Abstract
Background
Juvenile myoclonic epilepsy (JME) is the most common syndrome of idiopathic generalized epilepsy. Although resting-state functional magnetic resonance imaging (rs-fMRI) studies have found thalamocortical circuit dysfunction in patients with JME, the pathophysiological mechanism of JME remains unclear. In this study, we used three complementary parameters of rs-fMRI to investigate aberrant brain activity in JME patients in comparison to that of healthy controls.
Methods
Rs-fMRI and clinical data were acquired from 49 patients with JME undergoing monotherapy and 44 age- and sex-matched healthy controls. After fMRI data preprocessing, the fractional amplitude of low-frequency fluctuation (fALFF), regional homogeneity (ReHo), and degree centrality (DC) were calculated and compared between the two groups. Correlation analysis was conducted to explore the relationship between local brain abnormalities and clinical features in JME patients.
Results
Compared with the controls, the JME patients exhibited significantly decreased fALFF, ReHo and DC in the cerebellum, inferior parietal lobe, and visual cortex (including the fusiform and the lingual and middle occipital gyri), and increased DC in the right orbitofrontal cortex. In the JME patients, there were no regions with reduced ReHo compared to the controls. No significant correlation was observed between regional abnormalities of fALFF, ReHo or DC, and clinical features.
Conclusions
We demonstrated a wide range of abnormal functional activity in the brains of patients with JME, including the prefrontal cortex, visual cortex, default mode network, and cerebellum. The results suggest dysfunctions of the cerebello-cerebral circuits, which provide a clue on the potential pathogenesis of JME.
Similar content being viewed by others
Background
Juvenile myoclonic epilepsy (JME) is the most common syndrome of idiopathic generalized epilepsy (IGE) [1]. JME is characterized by myoclonic jerks, sometimes with generalized tonic-clonic seizures and less frequently with absence seizures. It typically starts during adolescence and has a better prognosis than symptomatic epilepsy [2]. The electroencephalographic (EEG) characteristics of JME are 3–4-Hz generalized polyspike-wave or spike-wave discharges, and routine brain magnetic resonance imaging (MRI) usually presents no structural abnormalities [3, 4]. Resting-state functional MRI (rs-fMRI) reveals intrinsic spontaneous neural activity without performing a task and thus aids in investigation of the possible pathogenesis of JME according to blood oxygen level-dependent signals at the whole-brain level [5].
The rs-fMRI is widely used to explore various neurological conditions using different analytical parameters. The amplitude of low-frequency fluctuation (ALFF), the fractional ALFF (fALFF), the regional homogeneity (ReHo) and the degree centrality (DC) measure local brain functions from various aspects and introduce a complementary relationship. The fALFF reflects the intensity of spontaneous neuronal activity at the voxel level, ReHo measures the synchronization of activity at a given voxel with its neighboring voxels, and DC demonstrates the importance of a node within a cerebral network and quantifies the centrality of node directly [6,7,8]. Using these parameters, a previous study has found functional changes and dysconnectivity among brain regions, especially in the thalamocortical circuit, in JME patients, thus reflecting different intrinsic neural activities at different levels in JME [9]. Additionally, widespread cortical (e.g., in the temporal, occipital, cingulate, and insular cortices) and subcortical (e.g., in the pallidum, hippocampus, and putamen) regional abnormalities have been evidenced in patients with JME, suggesting that brain dysfunction in JME occurs over a range of thalamofrontal regions [10, 11]. However, previous studies only used a single rs-fMRI parameter to investigate local brain activity alterations in JME patients [10, 12, 13].
In this study, we investigated aberrant brain functional activities in a group of JME patients in comparison to healthy controls (HCs), using three complementary metrics. To prevent confounding effects from multiple antiepileptic drugs, only JME patients undergoing monotherapy of valproate (VPA) or levetiracetam (LEV) were included in this study.
