Abstract
Background
Malaria has been identified as a significant public health burden, exhibiting a high risk of death and morbidity. In sub-Saharan Africa, most young children attending primary healthcare facilities are commonly diagnosed with malaria. Thus, introduction of malaria rapid diagnostic test (mRDT) kits and effective antimalarials has substantially improved the management of malaria cases. However, healthcare worker confidence and adherence to procedures dependent on malaria test results remain variable in high-burden settings due to lacking alternative point-of-care tests to diagnose other causes of fever. In this study, we compared the results of malaria screenings using mRDT and microscopy in febrile children presenting at a primary health facility.
Methods
This study was conducted at a primary health center in Owo, Ondo State, Nigeria. Children with fever were assessed for malaria by health staff and, where indicated, screened using Plasmodium falciparum histidine-rich protein-2 mRDT kits. Blood samples were collected on slides for microscopy and in hematocrit tubes for hematocrit determination simultaneously, whereas the mRDT test was done by routine health staff. Children found positive for malaria via mRDT were diagnosed as uncomplicated malaria cases and treated as outpatients using artemether-lumefantrine. Blood slides were read independently by two trained microscopists blinded to the mRDT results. The parasite densities were defined as average counts by both microscopists. We then assessed the sensitivity, specificity, and predictive value of mRDT for the diagnosis of malaria.
Results
We compared the test results of 250 febrile children who are under 15 years old. The test positivity rates were 93.6% (234/250) and 97.2% (243/250) using microscopy and rapid RDTs, respectively. The sensitivity and specificity of mRDT compared to microscopy were 100.0% and 43.8%, respectively, with a positive predictive value of 96.3% (95% CI 93.1–98.3). The hematocrit value was <30% in 64% of the children.
Conclusion
As per our findings, mRDTs have correctly detected infections in febrile children. Healthcare workers and caregivers should be encouraged to act in accordance with the test results by means of regular feedback on the quality of mRDTs in use in malaria case management.
Similar content being viewed by others
1 Introduction
Malaria, known to be caused by Plasmodium falciparum, remains a significant public health challenge worldwide, with an estimated 229 million cases in 2019 in 87 countries where it is endemic [1]. These figures remain high despite the marked reductions in the global burden relative to estimates at the start of the millennium [2]. Between 2000 and 2019, it was estimated that 1.5 billion malaria cases and 7.6 million malaria deaths were averted, most of which were in sub-Saharan Africa [1, 3]. The World Health Organization (WHO) African Region accounts for 94% of cases and 95% of deaths globally [1]. Nigeria has the highest burden in this region, accounting for 27% of cases and 23% of deaths due to malaria [1]. Reductions in malaria burden were brought about by the introduction of policies and strategies, such as the use of long-lasting insecticidal bed nets (LLITN), improved malaria diagnosis, use of artemisinin-based combination therapy, and improved surveillance [4, 5].
The differential impact of these interventions contributed to changes in the epidemiological profiles of malaria risk and transmission [6, 7]. Some countries and regions have made significant reductions in coping with the disease burden and are considering elimination, while the burden of malaria has remained the same or is on increase in others.
The current protocols for malaria case management require screening for infection before treatment in cases with associated symptoms such as a history of fever [8]. Malaria rapid diagnostic test (mRDT) kits were introduced to improve confidence in malaria diagnosis; these have been shown to reduce over-diagnosis and over-prescription for malaria [9]. In areas where malaria transmission is stable, most children are infected in the first year of life, and severe disease is widespread in the first 2 years of life. In older children and adults, infections tend to remain asymptomatic due to the acquisition of a degree of clinical immunity [10, 11]. In high-burden areas, confidence in test results among healthcare workers remains a challenge due to the overlap of symptoms and signs between malaria and other causes of febrile illnesses and the high risk of re-infection [12]. To improve healthcare worker confidence in the use of malaria RDTs and adherence to national guidelines with reference to diagnosis-based treatment for malaria, local evidence with regard to the validity of these tests is needed and should be updated regularly.
Thus, in this study, we compared the utility of malaria RDTs in the diagnosis of malaria in children under 15 years old presenting with fever at a primary healthcare facility in an area with perennial and high malaria transmission intensity.
