Abstract
Background
Healthcare workers (HCWs) are at the front line in battling infection transmission, such as that in coronavirus disease 19 (COVID-19). Additionally, they may act as potential carriers passing the virus on to others. Anti-spike (anti-S) antibodies for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are formed either as a result of infection or vaccination with both indicating immunity against future COVID-19 infection.
Aim
This study aimed to identify the prevalence of COVID-19 seropositivity among HCWs.
Methods
This cross-sectional study included 559 HCWs from 39 hospitals with variable degrees of COVID-19 exposure risk (depending on the occupation, department, and hospital type). Demographic data were recorded as well as history of COVID-19 infection and vaccination. Serum samples were collected and tested for SARS-CoV-2 spike antibodies.
Results
Anti-S positivity was found in 59.0% of the participating 559 HCWs, indicating a high level of seroprotection. Of the 559 HCWs, 34.1% had reported previous infection with COVID-19. Following infection, only 46 (24.0%) of those affected received vaccination. Anti-S seropositivity was found in 39.1% of participants who were unvaccinated and had no history of infection. Physicians had the highest median anti-S titers (58.0 relative units (RU)/mL), whereas pharmacists and office staff had the lowest (25.7 and 38.2 RU/mL, respectively).
Conclusions
Overall, 59.0% of the 559 HCWs were anti-S positive, indicating a relatively high seroprotective status. Among those who were unvaccinated and had no history of infection, 39.1% were seropositive for anti-S, denoting a high rate of silent/asymptomatic infections. Screening of HCWs for SARS-CoV-2 anti-S is recommended, along with the vaccination of seronegative individuals.
Similar content being viewed by others
1 Introduction
Coronavirus disease 19 (COVID-19) is a global pandemic wreaking havoc on public health. This pandemic has hit Egypt, with the first confirmed case officially announced on February 14, 2020 [1]. The related morbidity and mortality of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) necessitate the estimation of its prevalence among various high-risk groups [2]. The potential spread of illness among frontline healthcare workers (HCWs), who themselves are at risk while also acting as a reservoir of infection to their patients, colleague HCWs, and domestic family members, is a worldwide concern [3]. Asymptomatic infections in this vulnerable risk group pose a higher magnitude of the problem and higher transmission and mortality rates [4]. In one study, the risk of contracting COVID-19 was three times higher for patient-facing HCWs than for non-patient-facing HCWs and the general population. Additionally, HCWs had a sevenfold higher chance of contracting severe COVID-19 than non-HCWs [5].
HCWs are exposed to long working hours under significant pressure and insufficient resources (e.g., lack of personal protective equipment [PPE] and infrequent screening for COVID-19 exposure or infection clinical testing), in addition to the risk of close contact with patients with high viral load, due to the pandemic situation as an emergency. Furthermore, additional factors such as sleep deprivation, stress, and circadian inconsistency may impact their immune system, making them more susceptible to infection [3, 5].
Serosurveillance is a strategy of epidemiology that seeks to monitor the dynamics of disease transmission using serological tests (antibody testing) to generate data on the prevalence of infection and immunity in population groups. This can help to determine the level of antibodies required to achieve herd immunity and identify groups of susceptible individuals. It is also used to evaluate the duration of protective antibodies and determine the effectiveness of vaccination [6].
In the healthcare settings, serosurveillance studies are specifically needed to discover asymptomatic infections among HCWs, evaluate the utilization of PPE and infection control measures, identify risk factors for infection, assist policymakers in reducing infections in the healthcare settings, and assess vaccination coverage. The factors affecting COVID-19 exposure and seropositivity of HCWs include their occupation, the hospital type (whether dealing with patients with COVID-19 or not), and department in which HCWs are located [6, 7]. Therefore, prevention and control measures could be more targeted in health facilities based on the availability of institution-based risk stratification.
Several commercial SARS-CoV-2 antibody tests use specific viral antigens, with the spike protein being the most important. Anti-spike (anti-S) positivity denotes either previous infection or vaccination and indicates relative immunity against future reinfection [8].
