Abstract
The purple spaghetti-eel Moringua raitaborua lives on the sandy or muddy bottoms of estuaries, which are subject to rapid and wide changes in salinity, pH, and osmoregulatory and hypoxic conditions due to the influx of organic materials from sources of freshwater. The species has adapted to hypoxic environments by developing a thicker epidermis with stratified polygonal cells, club cells, two types of mucous cells (goblet and, oval cells), stratified cuboidal cells and dermis with abundant blood capillaries. Among them, histological modification of thinner dorsal, lateral, and ventral body skin to include abundant capillaries and well-developed dermal vascularization may provide cutaneous respiration, permitting survival in brackish waters with low levels of oxygen and variable environmental parameters.
Similar content being viewed by others
Introduction
Teleost species of fish exchange dissolved oxygen and carbon dioxide in aquatic environments through diverse respiratory adaptations such as gills (Park et al. 2014), skin (Glover et al. 2013), intestines (Park and Kim 2001), labyrinth organs (Zaccone et al. 2019), buccal cavities (Zaccone et al. 2018), swim bladders or hydrostatic organs (Zaccone et al. 2012), opercula (Summerfelt and Smith 1990), and lungs (Glass and Rantin 2009). Among them, the skin is a significant respiratory mediator that enables teleosts to absorb 5 to 30% of supplementary oxygens (Nilsson et al. 2004; Kim and Park 2011). This percentage rises to 50% in amphibious mudskippers, which spend much of their lives in air (Graham 2011). Teleosts have histologically adapted skin to allow for cutaneous respiration through the following mechanisms (Beon et al. 2013; Glover et al. 2013; Kim 2022): a thicker epidermis with diverse gland and large cells; the presence of intraepithelial blood capillaries; a defined lymphatic space at the basal layer of epidermis; well-vascularized connective tissue, an absence of scales; and other specific multicellular adaptations related to gas exchange.
The purple spaghetti eel Moringua raitaborua, which has an elongated and stubby body migrates from freshwater to seawater habitats in tropical zones in Nepal, India, Bangaladesh, and Philippines as they mature, and is most often found burrowed in the muddy bottoms of estuaries (Menes et al. 2010; Kottelat 2013; Behera et al. 2021). In general, coasts and estuarine zones have hypoxic water (2.8 mg O2 L-1 or lower) caused by excessive nutrient runoff, algal blooms, and stagnant water during dry season (Zhang et al. 2013; Mishra 2020). With such environmental conditions, brackish water–dwelling fishes are exposed to considerable skin stress that requires physiological tolerance of rapid changes in salinity, dissolved oxygen levels, pH, and water volume (Hu and Cai 2013; Robbins and Lisle 2018). While researching the histology of fishes inhabiting intertidal pools and estuarine standing water, dermal vascularization (as in Rhinogobius brunneus and Tridentiger brevispinis) was found in the skin of M. raitaborua (Kim 2022; Kim et al. 2022). This study aims to describe the skin structure and analyze the morphometry of the epidermal thickness and diffusion distance of M. raitaborua, along with their relevance to cutaneous respiration.
Materials and methods
Specimen collection
Five adult M. raitaborua individuals (20.2, 24.6, 28.3, 28.5, 32.0 cm in standard length, respectively) were purchased at a fish market (Aquapro) after being imported from India on December 16, 2021. For examination by light microscopy, the specimens were fixed in a 10% neutral buffered formalin solution at pH 7.4 for 1 day after receiving 0.05% tricaine methanesulfonate (MS-222, Sigma, St. Louis, MO, USA) as anesthesia in the laboratory. The experimental procedures strictly followed the rules of Jeonbuk National University Institutional Animal Care and Use Committee for animal ethics (License Number: CBNU-2023-00060).
