tara, and gallnut) and four historical leather samples from book bindings dating back to the Qajar period. Tannin extraction from both plants and leathers was performed using acetone–water solvent, and the samples were then subjected to FTIR spectroscopy. Pre-processing of the spectra included baseline correction, smoothing, and normalization. Principal component analysis (PCA) was used to identify the source of tannins based on FTIR results. FTIR was found to provide a good separation of condensed tannins from hydrolysable ones. However, PCA analysis allowed for the separation and identification of the type of plant used for tannin extraction. The examination of historical leather samples revealed that the primary classification based on the type of tannin is possible, but accurate identification faces challenges due to structural changes and degradation over time.
Graphical abstract

Similar content being viewed by others
1 Introduction
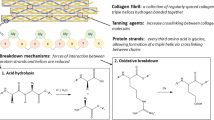
Leather artifacts, such as clothing, footwear, and practical items, have played a significant role in human history for thousands of years [1]. The examination and preservation of historical leather artifacts and their components provide valuable insights into past civilizations' technology, culture, and social practices. Tannins, a crucial component of leather, are essential in preserving and strengthening animal hides and skins' collagen fibers [2, 3]. Therefore, identifying tannins in historical leather is crucial for studying and preserving leather artifacts. The type of tannin can have an impact on the degradation mechanism of historical leathers. For instance, the use of condensed tannins, particularly mimosa extract, is one of the primary reasons for acid degradation in historical leather. Thus, it is crucial to identify the type of tannin present in leather to determine an effective conservation strategy [4, 5]. Additionally, the type of tannin used in leather production can impact the finished product's color, texture, and other properties, varying based on factors such as the region, time period, vegetation of the area, and cultural practices [6]. Thus, identifying tannins in historical leather can offer valuable insights into past civilizations' techniques, trade, materials, and social practices.
Vegetable tannins, which are natural compounds found in the bark, leaves, and fruits of various plants, belong to the polyphenol group of chemicals characterized by the presence of multiple phenolic hydroxyl groups in their structures. The chemical structure of vegetable tannins varies depending on the plant source and extraction method. Most vegetable tannins consist of a core structure of gallic acid, catechin, or ellagic acid. The resulting molecules can have different degrees of complexity and molecular weight, affecting their reactivity and solubility. Various plant species contain tannins, such as oak, chestnut, mimosa, quebracho, and tara, with tannin content and quality varying based on factors such as the plant's age, the season of harvest, and extraction method [3, 7,8,9].
Hydrolysable and condensed tannins are two broad categories of tannins traditionally used in the leather tanning process [3]. Hydrolysable tannins are common in the bark, leaves, and fruits of various plants, including sumac, oak, chestnut, tara, and myrobalan. In contrast, condensed tannins are found in many plant sources, including mimosa, quebracho, hemlock, alder, willow tree, and gambir [3, 5, 10]. However, hydrolysable tannins were generally used in leather production before the mid-nineteenth century [4, 11].
Identifying the tannins utilized in historical leather is a complex task due to the variation in materials and techniques used in leather production over time and across cultures, as well as degradation, contamination, and chemical changes that occur over time [12, 13]. Accurate tannin identification often requires a multidisciplinary approach combining chemical analysis with historical and archaeological research [14]. Various analytical techniques, including high-performance liquid chromatography (HPLC), spectrophotometry, Fourier transform infrared spectroscopy (FTIR), and mass spectrometry (MS), have been used to identify tannins in historical leather, along with several spot tests yielding satisfactory results [12, 14,15,16,17,18,19,20,21,22]. These methods offer information about tannin’s chemical composition and structure, allowing for the identification of specific tannin types.
Studies on tanning have mostly used spectroscopic methods and spot tests to identify the type of tannin used, with less emphasis on identifying the plant source. Recent research on tannins extracted from different plants has shown that chemometric methods, combined with spectroscopic techniques can classify extracts based on plant origin [23,24,25]. Accordingly, by analysing spectroscopic data and using appropriate chemometric methods can differentiate between tannins derived from different plant sources in leather. This study aims to identify the plant source used in historical leather tanning through primary classification using FTIR spectroscopy and chemometric methods. The study expands on previous research [6], which focused on modern leathers and condensed tannins, by focusing on historical leathers and plant sources containing hydrolysable tannins. The paper highlights the significance of identifying the sources of tannin in historical leather and aims to shed light on the lesser-studied aspects of historical leather works.