This study was performed to assess the magnitude of SARS-CoV-2 anti-S among a group of HCWs (including vaccinated and previously infected ones), which would reflect their immune status against future reinfections. This study also elucidated the relation between the occupation of HCWs, hospital type, and department and the SARS-CoV-2 anti-S seropositivity.
2 Methods
2.1 Study design
This cross-sectional survey was conducted throughout the period between January and June 2021. This period coincided with the second and third waves of the COVID-19 pandemic in Egypt.
2.2 Sample size calculation
A total sample size of 448 HCWs was required to estimate the mean prevalence of COVID-19 antibodies of 70%, with precision confirmation of 6% at 95% confidence level and a design effect of two. Following an extensive literature review, the sample size was estimated using Epi Info 7 software with the cited values [9]. A total of 559 participants were included in the study to increase the confidence of our estimate and have a greater precision.
2.3 Selection criteria
The HCWs included physicians, nurses, technicians, pharmacists, employees, office personnel, personal care workers, and faculty members. Our inclusion of such participants was based on the classification of the World Health Organization (WHO) for HCWs [10]. HCWs who declined to participate (via either blood sampling or questionnaire interview) were excluded from the study. Participants who had been vaccinated and those who had previously been infected with COVID-19 were included in the study. Previous history of COVID-19 infection was based on one or more of the following criteria: clinical symptoms, radiological results, and laboratory test results). The Egyptian national guidelines were used to determine the criteria for diagnosing infected cases.
2.4 Sampling technique
A total of 39 hospitals were randomly selected from 5 governorates using the multistage cluster sampling technique. At the first stage, the provinces and governorates were also chosen randomly using STATA software by adding the province and governorate names. The included provinces were Greater Cairo, Alexandria, and Delta. At the second stage, 14 government hospitals, 10 university hospitals, 8 private hospitals, and 7 medical insurance hospitals from the selected governorates were included. The number of each type of these hospitals was estimated to be proportionally allocated to the number of different types of hospitals in Egypt. A high number of hospitals (n = 39) were included to fulfill our sample size because several HCWs either declined or were attending to their patients and thus could not participate. A convenient sampling of HCWs was performed until the sample size was fulfilled.
2.5 Data collection methods and tools
A structured interview questionnaire sheet was designed and filled in for each participant. It included data on the occupation, hospital type, department name, history of COVID-19 diagnosis, and vaccination.
A pilot study was conducted before research implementation to test for the feasibility of recruitment as well as validation of the questionnaire. This was performed on a group of 20 HCWs in one of the university hospitals.
A 3-mL venous blood sample was collected from each participant for antibody testing. Serum samples were then separated by centrifugation at 3000 rpm and stored at − 20 °C until further processing. All samples were tested for anti-S.
The anti-SARS-CoV-2 QuantiVac sandwich enzyme-linked immunosorbent assay (ELISA) technique (EUROIMMUN, Lübeck, Germany) was used to detect the immunoglobulin class IgG against the S1 domain of the viral spike protein. According to the manufacturer's instructions, the results should be interpreted according to their relative unit (RU) results as follows: the titers of < 8 RU/mL were considered negative, those of ≥ 8 to < 11 RU/mL were considered borderline, and those of ≥ 11 RU/mL were considered positive. However, for the purpose of this study, the borderline results were considered positive for more accessible statistical analysis. Quantitative results were also expressed as quartiles because some samples had readings exceeding the highest calibrator in the kit (> 120 RU/mL), and thus, quartiles were used for correlations with quantitative variables.
2.6 Statistical analysis
After data extraction, these were revised, coded, and fed to IBM SPSS version 22 statistical software (SPSS Inc., Chicago, IL). All statistical analyses were performed using two-tailed tests. A p value of < 0.05 was considered statistically significant. The frequency and percent distribution of descriptive analysis was performed for all variables, including sociodemographic data, screening results, and immunity status. On the basis of the sensitivity and specificity of the used kits, as stated by their manufacturers, the adjusted prevalence rates were calculated using the crude prevalence rates to adjust any false-positive or false-negative results [11]. Cross-tabulation was performed to test some relations with serological findings among HCWs. The significance of relations was determined using an exact probability test for small frequency distribution.