Materials and methods
Participants
Forty-nine patients with JME and 44 sex- and age-matched HCs were recruited at the Epilepsy Center at West China Hospital of Sichuan University from January 2016 to April 2019. The inclusion criteria were: (1) having a diagnosis of JME by experienced neurologists according to the criteria of the International League Against Epilepsy guidelines, (2) having normal development, (3) having normal routine brain MRI, (4) with no history of psychiatric disorders, (5) with bilaterally synchronous 3–4-Hz generalized polyspike-wave or spike-wave discharges shown at least once by EEG examination, with a normal background, and (6) having been under monotherapy with VPA or LEV for > 3 months. Four patients were excluded due to the loss of original rs-fMRI images, resulting in 45 patients for further analysis. The HCs had no history of psychiatric or neurologic disorders. Nor did they have any structural lesions on brain MRI. This study was approved by the Local Ethics Committee of the West China of Sichuan University (No.2022[301]). All participants had provided written informed consent.
MRI acquisition
The fMRI data were obtained with a 3 T MR scanner (Siemens Medical Systems, Erlangen, Germany) with an 8-channel phased-array head coil. A T2-weighted gradient echoplanar imaging sequence was used with the following protocols: repetition time/echo time = 2000/30 ms, 30 axial slices per volume, 5-mm slice thickness, no slice gap, matrix = 64 × 64, field of view = 240 × 240 mm2, voxel size = 3.75 × 3.75 × 5 mm3, and flip angle = 90°, 200 volumes, 406 s. During scanning, participants were asked to relax and close their eyes.
The three-dimensional (3D) high-resolution T1-weighted anatomical images were acquired using a 3D-Magnetization Prepared Rapid Gradient Echo sequence: 176 sagittal slices, repetition time = 1900 ms, echo time = 2.26 ms, matrix = 256 × 256, voxel size: 1 × 1 × 1 mm3, thickness/gap = 1/0 mm, and flip angle = 90°. This session lasted for approximately 5 min.
Data preprocessing
All rs-fMRI data were preprocessed using the Data Processing and Analysis for Brain Imaging (DPABI) toolbox based on statistical parametric mapping (SPM12) in MATLAB. After discarding the first 5 points, slice timing and head motions were applied to the remaining 195 points. No patient had head translation movement > 3 mm or head rotation > 3°. The realigned mean functional images were coregistered to individual structural images. All functional images were subject to spatial normalization to the Montreal Neurological Institute space using the deformation fields derived from the transformed structural images (resampling voxel size = 3 × 3 × 3 mm3). The quality of the registration operation was checked, including coregistration and spatial normalization. No patient was excluded due to registration error. The linear trend of the time course was removed. Nuisance covariates, including the Friston 24-parameter model, white matter and cerebrospinal fluid signals, were regressed out, followed by band-pass filtering (0.01–0.08 Hz).
fALFF, ReHo and DC calculation
The fALFF values were calculated using DPABI. The filtered time series for each voxel was converted into the frequency domains using Fast Fourier Transform. The square root of the power spectrum was calculated and averaged across 0.01–0.08 Hz. This mean square root was defined as the ALFF [14]. The fALFF was the ratio of the ALFF at 0.01–0.08 Hz band to the ALFF across the whole frequency range (0.01–0.25 Hz) [7]. The fALFF is usually less sensitive to ventricles and blood vessels and reflects the intensity of spontaneous neuronal activity at a given voxel.
ReHo values were calculated using DPABI and were based on Kendall’s coefficient of concordance to measure the local synchronization of the time series of the nearest 26 voxels [6]. ReHo represents the consistency of the brain activity in adjacent voxels [6].
DC values were calculated using DPABI. The time course of each voxel within the whole-brain mask was correlated with the time course of every other voxel, and DC measures the strength of correlation to a given voxel [15]. Each voxel represents a node on the graph, and each significant FC in any voxel pair is an edge. The weighted DC is the sum of weights from edges connecting to a node [8]. Significant connections for calculating the DC were identified as having a correlation coefficient threshold of r > 0.25. This threshold was selected to eliminate counting voxels with low temporal correlations due to signal noise [15]. DC refers to the brain’s intrinsic connectivity in relation to the global information integration function [16].