2 Methods
2.1 Study area and population
This cross-sectional study was conducted from August to October 2020 at the Oke-Mapo Primary Health Center in Owo, Ondo State, Southwestern Nigeria. The town is located 305 m above sea level and has a population of about 222,000. The clinic is generally focused on the treatment of common childhood infections with referrals for more serious conditions to the district and federal hospitals in Owo. Malaria treatment guidelines in Nigeria recommend screening individuals presenting with a history of fever in the past 48 h or having a measured temperature of >37.5°C for malaria using a Plasmodium falciparum histidine-rich protein 2 (HRP-2)-based mRDT kit or microscopy, followed by treatment with artemisinin-based combination therapy if the test returns positive [13]. The mRDT kit available at the clinic at the time of the study was the Standard Diagnostics (SD Bioline®), malaria Ag P.f RDT kit.
Informed consent were provided by the caregivers for children aged less than 15 years where the decision to screen for malaria was made by the attending healthcare worker. Once permission was given, we documented the child’s age, gender, and complaints on presentation. In addition to the blood sample collected for the mRDT test, we collected three additional drops of blood on a slide for later malaria screening via microscopy. The slides were labeled with the participant’s initials and screening number.
The blood smear was dried at room temperature and stained with 3% Giemsa stain for 30 min and was thereafter rinsed and dried. The stained slides were independently examined under the microscope by two laboratory scientists, and up to 100 high-power fields were viewed before the sample was declared negative. When a parasite was seen, quantification was done by counting parasites in relation to 200 white blood cells and assuming an average white cell count of 8000 cells/μL, following published protocols [14]. For the hematocrit (PCV) determination, microhematocrit tubes were filled to the predefined mark and centrifuged for 5 min at 11,000 rpm in a microhematocrit rotor (Hawksley and Sons Ltd, Sussex, UK), and the PCV value was measured with a hematocrit reader.
To determine the sample size for this study, we assumed a 5% discordance in results between microscopy [15] and mRDT. We estimated that in 250 paired samples, we would be able to detect this level of discordance with a 90% power and a 5% significance level. This number included an additional 10% increase in the estimate to account for samples that were destroyed during processing.
Data collected on paper forms were entered in an access database and were verified against the source document. We presented a description of the study population and the association between malaria covariates, including age and gender, assessed using a regression model. We also determined the sensitivity, specificity, and predictive values of mRDT compared to microscopy.
3 Results
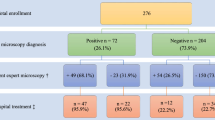
We screened 250 children with a history of fever for malaria using both mRDTs and microscopy. Of those enrolled, 46.0% (115/250) were female (Table 1). The mean (standard deviation, [SD]) age of the children was 5.52 years (±4.34), with 53.6% (134/250) aged less than 5 years (Fig. 1). The mean (SD) packed cell volume in the cohort was 28.9% (±5.8); there was no significant difference in the mean PCV in children aged below and above 5 years (mean 29.1% (±5.7) and mean 28.7% (±5.8) were recorded respectively; P=0.84), with 63.4% (159/250) of children having PCV < 30%.
From the mRDT results, 97.2% (243/250) were found to be positive for malaria and were treated with artemether-lumefantrine; 53.1% (129/243) were children younger than 5 years. Children over 5 years were twice more likely to have had a positive test (OR 2.21, 95% CI 0.42–11.61, p = 0.349) than those younger than 5 years.
Microscopy screening showed that 93.6% (234/250) were positive, with a mean (SD) parasite density of 2446 (2634))/μL. Children over 5 years old were more likely to have a positive slide result (OR 1.99, 95% CI 0.67–5.89, p = 0.217), although mean parasite densities were not different between these age groups after adjusting for sex and PCV level (adjusted OR 1.1, 95% CI 0.96–1.26, p = 0.166).
A comparison of test results between mRDT and microscopy showed them to be consistent in 241 paired samples: 234 positive and 7 negative (Spearman’s Rho 0.649, p < 0.001). The sensitivity and specificity of mRDT relative to microscopy were 100.0% and 43.8%, respectively (Table 2). The positive and negative predictive values for mRDTs were 96.3% (95% CI 93.1–98.3) and 100.0%, respectively.
4 Discussion
Malaria diagnosis is available in most primary healthcare settings because of the ease of use and the stability of RDT cassettes, across a range of temperature and humidity. Results are also easy to interpret. In 2012, the WHO launched the Test, Treat, Track (T3) initiative to scale up diagnostic testing, treatment, and surveillance for malaria in endemic countries. This program was developed to ensure that all malaria patients receive prompt treatment. Furthermore, T3 was developed to improve surveillance for malaria cases and deaths and help Ministries of Health to determine which areas or population groups are most affected and help target resources where they are most needed. However, the use of malaria diagnostics remains to be limited, and opinions are divided on its utility [16, 17].