3 Results
A total of 559 HCWs were included. The majority of participants (85.2%) were from Alexandria Governorate and urban areas (95.2%), and 73.5% were women. The most common age group was 30–39 years (36.1%), followed by 50+ years (29.2%). The majority of HCWs (62.3%) were from general, multipurpose hospitals, whereas 18.4% were from COVID-19 isolation hospitals and 14.8% were from hospitals performing screening for COVID-19. Only 11.1% of participants were from hospitals not dealing with patients with COVID-19. Regarding occupation, the majority of HCWs were physicians (42.8%), whereas 17.9% were nurses and 12.2% were pharmacists (Supplementary Table 1).
HCWs were categorized into four groups as follows: neither vaccinated nor previously infected (n = 271 [48.5%]), previously infected and unvaccinated (n = 145 [25.9%]), vaccinated and not previously infected (n = 97 [17.4%]), and vaccinated with a history of infection prior to vaccination (n = 46 [8.2%]). The overall anti-S positivity among the 559 HCWs was 59.0% (83.9% among vaccinated and 50.5% among unvaccinated) (Table 1).
The highest crude prevalence for anti-S results according to department was for those working in radiology departments (66.7%; 95% confidence interval (CI), 48.7–94.3%), followed by wards (58.6%; 95% CI, 50.4–75.2%). Conversely, the lowest crude prevalence for anti-S results according to department was recorded among HCWs working in infection control units (22.2%; 95% CI, 1.3–51.5%) and blood banks (25%; 95% CI, 1.5–70.1%) (Table 2).
The highest crude prevalence for anti-S results according to the hospital type was among those working in pulmonology hospitals (65.7%; 95% CI, 55.4–85.6%), followed by HCWs working in hospitals performing screening for COVID-19 (56.2%; 95% CI, 49.1–71.5%). Conversely, seropositivity for anti-S was lowest among HCWs working in outpatient clinics (37.5%; 95% CI, 6.2–74.2%) (Table 3).
Among HCWs who neither were vaccinated nor had a previous infection, 60.9% were negative for anti-S, whereas 39.1% were positive for anti-S. Among HCWs who had a history of COVID-19 infection and no vaccination, 28.3% were negative, whereas 71.7% were positive. Vaccinated HCWs who reported no previous history of infection showed 77.3% anti-S positivity, and this value increased to 97.8% in the group who reported having both vaccination and previous infection. Significant differences were recorded among the anti-S results in the four groups (p = 0.001) (Table 4).
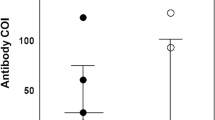
Regarding occupation, physicians had the highest median anti-S titers (58.0 RU/mL). The lowest titers were found among pharmacists and office staff (25.7 and 38.2 RU/mL, respectively) (p = 0.254) (Fig. 1). Regarding hospital department, the highest median anti-S titers were found among those working in infection control, radiology, and emergency departments (112.5, 79.7, and 67.2 RU/mL, respectively). In contrast, the lowest median titers were found among those working in hospital pharmacies (Fig. 2 and Supplementary Table 2).
4 Discussion
Although people of all ages and occupations can be infected with SARS-CoV-2, HCWs probably have the highest risk of contracting the infection [4, 7, 9]. The asymptomatic and presymptomatic portions of SARS-CoV-2 infections are estimated to be up to 30% [12]. The anti-spike status of HCWs can reflect acquired immunity resulting either from exposure or vaccination. The seroconversion rate among frontline providers could provide valuable information regarding the effectiveness of the PPE strategy [13]. The current study was conducted to assess SARS-CoV-2 anti-spike IgG seroprevalence among a group of HCWs. Differences in seroprevalence of anti-spike frequency regarding hospital type, department, and occupation were also investigated.