After calculation, the data were normalized by dividing the fALFF, ReHo and DC value of each voxel to the global average fALFF, ReHo and DC value with a whole-brain mask. Finally, all normalized images were smoothed with a Gaussian kernel (4-mm full width at half maximum).
Statistical analysis
The age, sex, and clinical features of participants were analyzed using the SPSS 21.0 (IBM, Armonk, NY). Group difference in age was tested using two-sample t-tests; group difference in sex was analyzed with chi-square test. To identify the abnormalities of brain activity in patients with JME, the normalized fALFF, ReHo and DC maps were included for the second-level random-effects analysis. Two-sample t-tests were performed, with age and sex as nuisance covariates to eradicate their respective contributions. The T-map threshold was set at a voxel level of P < 0.005 and a cluster level of P < 0.05, by using the Gaussian random field theory for multiple comparisons. The values of clusters showing significant group-differences in the mean fALFF/ReHo/DC were extracted for correlation analysis. Pearson correlation analysis assessed the relationships between the fALFF, ReHo or DC and clinical features, including seizure-onset age and epilepsy duration. Spearman’s correlation analysis was used to estimate the relationships between fALFF, ReHo or DC and the VPA or LEV dosage.
Results
Demographics and clinical characteristics
Table 1 shows the demographics and clinical characteristics of the JME patients (n = 45) and the HCs (n = 44). There was no significant difference in sex or age between the two groups. All JME patients received a single type of antiepileptic drugs, including 25 receiving the LEV monotherapy and 20 receiving the VPA monotherapy.
There was no significant difference in age between the VPA and LEV monotherapy groups. In the LEV groups, 20% of the patients (n = 5) were male, while 70% of the patients (n = 14) were male in the VPA group (Supplementary Table S1).
Between-group comparisons of functional alterations
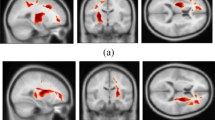
Compared with the HCs, the patients with JME showed decreased fALFF in the left cerebral hemisphere, including the fusiform/lingual gyrus, middle occipital/superior parietal gyrus, inferior parietal lobe (IPL), right cuneus/superior occipital gyrus, and cerebellum, and increased fALFF in the left inferior temporal gyrus (Fig. 1, Table 2).
The group differences of fractional amplitude of low-frequency fluctuation (fALFF) between juvenile myoclonic epilepsy patients with monotherapy and healthy controls. Cold colors indicate decreased fALFF in the left fusiform/lingual gyrus, the middle occipital/superior parietal gyrus, the inferior parietal lobe, the right cuneus/superior occipital gyrus, and the cerebellum. Warm colors indicate an increase in fALFF in the left inferior temporal gyrus (voxel-level P < 0.005, cluster-level P < 0.05, corrected for Gaussian random field theory)
The JME patients also exhibited lower ReHo in the cerebellum, the fusiform/lingual gyrus, the middle occipital gyrus, and the IPL (Table 2, Fig. 2). No regions showed increased ReHo in the JME patients compared to the controls.
Aberrant regional homogeneity (ReHo) in the juvenile myoclonic epilepsy patients with monotherapy compared with healthy controls. Cold colors indicate decreased fALFF in the left fusiform/lingual gyrus, the middle occipital gyrus, and the inferior parietal lobe. (voxel-level P < 0.005, cluster-level P < 0.05, corrected for Gaussian random field theory). No regions showed increased ReHo
Compared with the HCs, the JME patients displayed reduced DC in the left lingual/fusiform gyrus, cerebellum, and the right IPL, and increased DC in the right orbital middle frontal gyrus (Table 2, Fig. 3).