2 Materials and methods
2.1 Tannin extraction
Tannins used in the study were extracted from five sources, sumac, oak, tara, valonia, and gallnut. Different parts of the sumac plant, including fruit, leaf, and bark, were used. The four historical leather specimens obtained from Qajar book bindings were also studied, and their characteristics are presented in Table 1. Although slightly less flexible, these leathers were in relatively good condition with regard to their appearance. Examination of the hair follicle arrangement in all four bookbindings indicated the use of goat skin.
To extract tannins from plants, 200 mg of the powdered plant substance was mixed with 10 mL of a 70% acetone–water solution in a sealed container and placed in an ultrasonic bath. The Makkar [26] method was used to perform the extraction in two 10-min intervals, with a 5-min rest between them. The extract was filtered using Whatman filter paper grade 42, followed by centrifugation at 3000 rpm for 10 min. The centrifuged solution was separated and then placed in an oven at 70 °C for one hour to remove the solvent.
For tannin extraction from leather, a water–acetone solution (1:1) was used, and samples were collected from the collagen fibers of the reticular layer. For every 10 mg of leather fiber, 1 mL of the solution was added to the sample [17, 18]. The extraction was carried out in a sealed container at standard laboratory temperature over a period of 48 h, during which the samples were placed on a shaker. Afterward, the solution was filtered, followed by centrifugation and solvent removal, as described in the previous paragraph for tannin extraction from plants.
2.2 FTIR spectroscopy and principal components analysis
Fourier transform infrared spectroscopy (FTIR) was utilized to investigate the molecular structure of the extracts. The tannins extracted from the plants and historical leathers were analysed using the KBr pellet method. In this method, the extract and KBr powder were mixed in a ratio of 1:100 w/w to prepare the sample plates. The FTIR analysis was performed using a Jasco 680-plus FTIR spectrometer (Jasco Inc., Japan), and the spectra were recorded in 64 scans with a resolution of 2 cm−1 in the range of 400–4000 cm−1.
The software Originpro2021 was used for spectrum analysis. Before analysis, the spectra were pre-processed by correcting the baseline in the range of 650–1850 cm−1, applying a 25-point smoothing, and normalizing the data to the standard normal distribution using Z-scores (standardize to n(0,1)). The corrected spectra were subjected to Principal Component Analysis (PCA) using the correlation matrix, from which three components were extracted. The discriminatory power of the different components in two dimensions was evaluated. For the PCA analysis, a total of 31 samples were examined, including 27 plant extracts and 4 historical leather extracts. The analysis utilized 1885 variables obtained from FTIR spectra within the range of 900–1850 cm−1. Additonal file 1 contains the data utilized in the PCA analysis.
3 Results and discussion
Examination of spectral data has confirmed the presence of tannins in all samples. Figure 1a depicts exemplary FTIR spectra of gallnut and mimosa extracts. Tannins can be detected in FTIR spectra by observing the characteristic absorption bands at 1610 and 1450 cm−1 related to aromatic ring stretching vibration, 1210 and 1040 cm−1 related to C–O stretching vibration, and 1510 cm−1 related to the skeletal vibration of aromatic rings. Condensed and hydrolysable tannins exhibit their unique peaks in the spectra. The presence of two peaks attributed to the vibration of C=O stretching bonds of free gallic acid at 1710 cm−1 and the vibration of C–O–C stretching and OH deformation bands at 1330 cm−1 indicate the presence of hydrolysable tannins, which can be identified as gallotannins if additional absorption bands at 1073 cm−1 (aryl phenolic ester C–O–C symmetric stretching), 870 cm−1 (C–C stretching), and 760 cm−1 (sugar ring breathing vibration) appear. Otherwise, they can be considered ellagitannins. Conversely, condensed tannins contain an oxygenated heterocyclic ring that produces characteristic vibrations, a peak around 1285 cm−1 due to the asymmetric stretching vibration of the pyran ring. When this signal is coupled with strong bands at about 1160 and 1115 cm−1, and weak bands at about 976 and 842 cm−1, in addition to the characteristic bands of tannin, the extract can be unambiguously assigned to the condensed tannins group [3, 6, 14, 19, 23, 25, 27]. Based on the provided information, Fig. 1b presents a flowchart that suggests using FTIR spectroscopy to determine the type of tannin. Accordingly, the analysis of the FTIR spectra of all samples confirms the presence of hydrolysable tannins.