Of the 559 HCWs, 34.1% had a positive history of COVID-19 infection. Although this percentage is higher than several other reported results (3.7 to 20%) in Egypt [4, 14, 15], it is consistent with that recorded by El-Sokkary et al. (yet their sample size was smaller [n = 82 HCWs], all were working in isolation hospitals) [16]. Two studies, one in Germany [17] and the other in the Netherlands [18], reported much lower infection rates among HCWs (5.4% and 1%, respectively), reflecting strict adherence to infection control measures. High infection rates among HCWs, if proven to be work-related, are attributed to their higher exposure as well as a need for more diligent infection control measures and the use of PPE. The WHO recommends guidelines for PPE use in the COVID-19 pandemic, including universal masking, with respirator (N95 or equivalent) utilization only during approved aerosol-generating procedures [19]. However, the implementation of such guidelines is often limited by resource availability. Comparisons of the COVID-19 infection rates among HCWs across countries may not be valid because of the differences in health system structure and organization, availability and enforcement of healthcare protocols and PPE, and differences in declared COVID-19 prevalence rates between countries [20].
Only 18.4% of HCWs included in our study were from COVID-19 isolation hospitals. This relatively small proportion may explain the low vaccination rate (25.6%) among our HCWs since at the time of our study; vaccination was prioritized, when available, for HCWs in isolation hospitals. Currently, several vaccines are readily available for all HCWs and the general population.
Vaccinated HCWs who reported no previous history of infection showed 77.3% anti-S positivity, and this value increased to 97.8% in the group who reported having both vaccination and previous infection. This highlights the hybrid immunity offered by vaccination and natural infection compared with that by vaccination alone [21].
Only 24.0% of those with previous COVID-19 infection received vaccination following infection, whereas the rest did not. The reasons for not obtaining the vaccine following infection may be the lack/delay of vaccine delivery at the time of the study (local vaccine manufacturing had not yet started at that time) or lack of confidence of the HCWs in the importance of vaccination following infection. Guidelines in vaccine delivery recommend that, following COVID-19 infection, a period of 90 days passes before vaccination if monoclonal antibodies or convalescent plasma were received; otherwise, 14 days is sufficient. Those factors may have contributed to the low vaccination rate among those with previous infections. The reasons for not taking the vaccine were not tackled in the current study.
The overall anti-S positivity among the 559 HCWs was 59.0%, denoting a relatively high seroprotective status. This high rate might be over-estimated due to the inclusion of borderline along with the positive anti-S cases. However, this inclusion was important owing to the importance of borderline results and their partial protective role against COVID-19 infection. Such inclusion might explain our high seroprevalence rates compared to other similar studies.
Among those who neither were vaccinated nor had a history of infection, 39.1% were seropositive for anti-S, denoting the high rate of silent and asymptomatic infections. Slightly higher rates (40.3%) were even reported in a similar study by El-Sokkary et al. in Egypt among unvaccinated HCWs [16], but all of those were HCWs in isolation hospitals only. In contrast, our study included heterogeneous hospital types; therefore, our rate would be considered high for such hospital types. Nevertheless, lower rates were reported in a study in Saudi Arabia (14.8%) [16]. Our high rates emphasize the importance of serosurveillance for anti-S among HCWs, not merely counting on screening for clinical symptoms of COVID-19. Serosurveillance is necessary for several reasons, including the prevention of complications among vulnerable individuals and assessment of infection control measures in healthcare facilities.