The group differences of degree centrality (DC) between juvenile myoclonic epilepsy patients with monotherapy and healthy controls. Cold colors indicate decreased DC in the left fusiform/lingual gyrus, the right inferior parietal lobe, and cerebellum, Warm colors indicate an increase of DC in the right orbitofrontal cortex (voxel-level P < 0.005, cluster-level P < 0.05, corrected for Gaussian random field theory)
We further compared these parameters between the VPA and the LEV monotherapy groups. Compared with the VPA group, the JME patients with the LEV monotherapy showed decreased fALFF in the left middle temporal gyrus with 55 voxels in size (Supplementary Fig. S1). No significant differences were detected in ReHo and DC.
Correlation analysis
No significant correlations were found between the age of seizure onset or epilepsy duration and the value of fALFF, ReHo or DC in the brain regions that showed significant differences between groups (P > 0.05, corrected for false discovery rate).
Discussion
In this study, we used rs-fMRI indices from three dimensions to investigate focal and global alterations of functional activity in patients with JME. The results showed reduced localized spontaneous brain activity and degree of global connectivity predominantly in the posterior regions of brains of JME patients, including the occipitoparietal cortex and cerebellum, compared with the HCs. This suggests that the functional abnormalities associated with JME have extended beyond the prefrontal lobe. Our findings indicate alterations of the cerebello-cerebral circuits, which may provide new information on the potential neuropathology of JME.
Here, we found that the patients with JME had reduced fALFF, ReHo, and DC in the parietooccipital cortex, especially in the IPL and visual cortex. Previous rs-fMRI studies have demonstrated aberrant neural activities in the parietooccipital cortex in patients with JME [12], childhood absence epilepsy [17], and generalized tonic-clonic seizures only [18]. Studies using both EEG recording and fMRI showed that the JME patients display impaired connections between the lower-level sensory systems (including the primary visual and extravisual networks) and higher-order cognitive networks (including the default mode network [DMN]) [19]. Simultaneous acquisition of EEG-fMRI showed significantly reduced connectivity between DMN and the occipital cortex in patients with IGE and uncontrolled seizures [20]. Studies have also reported aberrant cortical thickness and gray matter volume in the occipital lobe in patients with JME, mainly in the fusiform, lingual gyrus, and lateral occipital cortex [21, 22]. Our results of decreased functional activity and global connectivity in the occipital cortex are consistent with those findings. We speculate that the abnormalities in the occipital cortex are the underlying pathomechanisms of JME.
The IPL and cuneus are the posterior elements of the DMN, which involves self-awareness, higher-order cognition, and modulation of attentions [23]. Previous studies have reported DMN dysfunction and decreased functional connectivity (FC) between the thalamus and DMN in JME patients [11]. In addition, each subtype of IGE shows an apparent reduction in FC within the DMN [24]. Another study showed generalized spike and wave discharge (GSWD)-related deactivations in the DMN, suggesting that GSWDs may interrupt the functions of the DMN [25]. Dynamic effective connectivity and independent component analyses showed complex alterations of DMN and its components in drug-naïve JME patients, indicating DMN dysfunction in the early stage of JME [26]. Interestingly, another study found significantly reduced DMN connectivity in VPA-resistant IGE patients with uncontrolled seizures, compared with responsive patients and HCs [20]. In this study, the concurrent decreases of fALFF, ReHo, and DC in the DMN imply aberrant local spontaneous brain activity and reduced global connectivity in JME patients. Thus, the DMN abnormalities might indicate disorders of essential brain function in JME patients.