a FTIR spectra of mimosa and gallnut extracts as sources of condensed and hydrolysable tannins, respectively. In these spectra, the characteristic absorption bands of tannins, condensed tannins, hydrolysable tannins, and gallotannins are marked with green, purple, blue, and orange highlights, respectively. b The flowchart shows the identification of the tannin type based on the absorption bands in the FTIR spectrum
The classification of the studied extracts was done using PCA analysis. The PCA score plot, PC1 versus PC2, obtained from the analysis of raw FTIR spectra of the extracts is presented in Fig. 2. PC1 and PC2 represent approximately 80% and 10% of the total variance, respectively. As is clear, the score plot indicates that there is a good correlation between some extracts, such as gallnut or sumac leaves clusters. However, the PCA analysis of raw spectra does not effectively separate different sources of the studied plant tannins. Therefore, a preprocessing step is required on these spectra.
To prepare the spectral data for principal component analysis, three pre-processing stages were conducted, as illustrated in Fig. 3. The first stage involved uniformly correcting the baseline with respect to the spectrum index peaks using the second derivative. Subsequently, the corrected spectra were smoothed to eliminate noise. Various normalization methods were evaluated, and it was determined that the Z-score normalization method produced the most desirable outcomes for this study. Overall, the pre-processing steps executed on the spectra played a crucial role in reducing the impact of noise and variations, resulting in a more reliable classification of plant tannin extracts.
PCA analysis was performed on the spectral data, and the results revealed that the first three principal components (PC1, PC2, and PC3) accounted for 71.29%, 12.06%, and 8.36% of the variance, respectively. The cumulative variance of these three components was 91.71%. Loadings of these principal components in the range of 900–1800 cm−1 are illustrated in Fig. 4a. Notably, PC2 exhibited a strong negative correlation around 1150–1300 cm−1, which is associated with C–O stretching vibrations, while PC3 showed a similar correlation around 1300–1400 cm−1, related to C–O–C stretching vibrations and O–H deformation bands.
In Fig. 4a, b PCA score plot is displayed, showing the separation of extracts into three distinct clusters based on PC1 (accounting for 71.29% of total variance) and PC2 (accounting for 12.06% of total variance). The first cluster consists of oak bark extracts, primarily isolated by PC2. The second cluster includes tara and valonia extracts, which are relatively separated but highly correlated. The third cluster comprises extracts from various parts of sumac and gallnut. However, the differentiation of tannin extracts is much more apparent in the PCA score plot using PC1 versus PC3 (accounting for 8.36% of total variance), as depicted in Fig. 4c. PC3 has resulted in better separation of valonia from tara and has also enabled differentiation of different sumac extracts (leaf, fruit, and bark) from each other and gallnut.
The plot shown in Fig. 3d displays the scores of PC2 versus PC3, which account for a small percentage of the total variance (around 20%), but are effective in distinguishing between different extracts. PC2 effectively separates oak and tara extracts (positive scores) from the extracts of sumac (bark, leaves, and fruit), gallnut, and valonia (negative scores). On the other hand, PC3 distinguishes between the tannin extracts of different parts of sumac. Specifically, the tannin extract from sumac leaves is assigned positive scores, while that of the fruit is assigned negative scores, and the tannins from the plant skin are assigned both positive and negative scores. Moreover, these two principal components lead to the separation of gallnut and valonia tannins from each other as well as other extracts.
It is worth noting that the segregation of the extracts into distinct categories may be due to variations in their chemical compositions. Tannins are a complex mixture of compounds with diverse molecular structures, which result in their distinct physical and chemical characteristics. Therefore, these findings have significant implications for the creation of tannins with specific attributes, depending on their intended purpose. For instance, separating gallnut and valonia tannins from each other can be advantageous in generating tannins with unique properties that cannot be obtained from a single source. Nevertheless, further research is necessary to confirm these findings and investigate their potential applications.