Several studies have investigated the work related factors in hospitals for anti-S seropositivity. Piccoli et al. reported that seropositivity was significantly higher in high-risk occupations (i.e., physicians and nurses) than in low-risk ones (i.e., personal care workers and administrative staff) (10.11% vs. 6.56%; odds ratio (OR) = 1.75) [20]. A study in Egypt reported that SARS-CoV-2 seropositivity was associated with being a physician and exposure to patients with COVID-19 in isolation hospitals for longer durations (> 3 months) [16]. In our study, the crude prevalence for anti-S seropositivity among unvaccinated physicians was not high compared with that in other occupations (48.6%; 95% CI, 44.1–60.1%); however, they had the highest median anti-S titers (58.0 RU/mL). Together, these findings suggest that personal care workers, nurses, and pharmacists have higher probabilities to contract the infection, but physicians are more likely to have higher viral loads. Our findings did not reach statistical significance (p = 0.254) but may be proven in other studies with larger sample sizes. This indicates that physicians may be exposed to higher viral loads because of their direct and repeated contact with patients with COVID-19, with higher probabilities of severe infection due to high viral load. High antibody levels indicate exposure to high viral loads. Hospital office staff and pharmacists do not carry the same risk as physicians of high viral load do because of their less risky exposure to patients. Physicians should use more PPE and infection control measures.
Regarding the department in the hospital, differences in titers among departments did not reach statistical significance (p = 0.069). Radiology departments could be considered high-risk areas in hospitals because of the repeated exposure to patients with COVID-19 during the performance of chest imaging. Emergency departments may also pose a risk for HCWs contracting COVID-19 because of this department’s “emergency” nature, where effective infection control measures taken by patients may not be promptly adequate. These data help to stratify the risks of COVID-19 exposure inside hospitals according to the nature of departments. More diligent measures should be taken in departments with high seroprevalence. Piccoli et al. reported that the association between hospital site and SARS-CoV-2 seroprevalence was no longer significant (p = 0.932) after mutual adjustment for ward/unit type and HCW occupation [20].
Our highest crude prevalence for anti-S results was among those working in pulmonology hospitals (65.7%; 95% CI, 55.4–85.6%), whereas the lowest was among HCWs working in outpatient clinics (37.5%; 95% CI, 6.2–74.2%). Similarly, Scozzari et al. [21] documented that HCWs in COVID-19 wards showed higher anti-S frequency than those in non-COVID-19 wards. A study reported that HCWs with the most exposure to patients with COVID-19 were not at higher risk for developing anti-S than those with limited COVID-19 exposure, and the authors attributed this to their strict institutional adherence to the WHO guidelines for PPE use [13]. Despite efforts to protect high-risk first-line HCWs, more infection control measures should still be taken.
4.1 Study strengths and limitations
To the best of our knowledge, similar studies on seroprevalence of SARS-CoV-2 among HCWs are limited in Egypt. This study also investigated the anti-S titers in relation to the hospital type, occupation, and department. Higher anti-S titers indicate exposure to higher viral loads (among unvaccinated individuals), highlighting the inadequacy in the use of PPE.
The COVID-19 vaccination first became available only during participant recruitment and not before research initiation. Because the situation has changed considerably in recent months, the vaccination coverage indicated in this article no longer reflects the current picture. Borderline cases were included with the positive ones in the statistical analysis, and this may explain the higher prevalence rate compared with those reported by other studies. Governorates included in our study were from Lower Egypt, limiting the generalization of our results. Further studies including HCWs from Upper Egypt governorates would provide a more comprehensive view of the overall seroprevalence in Egypt.
5 Conclusions
HCWs in our study had an overall 59.0% anti-S seroprevalence rate including those who received COVID-19 vaccines. Anti-S seroprevalence reached 83.9% among vaccinated HCWs (some received only one dose at recruitment time), and it was 50.5% among unvaccinated HCWs. Among those who neither were vaccinated nor had a history of infection, 39.1% were seropositive for anti-S, denoting the high rate of silent and asymptomatic infections. The prevalence may even be higher after adjustment. Personal care workers, those working in pulmonology hospitals and radiology units, had the highest anti-S prevalence rates.