Microscopic investigation
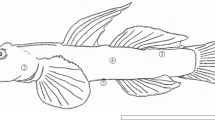
Each skin region (operculum, dorsal body, lateral body, ventral body; Fig. 1) of M. raitaborua specimens fixed with formalin solution was dissected to approximately 0.5 cm2, respectively. Each tissue was processed through an ascending series of concentrations (50–100%) of alcohol for 1 h, cleared with xylene, and then embedded in ordinary paraffin at 65 ℃. The paraffin-embedded tissue blocks were serially sectioned at 5 cm intervals with a microtome (Jung Histocut, model 820-II, Leica, Wetzlar, Germany) and mounted on microscope glass slides. Each section was then deparaffinized in xylene, dehydrated through descending alcohol concentrations (100–50%), and stained with hematoxylin and eosin (H&E) and Masson’s trichrome to confirm blood capillaries, connective tissue, basement membranes, and specific cells. Images of stained tissues were acquired with a light microscope (Imager A1, Carl Zeiss, Germany) and analyzed in Axio Vision (LE REL. 4.5, Carl Zeiss).
Statistical analysis
A regional comparison of epithelial thickness and diffusion distance (the shortest distance from a capillary to the skin’s surface) of each skin sample was performed using PASW SPSS statistical software (SPSS version 18.0, IBM, Armonk, NY, USA). The normality and homogeneity of variance for all samples was verified by Kolmogorov–Smirnov or Shapiro–Wilk test and Levene’s test (P > 0.05). One-way analysis of variance (ANOVA) with Tukey’s honestly significant difference test was used to compare the mean of data for epithelial thickness and diffusion distance. An analysis of covariance (ANCOVA) was utilized for statistical analysis of diffusion distance based on covariate epithelial thickness. The Pearson correlation coefficient was used to determine the positive linear association between two factors.
Results
Histology
The skin of M. raitaborua individuals was classified into two main parts, the epidermis (ED) and dermis (DM), which are separated by a basement membrane (Figs. 2 and 3).
Histological characteristics of the operculum and dorsal body skin of Moringua raitaborua, stained with Hematoxylin and Eosin (A and C), Masson’s trichrome (B and D). A and B, the operculum consisting of the epidermis (ED) having the outermost surface layer with stratified polygonal cells (SPC), the stratum spinosum with club cells (CC), two types of mucous cells (MCI and MCII), and the stratum germinativum (SG) with stratified cuboidal cells (SCC), the dermis (DM) having the stratum laxum (SL) with blood capillaries (yellow asterisk) and the stratum compactum (SC); C and D, the dorsal body having ED with SPCs, CCs, MCI, MCII, SCCs and DM having SL with abundant blood capillaries. All bars indicate 50 μm
Histological characteristics of the lateral and ventral body skin of Moringua raitaborua, stained with Hematoxylin and Eosin (A and C), Masson’s trichrome (B and D). A and B, the lateral body consisting of the epidermis (ED) with stratified polygonal cells (SPC), club cells (CC), two types of mucous cells (MCI and MCII), stratified cuboidal cells (SCC) and the dermis (DM) with blood capillaries (yellow asterisk); C and D, the ventral body consisting of ED with SPC, CC, MCII, SCC and DM with blood capillaries. All bars indicate 50 μm
The epidermis consisted of the outermost surface layer (OS), stratum spinosum (SS), and stratum germinativum (SG) (Fig. 2A). The OS is an upper region built of stratified polygonal cells (SPC). The SS is a thicker region with diverse multi-cells such as club cells (CCs), and mucous cells (MCs). The SG consisted of a single basal layer of stratified cuboidal cells (SCCs) at the bottom of the ED. The SPCs had a polygonal cell with a violet nucleus and faint cytoplasm when stained with H&E, and a purple nucleus and weak blush cytoplasm when stained with Masson’s trichrome (Figs. 2A–C and 3). The CCs had a wide cylindrical body with small violet nucleus and pink cytoplasm by H&E and small violet nucleus and faint cytoplasm by Masson’s trichrome, and constituted most of the SS in the dorsal body, lateral body, and ventral body (Figs. 2 and 3). The MCs were classified into two types of unicellular gland cell (MCI and MCII). MCI was an elongated tubular cell extending from the basement membrane to the surface and showed an upper part (purple with H&E and blush with Masson’s trichrome) and a reddish lower part of the cell body and the basal position of nucleus. These were more abundant and larger in the operculum than in other skin regions (Fig. 2 A). MCII had a flattened nucleus at the cell bottom and wide cytoplasm (hazy color with H&E and blush with Masson’s trichrome) (Figs. 2A, B, and D and 3B, C, and D). The SCCs were small but densely arranged cells on the basement membrane, with a larger nucleus and narrower cytoplasm (Fig. 2).