The orange star symbols in the score plots presented in Fig. 4 correspond to extracts of historical leathers. As previously mentioned, the FTIR spectrum reflects the chemical structure of tannins present in the extracts. However, it is important to consider the effects of degradation caused by the passage of time, such as oxidation, on historical samples [12, 13]. These changes can alter the characteristics of the chemical structure and, as a result, the FTIR spectrum. Therefore, the possibility of the effect of these structural changes on the results should be taken into account, which can make the identification of the plant used in historical leather tanning a challenging task. Nevertheless, the principal component analysis displayed in Fig. 4c, d has positioned two samples of these leathers near the oak extract cluster and two samples near the gallnut extract cluster, indicating the possibility of using these tannin sources in the tanning of these four historical leather samples.
In support of these results, we can also refer to the traditional and historical tanning practices in Iran, where compounds such as gallnut, oak, and sumac have commonly been used in the vegetable tanning of historical leathers [14, 28, 29]. Based on this, it appears that the method investigated, which includes FTIR and PCA, provides an acceptable solution, at least for the initial classification of leathers based on the diversity of tannin sources present in them. However, the necessity of additional analyses, especially chromatographic methods coupled with mass spectrometry, cannot be ignored for the accurate identification of plant sources.
4 Conclusion
In conclusion, our study has provided valuable insights into differentiating tannin extracts based on their chemical composition using FTIR and PCA. We have successfully identified the presence of hydrolysable tannins in all samples, and the application of principal component analysis has facilitated the separation of extracts into distinct groups based on their plant source. Notably, the PCA score plots have effectively distinguished between various tannin extracts derived from different plants along with different plant parts, including leaves, bark, and fruit.
Furthermore, our analysis of historical leather samples has revealed the potential use of tannin sources such as gallnut and oak in tanning, which is consistent with traditional and historical tanning practices in Iran. These findings provide promising avenues for future research to validate their applications in the tanning industry and explore their potential use in studying historical leathers.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Doyon L, Faure T, Sanz M, Daura J, Cassard L, d’Errico F. A 39,600-year-old leather punch board from Canyars, Gavà, Spain. Sci Adv. 2023;9(15):0834.
Farooq U, Shafi A, Akram K, Hayat Z. Fruits and nutritional security. In: Srivastava AK, Hu C, editors. Fruit crops: diagnosis and management of nutrient constraints. Elsevier; 2020. p. 1–12.
Falcão L, Araújo MEM. Vegetable tannins used in the manufacture of historic leathers. Molecules. 2018;23(5):1081.
Koochakzaei A, Achachluei MM. Red stains on archaeological leather: degradation characteristics of a shoe from the 11th–13th centuries (Seljuk period, Iran). J Am Inst Conserv. 2015;54(1):45–56.
Koochakzaei A, Ahmadi H, Mallakpour S. An experimental comparative study of the effect of skin type on the stability of vegetable leather under acidic condition. J Am Leather Chem Assoc. 2018;113(11):345–51.
Koochakzaei A, Bidgoli MS, Safapour S. Feasibility assessment of the identification of the source of condensed tannins in leathers by FTIR spectroscopy and chemometrics. J Am Leather Chem Assoc. 2022;117(12):515–9.
Hagerman AE, Butler LG. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981;256(9):4494–7.
Mooney HA, Harrison AT, Morrow PA. Environmental limitations of photosynthesis on a California evergreen shrub. Oecologia. 1975;19(4):293–301.
Garro Galvez JM, Riedl B, Conner AH. Analytical studies on Tara tannins. Holzforschung. 1997;51(3):235–43.
Jayanegara A, Goel G, Makkar HPS, Becker K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim Feed Sci Technol. 2015;209:60–8.
Liénardy A, Van Damme P. Inter folia: manuel de conservation et de restauration du papier. Belgium: Institut royal du patrimoine artistique; 1989.