Screening of HCWs for SARS-CoV-2 anti-S is recommended together with vaccination of seronegative individuals. Completion of vaccine doses of all HCWs is recommended for full protection. Special attention should be paid to HCWs at higher risk of contracting COVID-19 infection, including those in high-risk departments or occupations.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ELISA:
-
Enzyme-linked immunosorbent assay
- HCWs:
-
Healthcare workers
- IRB:
-
Institutional Review Board
- PPE:
-
Personal protective equipment
- RU:
-
Relative unit
- WHO:
-
World Health Organization
References
World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 26. 2020 [Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200215-sitrep-26-covid-19.pdf?sfvrsn=a4cc6787_2. [Accessed 21 Sept 2021]
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–93. https://doi.org/10.1001/jama.2020.12839.
Cag Y, Erdem H, Gormez A, Ankarali H, Hargreaves S, Ferreira-Coimbra J, et al. Anxiety among front-line health-care workers supporting patients with COVID-19: a global survey. Gen Hosp Psychiatry. 2021;68:90–6. https://doi.org/10.1016/j.genhosppsych.2020.12.010.
Abdelmoniem R, Fouad R, Shawky S, Amer K, Elnagdy T, Hassan WA, et al. SARS-CoV-2 infection among asymptomatic healthcare workers of the emergency department in a tertiary care facility. J Clin Virol. 2021;134:104710. https://doi.org/10.1016/j.jcv.2020.104710.
Mutambudzi M, Niedwiedz C, Macdonald EB, Leyland A, Mair F, Anderson J, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK biobank participants. Occup Environ Med. 2021;78(5):307–14. https://doi.org/10.1136/oemed-2020-106731.
Infectious Diseases Society of America: Guidelines on the Diagnosis of COVID-19: Serologic Testing. Infectious Diseases Society of America. 2020 [cited 10-8-2021]. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-serology/.
Dzinamarira T, Mhango M, Dzobo M, Ngara B, Chitungo I, Makanda P, et al. Risk factors for COVID-19 among healthcare workers: a protocol for a systematic review and meta-analysis. PLoS One. 2021;16(5):e0250958. https://doi.org/10.1371/journal.pone.0250958.
Verissimo CDM, O'Brien C, Corrales JL, Dorey A, Cwiklinski K, Lalor R, et al. Improved diagnosis of SARS-CoV-2 by using nucleoprotein and spike protein fragment 2 in quantitative dual ELISA tests. Epidemiol Infect. 2021;149(E140):1–11. https://doi.org/10.1017/S0950268821001308.
Singhal T, Shah S, Naik R, Kazi A, Thakkar P. Prevalence of COVID-19 antibodies in healthcare workers at the peak of the pandemic in Mumbai, India: a preliminary study. Indian J Med Microbiol. 2020;38(3-4):461–3. https://doi.org/10.4103/ijmm.IJMM_20_308.
World Health Organization. Classifying health workers: mapping occupations to the international standard classification. Geneva; 2010. [updated 2-9-2022. 1-14]. Available from: https://www.who.int/hrh/statistics/Health_workers_classification.pdf
Sempos CT, Tian L. Adjusting coronavirus prevalence estimates for laboratory test kit error. Am J Epidemiol. 2021;190(1):109–15. https://doi.org/10.1093/aje/kwaa174.
Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–90. https://doi.org/10.1056/NEJMoa2008457.
Hunter BR, Dbeibo L, Weaver CS, Beeler C, Saysana M, Zimmerman MK, et al. Seroprevalence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) antibodies among healthcare workers with differing levels of coronavirus disease 2019 (COVID-19) patient exposure. Infect Cont Hosp Ep. 2020;41(12):1441–2. https://doi.org/10.1017/ice.2020.390.
Mostafa A, Kandil S, El-Sayed MH, Girgis S, Hafez H, Yosef M, et al. Universal COVID-19 screening of 4040 health care workers in a resource-limited setting: an Egyptian pilot model in a university with 12 public hospitals and medical centers. Int J Epidemiol. 2021;50(1):50–61. https://doi.org/10.1093/ije/dyaa173.
Mostafa A, Sabry W, Mostafa NS. COVID-19-related stigmatization among a sample of Egyptian healthcare workers. PLoS One. 2020;15(12):e0244172. https://doi.org/10.1371/journal.pone.0244172.