The DM comprised the SL and SC. The dermis contained a few capillaries, with one to four blood cells just below the basement membrane in the operculum (Fig. 2A and B), and featured well-developed vascularization, with numerous blood cells among dermal collagen fibers the dorsal, lateral, and ventral body (Figs. 2C–D and 3).
Morphometry
Measurement of epithelial thickness revealed a regional difference: the operculum was the thickest (mean = 315.4 ± standard deviation [SD] = 24.7; range = 258.5–358.0), with the lateral body (241.9 ± 30.6; 187.9–295.2) and dorsal body (238.0 ± 15.1; 216.8–283.0) exhibiting similar values, and the ventral body was the thinnest (191.9 ± 32.1; 139.1–263.1). These measurements showed a highly significant difference in epithelial thickness (one-way ANOVA, df = 3, f = 111.457, p < 0.001; Fig. 4A). The diffusion distance also included a relative difference between four regions: the operculum was associated with the highest value (346.1 ± 32.0; 257.3–409.2), followed by the lateral body (262.9 ± 30.3, 216.5–319.7), dorsal body (258.1 ± 23.5; 208.7–309.5), ventral body (208.1 ± 38.7, 148.8–281.5). These showed a highly significant difference in diffusion distance (one-way ANOVA, d f = 3, f = 98.259, p < 0.001; Fig. 4A). The diffusion distance between the four skin regions was strongly affected by epithelial thickness as a covariate (ANCOVA, df = 3, f = 13.671, p < 0.001; Fig. 4B). The two factors were highly and positively correlated in the four skin regions (Pearson’s correlation coefficient, r = 803, p < 0.001; Fig. 4B).
Regional comparison of epithelial thickness and diffusion distance of Moringua raitaborua skin. A line and bar graphs for relative difference between four skin regions (operculum, dorsal body, lateral body, ventral body); B a scatterplot graph showing a correlation between epithelial thickness (x-axis, n = 20) and diffusion distance (y-axis, n = 20) in each skin region. Red circle, operculum; yellow triangle, dorsal body; X, lateral body; blue diamond, ventral body. DD, diffusion distance; MED, a measured value of epithelial thickness and diffusion distance
Discussion
Fish skin is a multi-functional envelope that acts as physical barrier to potential bacterial infections (Zhang et al. 2021), abrasion (Lv et al. 2023), sensory system (Mogdans 2019), color expression (Vissio et al. 2021), much more freedom motion (Clark et al. 2016), acid-base regulation (Perry and Gilmour 2006), excretion of nitrogenous compounds (Wood 1993), and osmoregulation (Marshall 2012). Such physiologies are well-supported by the ED with SPCs, CCs, MCs, and SCCs confirmed in this study. Among them, two unicellular secretary glands, CCs and MCs, not only produce alarm-clue chemicals (proteins and pheromones such as serotonin and 5-HT) with cytoplasmic membrane breakage for antipredator response in conspecifics (Zaccone et al. 1990; Carreau-Green et al. 2008; Manek et al. 2013) but also engage in defense against pathogens that can penetrate the skin (Pollock 2011) and repair damaged tissues with chondroitin and keratin (Damasceno et al. 2012). They can help oxygen penetrate deeper toward the dermal matrix of connective tissue due an abundance of water and acidophilic proteins of a positive ion (Jakubowski 1958; Mittal and Munshi 1971; Park 2002). These reports indicate that CCs and MCs of M. raitaborua may constitute a cytological delivery system for efficient oxygen diffusion or storage in cutaneous respiration, and act as a skin protector against harmful substances a fish migrating can expect in encounter in contaminated habitats.
M. raitaborua also had two types of MCs: elongated (MCI, ii) and oval (MCII). The elongated MCI is a goblet mucous cell due to its nucleus position, cell morphology, and histochemistry, and has been reported in the skin of other teleosts (Rakers et al. 2011; Mohamed et al. 2020; Abolfathi et al. 2022). Fishelson (1996) noted that abundant goblet cells of the skin of the marine eel Siderea grisea skin are relevant to skin-damage reduction during movement on a hard substrate and the initiation of digging into the substrate. Elsheikh (2012) confirmed that goblet cell secretion of Oreochromis niloticus protects the epidermis of the buccal cavity from physical abrasion during feeding. These findings support the presence of more MCIs of the operculum of M. raitaborua, which feeds on burrowing fish or invertebrates living in the sand and dig into bottom substrate using its head as ecology, at least in genus Moringua (Smith 1997).