Sebestyén Z, Jakab E, Badea E, Barta-Rajnai E, Şendrea C, Czégény Z. Thermal degradation study of vegetable tannins and vegetable tanned leathers. J Anal Appl Pyrol. 2019;138:178–87.
Vyskočilová G, Ebersbach M, Kopecká R, Prokeš L, Příhoda J. Model study of the leather degradation by oxidation and hydrolysis. Herit Sci. 2019;7:26.
Koochakzaei A, Mallakpour S. Identification of surface dyeing agents of two bookbinding leathers from 19th-century Qajar, Iran, using LC–MS, μXRF and FTIR spectroscopy. Archaeometry. 2023;65(3):603–16.
Abdel-Maksoud G. Analytical techniques used for the evaluation of a 19th century quranic manuscript conditions. Measurement. 2011;44(9):1606–17.
Sebestyén Z, Badea E, Carsote C, Czégény Z, Szabó T, Babinszki B, et al. Characterization of historical leather bookbindings by various thermal methods (TG/MS, Py-GC/MS, and micro-DSC) and FTIR-ATR spectroscopy. J Anal Appl Pyrol. 2022;162:105428.
Wouters J. High-performance liquid chromatography of vegetable tannins extracted from new and old leathers. In: Proceedings of the 10th Triennal meeting ICOM committee for conservation; 1993, 22–27 August Washington, DC, USA. London, UK: James & James for ICOM-CC.
Falcão L, Araújo MEM. Tannins characterization in historic leathers by complementary analytical techniques ATR-FTIR, UV-Vis and chemical tests. J Cult Herit. 2013;14(6):499–508.
Falcão L, Araújo MEM. Application of ATR–FTIR spectroscopy to the analysis of tannins in historic leathers: the case study of the upholstery from the 19th century Portuguese Royal Train. Vib Spectrosc. 2014;74:98–103.
Falcão L, Araújo MEM. Tannins characterisation in new and historic vegetable tanned leathers fibres by spot tests. J Cult Herit. 2011;12(2):149–56.
Melniciuc N, Pui A, Florescu MS. FTIR spectroscopy for the analysis of vegetable tanned ancient leather. Eur J Sci Theol. 2006;2(4):49–53.
Koochakzaei A, Oudbashi O. A courtly brocade belt belonging to Qajar period (1789–1925). Iran Herit Sci. 2023;11:29.
Grasel FS, Ferrão MF, Wolf CR. Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochim Acta A Mol Biomol Spectrosc. 2016;153:94–101.
Grasel FS, Ferrão MF, Wolf CR. Ultraviolet spectroscopy and chemometrics for the identification of vegetable tannins. Ind Crops Prod. 2016;91:279–85.
Ricci A, Parpinello GP, Olejar KJ, Kilmartin PA, Versari A. Attenuated total reflection mid-infrared (ATR-MIR) spectroscopy and chemometrics for the identification and classification of commercial tannins. Appl Spectrosc. 2015;69(11):1243–50.
Makkar HPS. Treatment of plant material, extraction of tannins, and an overview of tannin assays presented in the manual. In: Makkar HPS, editor. Quantification of tannins in tree and shrub foliage: a laboratory manual. Dordrecht: Springer; 2003. p. 43–8.
Fernández K, Agosin E. Quantitative analysis of red wine tannins using Fourier-transform mid-infrared spectrometry. J Agric Food Chem. 2007;55(18):7294–300.
Wulff HE. The traditional crafts of Persia. Cambridge: MIT Press; 1966.
Parsa A. Medicinal plants and drugs of plant origin in Iran. IV Plant Food Hum Nutr. 1960;7:65–136.
Acknowledgements
The authors would like to thank authorities of Tabriz Central Library for their cooperation in preparing historical samples.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AK designed the work process and analysed and interpreted the data. MS participated in extract preparation and experimental. All authors discussed the results and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. The data utilized in the multivariate analysis consisted of preprocessed FTIR spectra.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koochakzaei, A., Sabaghian, M. Tannin characterization and sourcing in historical leathers through FTIR spectroscopy and PCA analysis. Collagen & Leather 5, 21 (2023). https://doi.org/10.1186/s42825-023-00128-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-023-00128-4