El-Sokkary RH, Daef E, El-Korashi LA, Khedr EM, Gad D, Mohamed-Hussein A, et al. Sero-prevalence of anti-SARS-CoV-2 antibodies among healthcare workers: a multicenter study from Egypt. J Infect Public Heal. 2021;14(10):1474–80. https://doi.org/10.1016/j.jiph.2021.09.011.
Schwierzeck V, Correa-Martinez CL, Schneider KN, Mellmann A, Hennies MT, Hafezi W, et al. SARS-CoV-2 in the employees of a large university hospital. Dtsch Arztebl Int. 2020;117(19):344–5. https://doi.org/10.3238/arztebl.2020.0344.
Kluytmans-van Den Bergh MF, Buiting AG, Pas SD, Bentvelsen RG, Van Den Bijllaardt W, Van Oudheusden AJ, et al. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020;3(5):e209673. https://doi.org/10.1001/jamanetworkopen.2020.9673.
WHO. Infection prevention and control during healthcare when COVID-19 is suspected Interim guidance. 2020 [cited 1-11-2021]. Available from: https://www.who.int/publications/i/item/10665-331495. [Accessed 30 Sept 2021].
Piccoli L, Ferrari P, Piumatti G, Jovic S, Rodriguez BF, Mele F, et al. Risk assessment and seroprevalence of SARS-CoV-2 infection in healthcare workers of COVID-19 and non-COVID-19 hospitals in southern Switzerland. Lancet Reg Health Eur. 2021;1:100013. https://doi.org/10.1016/j.lanepe.2020.100013.
Scozzari G, Costa C, Migliore E, Coggiola M, Ciccone G, Savio L, et al. Prevalence, persistence, and factors associated with SARS-CoV-2 IgG seropositivity in a large cohort of healthcare workers in a tertiary care university hospital in northern Italy. Viruses. 2021;13(6):1064. https://doi.org/10.3390/v13061064.
Acknowledgements
The authors are grateful to all participants and authorities and to the Science and Technology Development Fund (STDF) for supporting this study.
Funding
This study was supported financially by the Science and Technology Development Fund (STDF), Egypt (project no.: 43834).
Author information
Authors and Affiliations
Contributions
EMEl-G: (1) conception and design of the practical work and analysis and interpretation of data (2) critical revision of the manuscript for important intellectual content (3) final approval of the manuscript to be published (4) procurement of funding (5) provision of administrative, technical, and material support; and (6) study supervision. AGF: (1) contribution to design of the manuscript (2) drafting of the manuscript (3) final approval of the manuscript to be published and (4) study supervision. MHH: (1) substantial contributions to the ELISA testing and (2) final approval of the manuscript version to be published. FC: (1) contribution to the research idea and (2) final approval of the manuscript to be published. SF (1) contributions to analysis and interpretation of data (2) drafting of the tables and figures and (3) final approval of the manuscript to be published. EAO: (1) ELISA testing (2) result interpretation, (3) abstract drafting and (4) final approval of the manuscript to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in compliance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) Committee, Faculty of Medicine, Alexandria University (IRB number, 00012098; FWA number, 00018699; serial number, 0305136) and the Ethics Committee of the High Institute of Public Health, Alexandria University, on 24 November 2020. Administrative approval was taken from each healthcare setting before study onset. Anonymity and confidentiality of participants were ensured, and written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Socio-demographic data of 559 health care workers, Egypt, 2020. Supplementary Table 2: Distribution by quartiles of SARS-CoV-2 anti-S titres among 416 unvaccinated SARS-CoV-2 anti-S positive HCWs
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Ghitany, E.M., Farghaly, A.G., Farag, S. et al. Prevalence of severe acute respiratory syndrome coronavirus 2 spike antibodies in some healthcare settings in Egypt. J. Egypt. Public. Health. Assoc. 97, 11 (2022). https://doi.org/10.1186/s42506-022-00106-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42506-022-00106-4