Many amphibious fishes exhibiting cutaneous respiration contain a thicker ED produced by large secretary cells as follows: 38.4–156.8 μm thick, a freshwater goby Rhinogobius brunneus (Kim et al. 2022); 35.4–150 μm, a trident goby Tridentiger brevispinis (Kim 2022); 136.3–195.5 μm, a mud loach Misgurnus mizolepis (Park et al. 2001); 146–495 μm, a torrent catfish Liobagrus mediadiposalis (Park et al. 2003a); 59.0 μm 297.0 μm, an eel goby Odontamblyopus lacepedii (Park et al. 2003b), M. raitaborua (246.8 ± 51.5 μm, 139.1–358.0; mean ± SD, range) with CCs and MCs. Reduced diffusion distance of the skin also is strong evidence that confirms more rapid gas-exchange, as measured by an ascending vascularization that represents two histological categories in its occurrence position (Glover et al. 2013): (i) intraepidermal blood capillaries of the outermost surface layer (Mastcembellus pancalus with a mean diffusion distance of 34.0 μm; Mittal and Munshi 1971; Periophthalmus modestus with a mean of 1.4 μm; Park et al. 2000), the middle layer (Liobagrus mediadiposalis with a mean of 169 μm; Park et al. 2003a), and the stratum germinativum (Rhinogobius brunneus with a range of 35.0–202.6 μm; Kim et al. 2022), and (ii) well-developed dermal vascularization among collagen fibers of SL just below the basement membrane (Pseudobagrus brevicorpus, with a range of 19.9–399.4 μm, Park et al. 2010; abd Tridentiger brevispinis, with a range of 51.4–216.9, Kim 2022) (Kazerouni and Khodabandeh 2010; Romano et al. 2019). In this study, M. raitaborua showed reduced diffusion distance (268.8 ± 58.7 μm), which was similar to and affected strongly by ET (covariance, P < 0.001) in all skin regions, indicating that capillaries of M. raitaborua can get close to the basement membrane of the SL. For such a histological character, Park et al. (2003a) suggest that a reduced diffusion distance (mean = 169 μm, range = 22.5–220) by dermal vascularization as well as intraepidermal blood capillaries in L. mediadiposalis are meaningful histological modifications for fish that enable them to survive in frequently hypoxic habitats. Ba-Omar and AI-Riyami (2009) reported that rich dermal vascularization below the epidermis and in the dermis of an amphibious benny, Istiblennius edentulous, facilitates efficient gas exchange for cutaneous respiration. Thinner dorsal, lateral, and ventral bodies of M. raitaborua with reduced diffusion distance by ascending blood capillaries and well-developed vascularization may be collectively represent major skin region for gas-exchange and the supply of deficient oxygen through cutaneous respiration.
Conclusions
The purple spaghetti eel M. raitaborua has a thicker epidermis (the operculum was the thickest, at 315.4 ± 24.7, 258.5–358.0 [mean ± SD, range], the ventral body was the thinnest at 191.9 ± 32.1, 139.1–263.1) with stratified polygonal cells, club cells, and two types of mucous cells: elongated MCI goblet cells feature an upper part (purple with H&E staining and blush with Masson’s trichrome staining) and a reddish lower part of the cell body, and the basal position of nucleus, whereas MCII oval cells include a flattened nucleus at the cell bottom and wide cytoplasm (hazy color with H&E staining and blush with Masson’s trichrome staining), and stratified cuboidal cells. In particular, the dermis just below basement membrane in dorsal body, lateral body, and ventral body regions have abundant blood capillaries and well-developed dermal vascularization. These findings demonstrate the eel’s adaptation to cutaneous respiration to obtain supplementary oxygen in hypoxic muddy regions of brackish-water estuaries.
Availability of data and materials
Not applicable.