We also observed significantly decreased fALFF, ReHo, and DC in the cerebellum. Numerous neuroimaging studies have identified structural and functional abnormalities in the cerebellum of JME patients [10, 12, 27]. The cerebellum also shows enhanced FC with the thalamus [11], and decreased FC with the frontalparietal cortex [28], indicating that the cerebellum affects the thalamocortical circuit functions in JME. Furthermore, the JME patients demonstrate alterations of efferent and afferent fibers between the cerebellum and different regions of the cerebral cortex [28]. These findings indicate disrupted cerebello-cerebral connectivity in JME. Simultaneous EEG-fMRI revealed activations in the cerebellum during GSWDs [25, 29], and functional coupling among the cerebellum, thalamus, frontal cortex, and sensorimotor-related regions [30]. Therefore, the cerebellum may be involved in the generation and propagation of epileptic activities. Increasing studies have suggested that the cerebellum, which has widespread connections with the prefrontal, temporal, parietal and occipital cortices, is associated with cognitive function and emotional processing [31]. The fALFF, ReHo, and DC changes in the cerebellum and cerebral cortices in our study might be related to the disrupted cerebello-cerebral circuit.
DC reflects the importance of voxels in brain functional connectivity alterations and represents the most localized and directly quantifiable measure of centrality. Altered DC in a brain region indicates an aberrant degree of its global connectivity. Compared with the other two metrics in this study, DC depicts the importance of the brain region in the whole brain and is less sensitive to aberrations. In contrast to the above-mentioned decreased DC in the occipital cortex and IPL, the orbitofrontal cortex exhibited increased DC. The orbitofrontal cortex is an important subdivision of the prefrontal cortex, and is connected to structures involved in emotion processing and cognition, such as the hippocampus, amygdala, and dorsolateral prefrontal cortex. Previous rs-fMRI studies have reported increased intrinsic activity and functional coupling in the prefrontal cortex in patients with JME [10, 12, 27]. During a verbal memory task, researchers observed low activations in the amygdala, hippocampus and frontotemporal cortices in JME patients [32]. Structural neuroimaging studies have confirmed increased cortical thickness in the prefrontal cortex of JME patients [33, 34]. Neuropsychological and neuroimaging studies have revealed that the JME patients present impaired behavioral functions, working memory, and executive functions, which are associated with a dysfunctional prefrontal lobe [35]. The increased degree of global connectivity in the prefrontal cortex reflects the role of the prefrontal cortex in regulating activities of the whole brain. Disruption of the prefrontal cortex might underlie the pathophysiological mechanisms of JME.
The limitations of our study included a small sample size and an unbalanced representation of patients receiving LEV and VPA monotherapies in a cross-sectional design. Thus, our results could not explain a direct casual relationship. Longitudinal follow-up of larger cohorts of JME patients undergoing monotherapy is needed to establish patterns of functional change in specific brain regions.
Conclusions
In summary, we demonstrated that JME patients undergoing monotherapy exhibited decreased functional activity in the occipitoparietal lobe and cerebellum, and increased functional activity in the prefrontal cortex compared with the HCs. JME involves a wide range of abnormal functional activities across the brain, including the DMN, the cerebellum, and the frontal and visual cortices. Our results suggest that combining the fALFF, ReHo, and DC metrics enables effective detection of brain functional abnormalities. The findings of dysfunctions of cerebello-cerebral circuits may provide a clue for understanding the potential pathogenesis of JME.
Availability of data and material s
All data can be obtained from the corresponding author on reasonable request.
Abbreviations
- ALFF:
-
Amplitude of low-frequency fluctuation
- DC:
-
Degree centrality
- DMN:
-
Default mode network
- EEG:
-
Electroencephalography
- fALFF:
-
Fractional amplitude of low-frequency fluctuation
- FC:
-
Functional connectivity
- fMRI:
-
Functional magnetic resonance imaging
- GSWDs:
-
Generalized spike and wave discharge
- HCs:
-
Healthy controls
- IGE:
-
Idiopathic generalized epilepsy
- IPL:
-
Inferior parietal lobe
- JME:
-
Juvenile myoclonic epilepsy
- LEV:
-
Levetiracetam
- MRI:
-
Magnetic resonance imaging
- ReHo:
-
Regional homogeneity
- rs-fMRI:
-
Resting-state functional magnetic resonance imaging
- VPA:
-
Valproate
References
Camfield CS, Striano P, Camfield PR. Epidemiology of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S15–7.