In this study, children presenting with fever in the past 48 h or having a measured temperature of >37.5°C were screened using routine PfHPR2 mRDT and microscopy. Children with positive mRDT results were diagnosed as having uncomplicated malaria cases and were treated by routine healthcare workers with artemether-lumefantrine and discharged as outpatients following the national treatment guideline. The test positivity rate for malaria using microscopy was 93.6%, with slightly higher rates in children over 5 years old (95.7%. 111/116). Older children also had higher mean parasite densities than those who were under 5 years old. The sensitivity of the mRDT was 100%, with a positive predictive value of 96.3%.
Furthermore, approximately 64% of the children examined were moderately anemic with a hematocrit <30% [18]. Malaria is an important cause of anemia in endemic areas, as seen from the hemolysis of infected and uninfected red blood cells [19]. In high malaria transmission settings such as Nigeria, most young children and many older children and adults’ exhibit reduced hemoglobin concentration due to malaria infection [20]. The finding of this research underscores the need to screen children with fever presenting at primary healthcare for malaria as well as anemia in malaria-endemic areas.
Jegede et al. showed that an integrated intervention involving RDTs and Artemisinin-based combination therapy (ACTs) by community health workers (CHWs) in Burkina Faso, Nigeria, and Uganda was feasible and acceptable to their communities [21]; however, there are concerns about providing treatment to children with a false-positive RDT test, resulting in the over-prescription of antimalarials and antibiotics [22, 23]. However, it has been found that withholding antimalarials from children with a negative test is safe even in highly endemic areas [18]. The patterns of malaria transmission continue to evolve as the awareness and use of control measures increase. Studies such as this one are useful because healthcare workers and caregivers need to be aware of the relative risk of malaria infection in their settings to sustain their confidence in the results of malaria tests and reduce the rates of misdiagnosis and over-prescription of antimalarials and antibiotics.
4.1 Study limitations
This study did not investigate other causes of fever in the participating children and there was no follow-up of treated participants to determine outcome after treatment.
5 Conclusion
Malaria remains to be a significant cause of clinic presentations for febrile illness in children under 15 years old. Malaria RDTs are very sensitive and are determined to correctly predict infection in children. Healthcare workers and caregivers should be encouraged to adhere to test results in the case management of malaria in highly endemic settings.
Availability of data and materials
Data set used will be made available on reasonable request to the corresponding author.
Abbreviations
- CHWs:
-
Community health workers
- mRDT:
-
Malaria rapid diagnostic test
- WHO:
-
World Health Organization
- HRP-2:
-
Histidine-rich protein 2
- PCV:
-
Packed cell volume
- Pf:
-
Plasmodium falciparum
References
WHO. World malaria report 2020: 20 years of global progress and challenges. Geneva; 2020. Available from: https://www.who.int/publications/i/item/9789240015791. Accessed 10 Nov 2021.
WHO. Achieving the malaria MDG target: reversing the incidence of malaria 2000-2015. Geneva, Switzerland; 2015. Available from: https://www.who.int/publications/i/item/9789241509442. Accessed 10 Nov 2021.
WHO. World malaria report 2015. Geneva: World Health Organization; 2015. Available from: http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf. Accessed 10 Nov 2021.
WHO. Global technical strategy for malaria 2016-2030 2015. Available from: http://www.who.int/malaria/publications/atoz/9789241564991/en/. Accessed 10 Dec 2021.
WHO. Disease surveillance for malaria control: an operational manual 2012. Available from: https://apps.who.int/iris/bitstream/handle/10665/44851/9789241503341_eng.pdf. Accessed 10 Nov 2021.
Trape JF, Tall A, Sokhna C, Ly AB, Diagne N, Ndiath O, et al. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis. 2014;14(6):476–88. https://doi.org/10.1016/S1473-3099(14)70712-1.
Woolhouse MEJ, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, et al. Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proc Natl Acad Sci U S A. 1997;94(1):338–42. https://doi.org/10.1073/pnas.94.1.338.
WHO. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2015. Available from: http://www.who.int/malaria/publications/atoz/9789241549127/en/. Accessed 10 Nov 2021.
Okebe JU, Walther B, Bojang K, Drammeh S, Schellenberg D, Conway DJ, et al. Prescribing practice for malaria following introduction of artemether-lumefantrine in an urban area with declining endemicity in West Africa. Malar J. 2010;9:180. https://doi.org/10.1186/1475-2875-9-180.
Høgh B. Clinical and parasitological studies on immunity to Plasmodium falciparum malaria in children. Scand J Infect Dis Suppl. 1996;102:1–53.
Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, et al. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51(2):123–37.
Budimu A, Emidi B, Mkumbaye S, Kajeguka DC. Adherence, awareness, access, and use of standard diagnosis and treatment guideline for malaria case management among healthcare workers in Meatu. Tanzania J Trop Med. 2020;2020:1918583. https://doi.org/10.1155/2020/1918583.
National Malaria Strategic Plan. 2014-2020. Available from: https://www.health.gov.ng/index.php?option=com_content&view=article&id=156&Itemid=527. Accessed 1 Nov 2021.
Greenwood BM, Armstrong JR. Comparison of two simple methods for determining malaria parasite density. Trans R Soc Trop Med Hyg. 1991;85(2):186–8. https://doi.org/10.1016/0035-9203(91)90015-Q.
Kotepui M, Kotepui KU, Milanez GDJ, Masangkay FR. Summary of discordant results between rapid diagnosis tests, microscopy, and polymerase chain reaction for detecting Plasmodium mixed infection: a systematic review and meta-analysis. Sci Rep. 2020;10(1):1–17. https://doi.org/10.1038/s41598-020-69647-y.
Ogunfowokan O, Ogunfowokan BA, Nwajei AI. Sensitivity and specificity of malaria rapid diagnostic test (mRDT CareStatTM) compared with microscopy amongst under five children attending a primary care clinic in southern Nigeria. Afr J Prim Health Care Fam Med. 2020;12(1):e1–8. https://doi.org/10.4102/phcfm.v12i1.2212.
Falade CO, Ajayi IO, Nsungwa-Sabiiti J, Siribié M, Diarra A, Sermé L, et al. Malaria rapid diagnostic tests and malaria microscopy for guiding malaria treatment of uncomplicated fevers in Nigeria and Prereferral cases in 3 African countries. Clin Infect Dis. 2016;63(suppl 5):S290–s7. https://doi.org/10.1093/cid/ciw628.
d’Acremont V, Malila A, Swai N, Tillya R, Kahama-Maro J, Lengeler C, et al. Withholding antimalarials in febrile children who have a negative result for a rapid diagnostic test. Clin Infect Dis. 2010;51(5):506–11. https://doi.org/10.1086/655688.
White NJ. Anaemia and malaria. Malar J. 2018;17(1):371. https://doi.org/10.1186/s12936-018-2509-9.
Oladeinde B, Omoregie R, Olley M, Anunibe J, Onifade A, Oladeinde O. Malaria and anemia among children in a low resource setting in Nigeria. Iran J Parasitol. 2012;7(3):31–7 PMID: 23109959. PMCID: PMC3469169.
Jegede AS, Oshiname FO, Sanou AK, Nsungwa-Sabiiti J, Ajayi IO, Siribié M, et al. Assessing acceptability of a diagnostic and malaria treatment package delivered by community health workers in malaria-endemic settings of Burkina Faso, Nigeria, and Uganda. Clin Infect Dis. 2016;63(suppl 5):S306–11. https://doi.org/10.1093/cid/ciw630.
Kabaghe AN, Visser BJ, Spijker R, Phiri KS, Grobusch MP, van Vugt M. Health workers’ compliance to rapid diagnostic tests (RDTs) to guide malaria treatment: a systematic review and meta-analysis. Malar J. 2016;15:163. https://doi.org/10.1186/s12936-016-1218-5.
Ukwaja KN, Aina OB, Talabi AA. Clinical overlap between malaria and pneumonia: can malaria rapid diagnostic test play a role? J Infect Dev Ctries. 2011;5(3):199–203 Available from: https://jidc.org/index.php/journal/article/view/945.
Acknowledgements
The authors would like to thank the healthcare workers, participants, and their caregivers for their support in this work.
Funding
No direct funding was received for this work.
Author information
Authors and Affiliations
Contributions
JO wrote the first draft of the manuscript; PA collected the data and reviewed the draft manuscript; OO developed the idea, supervised the data collection, analyzed the data, and reviewed the draft manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Federal Medical Centre, Owo, on 10/08/2020 before commencing the study (Ref: FMC/OW/380/VOL.LXXXIX/186). A written informed consent was obtained from the parents/guardian of participating children.
Consent for publication
Not applicable
Competing interests
We have no competing interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, P.O., Okebe, J. & Oduwole, O.A. Malaria rapid diagnostic tests for the case management of febrile children in Nigerian primary healthcare settings: a cross-sectional study. J. Egypt. Public. Health. Assoc. 97, 10 (2022). https://doi.org/10.1186/s42506-022-00105-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42506-022-00105-5