Abbreviations
- CC:
-
Club cell
- DM:
-
Dermis
- ED:
-
Epidermis
- MC:
-
Mucous cell
- OS:
-
Outermost surface layer
- SC:
-
Stratum compactum
- SCC:
-
Stratified cuboidal cell
- SG:
-
Stratum germinativum
- SL:
-
Stratified laxum
- SPC:
-
Stratified polygonal cell
- SS:
-
Stratum spinosum
References
M. Abolfathi, A. Akbarzadeh, A. Hajimoradloo, H.R. Joshaghani, N.W. Ross, Seasonal variations in the skin epidermal structure and mucosal immune parameters of rainbow trout skin (Oncorhynchus mykiss) at different stages of farming. Fish Shellfish Immunol 127, 965–974 (2022)
T. Ba-Omar, M.M. Al-Riyami, Integumentary histology of the amphibious blenny, isteblennius edentulus (Forester and Schneider, 1801). Sultan Qaboos Univ J Sci 14, 9–15 (2009)
R.K. Behera, S.R. Mohanty, S. Patro, A. Mohapatra, New Distributional record of Moringua raitaborua (Hamilton, 1822)-First record of the genus in the Chilika Lagoon, India. Inter J Mar Sci 37, 439–443 (2021)
M.S. Beon, M.K. Oh, Y.J. Lee, C.H. Kim, J.Y. Park, A comparative study on vascularization and the structure of the epidermis of an amphibious mudskipper fish, Scartelaos gigas (Gobiidae, Teleostei), on different parts of the body and the appendages. J Appl Ichthyol 29, 410–415 (2013)
N.D. Carreau-Green, R.S. Mirza, M.L. Martínez, G.G. Pyle, The ontogeny of chemically mediated antipredator responses of fathead minnows Pimephales promelas. J Fish Biol 73, 2390–2401 (2008)
A.J. Clark, C.H. Crawford, B.D. King, A.M. Demas, T.A. Uyeno, Material properties of hagfish skin, with insights into knotting behaviors. Biol Bull 230, 243–256 (2016)
E.M. Damasceno, J.C. Monteiro, L.F. Duboc, H. Dolder, K. Mancini, Morphology of the epidermis of the neotropical catfish Pimelodella lateristriga (Lichtenstein, 1823) with emphasis in club cells. Plos one 7, e50255 (2012)
E.H. Elsheikh, E.S. Nasr, A.M. Gamal, Ultrastructure and distribution of the taste buds in the buccal cavity in relation to the food and feeding habit of a herbivorous fish: Oreochromis niloticus. Tissue Cell 44, 164–169 (2012)
L. Fishelson, Skin morphology and cytology in marine eels adapted to different lifestyles. Anat Rec 246, 15–29 (1996)
M.L. Glass, F.T. Rantin, Gas exchange and control of respiration in air-breathing teleost fish. In: Cardio-respiratory control in vertebrates: comparative and evolutionary aspects, springer pp. 99–119 (2009)
C.N. Glover, C. Bucking, C.M. Wood, The skin of fish as a transport epithelium: a review. J Comp Physiol B 183, 877–891 (2013)
J.B. Graham, Respiratory adaptations for air-breathing fishes, in Encyclopedia of Fish Physiology, Energetics, Interactions with the Environment, Lifestyles, and Applications. ed. by A. Farrell, J.J. Cech, J.G. Richards, E.D. Stevens (Elsevier, New York, 2011), pp.1861–1874
X. Hu, W.J. Cai, Estuarine acidification and minimum buffer zone—a conceptual study. Geophys Res Lett 40, 5176–5181 (2013)
M. Jakubowski, The structure and vascularization of the skin of the pond-loach (Misgurnus fossilis L). Acta Biol Cracoviensia 1, 113–127 (1958)
E.G. Kazerouni, S. Khodabandeh, Effects of ultraviolet radiation on skin structure and ultrastructure in Caspian Sea Salmon, Salmo trutta Caspius, during alevin stage. Toxicol Environ Chem 92, 903–914 (2010)
H.T. Kim, Histology and morphometry of the skin of the trident goby Tridentiger brevispinis (Perciformes, Gobiidae). Appl Microscopy 52, 8 (2022)
C.H. Kim, J.Y. Park, Modified organs of air breathing fishes in Korea. Korean J Ichthyol 23, 250–254 (2011)