Yang H, Zhang J, Yang C, Wu D, Liu X, Lu H, et al. The long-term prognosis and predictors of epilepsy: a retrospective study in 820 patients. Acta Epileptologica. 2021;3(1):26.
Janz D. Juvenile myoclonic epilepsy. Epilepsy with impulsive petit mal. Cleve Clin J Med. 1989;56(Suppl Pt 1):S23–33 discussion S40–22.
Engel J Jr. International league against E. a proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE task force on classification and terminology. Epilepsia. 2001;42(6):796–803.
Lv H, Wang Z, Tong E, Williams LM, Zaharchuk G, Zeineh M, et al. Resting-state functional MRI: everything that nonexperts have always wanted to know. AJNR Am J Neuroradiol. 2018;39(8):1390–9.
Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22(1):394–400.
Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172(1):137–41.
Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, et al. Network centrality in the human functional connectome. Cereb Cortex (New York, NY: 1991). 2012;22(8):1862–75.
Wang Y, Berglund IS, Uppman M, Li TQ. Juvenile myoclonic epilepsy has hyper dynamic functional connectivity in the dorsolateral frontal cortex. Neuroimage Clin. 2019;21:101604.
Kim JH, Kim JB, Suh SI. Alteration of cerebello-thalamocortical spontaneous low-frequency oscillations in juvenile myoclonic epilepsy. Acta Neurol Scand. 2019;140(4):252–8.
Jiang S, Luo C, Gong J, Peng R, Ma S, Tan S, et al. Aberrant Thalamocortical connectivity in juvenile myoclonic epilepsy. Int J Neural Syst. 2018;28(1):1750034.
Jiang S, Luo C, Liu Z, Hou C, Wang P, Dong L, et al. Altered local spontaneous brain activity in juvenile myoclonic epilepsy: a preliminary resting-state fMRI study. Neural Plast. 2016;2016:3547203.
Zhong C, Liu R, Luo C, Jiang S, Dong L, Peng R, et al. Altered structural and functional connectivity of juvenile myoclonic epilepsy: an fMRI study. Neural Plast. 2018;2018:7392187.
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development. 2007;29(2):83–91.
Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29(6):1860–73.
Czaikowski BL, Liang H, Stewart CT. A pediatric FOUR score coma scale: interrater reliability and predictive validity. J Neurosci Nurs. 2014;46(2):79–87.
Yang T, Fang Z, Ren J, Xiao F, Li Q, Liu L, et al. Altered spontaneous activity in treatment-naive childhood absence epilepsy revealed by regional homogeneity. J Neurol Sci. 2014;340(1–2):58–62.
Liao W, Wang J, Xu T, Zhang ZQ, Ji GJ, Xu Q, et al. Altered relationship between thickness and intrinsic activity amplitude in generalized tonic-clonic seizures. Sci Bull. 2016;61(24):1865–75.
Dong L, Luo C, Zhu Y, Hou C, Jiang S, Wang P, et al. Complex discharge-affecting networks in juvenile myoclonic epilepsy: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2016;37(10):3515–29.
Kay BP, DiFrancesco MW, Privitera MD, Gotman J, Holland SK, Szaflarski JP. Reduced default mode network connectivity in treatment-resistant idiopathic generalized epilepsy. Epilepsia. 2013;54(3):461–70.
Park KM, Kim TH, Han YH, Mun CW, Shin KJ, Ha SY, et al. Brain morphology in juvenile myoclonic epilepsy and absence seizures. Acta Neurol Scand. 2016;133(2):111–8.
Park KM, Kim SE, Lee BI, Hur YJ. Brain morphology in patients with genetic generalized epilepsy: its heterogeneity among subsyndromes. Eur Neurol. 2018;80(5–6):236–44.