H.T. Kim, S.W. Yun, J.Y. Park, Histological studies on the skin of a freshwater goby Rhinogobius brunneus (Gobiidae) related to cutaneous respiration. J Ichthyol 62, 495–502 (2022)
M. Kottelat, The fishes of the inland waters of Southeast Asia: a catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bull Zool 27, 1–663 (2013)
Z. Lv, Q. Guo, Z. Deng, Z. Cao, J. Jiang, S. Chen, L. Gan, Lactiplantibacillus plantarum fermented broth improved survival of marble goby (Oxyeleotris Marmoratus) after skin abrasion by regulating skin mucus microbiota. Aquaculture 573, 739575 (2023)
A.K. Manek, M.C.O. Ferrari, R.J. Pollock, D. Vicente, L.P. Weber, D.P. Chivers, Within and between population variation in epidermal club cell investment in a freshwater prey fish: a cautionary tale for evolutionary ecologists. Plos One. 8, 1–8 (2013)
W.S. Marshall, Osmoregulation in estuarine and intertidal fishes. Fish Physiol Acad Press 32, 395–434 (2012)
C.C. Menes, J.D. Linaugo, J.O. Pacalioga, A.A. Bucol, The Anguilliform Eels (Pisces: Anguilliformes) of bago river and adjacent waters in Negros Occidental, Philippines. Silliman J 51, 89–103 (2010)
V. Mishra, Relative contribution of precipitation and air temperature on dry season drying in India, 1951–2018. J Geophys Res Atmos 125, e2020JD032998 (2020)
A.K. Mittal, J.S.D. Munshi, A comparative study of the structure of the skin of certain airbreathing fresh-water teleosts. J Zool Lond 163, 515–532 (1971)
J. Mogdans, Sensory ecology of the fish lateral-line system: morphological and physiological adaptations for the perception of hydrodynamic stimuli. J Fish Biol 95, 53–72 (2019)
M. Mohamed, R. Abdi, M.T. Ronagh, M.A. Salari Ali Abadi, Z. Basir, Comparative histomorphometry of dorsal, ventral and lateral skin in macroscopy, microscopy and free scale fish. Iran Veterinary J 16, 47–53 (2020)
G.E. Nilsson, J.P. Hobbs, P.L. Munday, S. Ostlund-Nilsson, Coward or braveheart: extreme habitat fidelity through hypoxia tolerance in a coral-dwelling goby. J Exp Biol 207, 33–39 (2004)
J.Y. Park, Morphology and histochemistry of the skin of the Korean spined loach, Iksookimia koreensis (Cobitidae), in relation to respiration. Folia Zool 51, 241–247 (2002)
J.Y. Park, I.S. Kim, Histology and mucin histochemistry of the gastrointestinal tract of the mud loach, in relation to respiration. J Fish Biol 58, 861–872 (2001)
J.Y. Park, I.S. Kim, S.Y. Kim, Histology of skin of the amphibious esh, Periophthalmus modestus. Korean J Biol Sci 4, 315–318 (2000)
J.Y. Park, I.S. Kim, S.Y. Kim, Morphology and histochemistry of the skin of the mud loach, Misgurnus mizolepis, in relation to cutaeneous respiration. Korean J Biol Sci 5, 303–308 (2001)
J.Y. Park, I.S. Kim, S.Y. Kim, Structure and histochemistry of the skin of a torrent catfish, Liobagrus mediadiposalis. Environ Biol Fish 66, 3–8 (2003)
K. Park, W. Kim, H.Y. Kim, Optimal lamellar arrangement in fish gills. Proc Nat Acad Sci U.S.A. 111, 8067–8070 (2014)
J.Y. Park, Y.J. Lee, I.S. Kim, S.Y. Kim, Morphological and cytochemical study on the skin of Korean eel goby, Odontamblyopus lacepedii (Pisces, Gobiidae). Korean J Biol Sci 7, 43–47 (2003)
J.Y. Park, M.K. Oh, E.J. Kang, C.H. Kim, M.S. Beon, On the vascularization and structure of the skin of a Korean bullhead Pseudobagrus brevicorpus (Bagridae, Teleostei) based on its entire body and appendages. J Appl Ichthyol 26, 64–70 (2010)
S.F. Perry, K.M. Gilmour, Acid–base balance and CO2 excretion in fish: unanswered questions and emerging models. Respir Physiol Neurobiol 154, 199–215 (2006)
R.J. Pollock, The effects of pathogens on club cell investment in fathead minnows, Pimephales promelas. Doctoral dissertation, University of Saskatchewan, Saskatoon, S.K (2011)
S. Rakers, M. Klinger, C. Kruse, M. Gebert, Pros and cons of fish skin cells in culture: long-term full skin and short-term scale cell culture from rainbow trout, Oncorhynchus mykiss. Eur J Cell Biol 90, 1041–1051 (2011)
L.L. Robbins, J.T. Lisle, Regional acidification trends in Florida Shellfish estuaries: a 20 + year look at pH, oxygen, temperature, and salinity. Estuaries Coasts 41, 1268–1281 (2018)
L.A. Romano, A.I. Lopez, J.R. Buitrago, V.F. Pedrosa, Histology of Juvenile skin of Lepidosiren paradoxa Fitzinger, 1837 (Sarcopterygii, Dipnoi), vol. 91 (Anais da Academia Brasileira de Ciências, 2019). e20190822
L.C. Smith, National Audubon Society Field Guide to Tropical Marine Fishes Caribbean, Gulf of Mexico, Florida, the Bahamas, Bermuda (Alfred A. Knopf, Inc, New York, NY, 1997)
R.C. Summerfelt, L.S. Smith, 1990. Anesthesia, surgery and related techniques, in Methods for fish biology. ed. by C.B. Schreck, P.B. Moyle (American Fisheries Society, Bethesda, Maryland, 1990), pp.213–272
P.G. Vissio, M.J. Darias, M.P. Di Yorio, D.I.P. Sirkin, T.H. Delgadin, Fish skin pigmentation in aquaculture: the influence of rearing conditions and its neuroendocrine regulation. Gen Comp Endocrinol 301, 113662 (2021)
C.M. Wood, Ammonia and urea metabolism and excretion, in The Physiology of Fishes. ed. by D. Evans (CRC Press, Boca Raton, 1993), pp.379–425
G. Zaccone, E.R. Lauriano, G. Capillo, M. Kuciel, Air-breathing in fish: air-breathing organs and control of respiration: nerves and neurotransmitters in the air-breathing organs and the skin. Acta Histochem 120, 630–641 (2018)
G. Zaccone, J. Maina, A. Germanà, G. Montalbano, G. Capillo, L. Aragona, M.J. Kuciel, E.R. Lauriano, J.M. Icardo, First demonstration of the neuroepithelial cells and their chemical code in the accessory respiratory organ and the gill of the sharptooth catfish, Clarias gariepinus: a preliminary study. Acta Zool 100, 160–166 (2019)
D. Zaccone, M. Sengar, E.R. Lauriano, S. Pergolizzi, L. Salpietro, A. Favaloro, L. Satora, K. Dabrowski, G. Zaccone, Morphology and innervation of the teleost physostome swim bladders and their functional evolution in non-teleostean lineages. Acta Histochem 114, 763–772 (2012)
G. Zaccone, G. Tagliafierro, S. Fasulo, A. Contini, L. Ainis, M.B. Ricca, Serotonin-like immunoreactivity in the epidermal club cells of teleost fishes. Histochemistry 93, 355–357 (1990)
J. Zhang, G. Cowie, S.W.A. Naqvi, Hypoxia in the changing marine environment. Environ Res Lett 8, 015025 (2013)
X.T. Zhang, Y.Y. Yu, H.Y. Xu, Z.Y. Huang, X. Liu, J.F. Cao, K.F. Meng, Z.B. Wu, G.K. Han, M.T. Zhan, L.G. Ding, W.G. Kong, N. Li, F. Takizawa, J.O. Sunyer, Z. Xu, Prevailing role of mucosal igs and B cells in teleost skin immune responses to bacterial Infection. J Immunol 206, 1088–1101 (2021)
Acknowledgements
No applicable.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
Hyun-Tae Kim designed and wrote the manuscript. The author edited and approved the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests relevant to the article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, HT. Histology and morphometry of the skin of purple spaghetti-eel Moringua raitaborua (Anguilliformes, Moringuidae). Appl. Microsc. 53, 10 (2023). https://doi.org/10.1186/s42649-023-00093-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42649-023-00093-6