Smallwood J, Bernhardt BC, Leech R, Bzdok D, Jefferies E, Margulies DS. The default mode network in cognition: a topographical perspective. Nat Rev Neurosci. 2021;22(8):503–13.
Parsons N, Bowden SC, Vogrin S, D'Souza WJ. Default mode network dysfunction in idiopathic generalised epilepsy. Epilepsy Res. 2020;159:106254.
Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci. 2005;102(42):15236–40.
Zhang Z, Liu G, Zheng W, Shi J, Liu H, Sun Y. Altered dynamic effective connectivity of the default mode network in newly diagnosed drug-naïve juvenile myoclonic epilepsy. Neuroimage Clin. 2020;28:102431.
Jia X, Ma S, Jiang S, Sun H, Dong D, Chang X, et al. Disrupted coupling between the spontaneous fluctuation and functional connectivity in idiopathic generalized epilepsy. Front Neurol. 2018;9:838.
Jiang S, Li X, Li Z, Chang X, Chen Y, Huang Y, et al. Cerebello-cerebral connectivity in idiopathic generalized epilepsy. Eur Radiol. 2020;30(7):3924–33.
Moeller F, Maneshi M, Pittau F, Gholipour T, Bellec P, Dubeau F, et al. Functional connectivity in patients with idiopathic generalized epilepsy. Epilepsia. 2011;52(3):515–22.
Qin Y, Jiang S, Zhang Q, Dong L, Jia X, He H, et al. BOLD-fMRI activity informed by network variation of scalp EEG in juvenile myoclonic epilepsy. Neuroimage Clin. 2019;22:101759.
Habas C. Functional connectivity of the cognitive cerebellum. Front Syst Neurosci. 2021;15:642225.
Caciagli L, Wandschneider B, Xiao F, Vollmar C, Centeno M, Vos SB, et al. Abnormal hippocampal structure and function in juvenile myoclonic epilepsy and unaffected siblings. Brain. 2019;142(9):2670–87.
Alhusaini S, Ronan L, Scanlon C, Whelan CD, Doherty CP, Delanty N, et al. Regional increase of cerebral cortex thickness in juvenile myoclonic epilepsy. Epilepsia. 2013;54(9):e138–41.
Tae WS, Kim SH, Joo EY, Han SJ, Kim IY, Kim SI, et al. Cortical thickness abnormality in juvenile myoclonic epilepsy. J Neurol. 2008;255(4):561–6.
Wolf P, Yacubian EM, Avanzini G, Sander T, Schmitz B, Wandschneider B, et al. Juvenile myoclonic epilepsy: a system disorder of the brain. Epilepsy Res. 2015;114:2–12.
Acknowledgements
We gratefully acknowledge all participants in this project. This study was supported by the National Natural Science Foundation of China (82101521 and 81871017) and the Brain Science Project of West China Hospital, Sichuan University (ZYJC21001).
Funding
This study was supported by the National Natural Science Foundation of China (82101521 and 81871017) and the Brain Science Project of West China Hospital, Sichuan University (ZYJC21001).
Author information
Authors and Affiliations
Contributions
LQ and YZ drafted and revised the manuscript; LQ, YZ, and DZ analyzed and interpreted the data; YZ, JR, DL, and XL collected the neuroimaging data; TY, QG, and DZ designed this study and revised the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This study was approved by the Local Ethics Committee of the West China of Sichuan University (No.2022[301]). All participants provided written informed consent.
Consent for publication
All participants gave consent for publication.
Competing interests
DZ, who is the associate editor of Acta Epileptologica, was not involved in the journal’s review of or decisions on this manuscript.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, L., Zhang, Y., Ren, J. et al. Altered brain activity in juvenile myoclonic epilepsy with a monotherapy: a resting-state fMRI study. Acta Epileptologica 4, 29 (2022). https://doi.org/10.1186/s42494-022-00101-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42494-022-00101-4