Abstract
The mitogen-activated protein kinase (MAPK) cascade MEK2-SIPK/WIPK is essential for immunity in Solanaceae plants. This cascade is tightly controlled to prevent harmful hyperactivation. However, the E3 ubiquitin ligases utilized by plants to reduce MEK2- SIPK/WIPK protein levels remain largely elusive. Here, we confirmed the essential role of Nicotiana benthamiana MEK2-SIPK/WIPK in resistance to the oomycete pathogen Phytophthora capsici. Using tobacco rattle virus (TRV)-based gene silencing, we screened prevalent plant U-box protein (PUB)-type E3 ligases with Armadillo (ARM) repeats to characterize those involved in Phytophthora resistance and MEK2-SIPK/WIPK degradation. We found that pub40 knockdown mutants exhibited significantly enhanced resistance to P. capsici. NbPUB40 was under ubiquitination and proteasomal degradation in planta, with two conserved sites (Cys28 and Val41) in the U-box domain being essential for its activity. NbPUB40 was shown to interact with the whole MEK2-SIPK/WIPK cascade and promote their degradation, the ubiquitination levels of which were also notably reduced in the pub40 mutant. Our results reveal a mechanism in which a PUB E3 ubiquitin ligase negatively regulates plant P. capsici resistance by destabilizing the MEK2-SIPK/WIPK cascade.
Similar content being viewed by others
Background
Plants have evolved sophisticated immune systems to sense the invasion of phytopathogens (Jones and Dangl. 2006). Cell surface-localized pattern recognition receptors (PRRs) are activated by pathogen-associated molecular patterns (PAMPs), leading to phosphorylation and activation of receptor-like cytoplasmic kinases (RLCKs) and causing multiple immune events (Zhang and Zhang. 2022). Rapid activation of mitogen-activated protein kinase (MAPK or MPK) cascades downstream of the RLCKs is central to the plant immune system (Su et al. 2021). Each of the three layers has multiple members to ensure signaling diversity. Stimulus perception by PRRs activates MAPK kinase kinases (MAPKKKs or MKKKs), MAPKKKs phosphorylate and activate downstream MAPK kinases (MAPKKs, MKKs, or MEKs), which further activates MAPKs through phosphorylation (Group. 2002). Activated MAPKs subsequently phosphorylate substrate proteins, including transcription factors, enzymes, and other proteins with distinct functions to induce immune responses (Jonak et al. 2002; Li et al. 2012a; Tsuda and Somssich. 2015; Furlan et al. 2017).
While PAMP-triggered immunity (PTI) induces rapid and instantaneous activation of MAPK cascades to enhance local immune responses, ETI ensures extended and persistent MAPK activation (Zipfel. 2009; Tsuda et al. 2013; Cui et al. 2015). However, hyperactivation of MAPKs is harmful to plants. Ectopic expression of MAPKs, such as NtMEK2DD (a constitutively active mutant of NtMEK2), StMEK1DD, GhMKK6, CA-StMPK7, and NtSIPK often causes excessive hypersensitive response (HR) in plants (Yang et al. 2001; Zhang and Liu. 2001; Kanzaki et al. 2003; Wang et al. 2017; Zhang et al. 2021). Therefore, MAPK signaling needs to be controlled tightly to prevent hyperactivation of defense responses. To this end, MAPK phosphatases dephosphorylate and inactivate MAPKs (Jiang et al. 2017). In potato, the protein tyrosine phosphatase StPTP1a dephosphorylates StMPK4/7 to inhibit resistance to Phytophthora infestans (Li et al. 2023a). Cotton microRNA ghr-miR5272a targets and represses the expression of GhMKK6 to attenuate plant immunity (Wang et al. 2017). In Arabidopsis thaliana, MAPK signaling can be inhibited by the competition between MAPKKK proteins YDA and MAPKKK3/5 for binding downstream MKKs (Sun et al. 2018).
MEK2-SIPK/WIPK is a well-known MAPK immunity cascade in Solanaceae plants (Yang et al. 2001). MEK2 shares the highest similarity with Arabidopsis MKK4 and MKK5, and SIPK and WIPK are orthologs of Arabidopsis MPK6 and MPK3, respectively (Asai et al. 2002). N-gene-mediated TMV resistance is compromised by the silencing of MEK2, SIPK, or WIPK in tobacco plants. Likewise, N. benthamiana MEK2 and SIPK participate in brassinosteroid (BR)-induced TMV resistance (Jin et al. 2003; Deng et al. 2016). Solanum lycopersicum MEK2-SIPK/WIPK cascade acts downstream of SlMAPKKKε, which is essential for resistance to Gram-negative bacterial pathogens (Melech-Bonfil and Sessa. 2010). Pathogens have targeted MEK2-SIPK/WIPK due to their importance in plant resistance. Although the regulation of the MEK2-SIPK/WIPK cascade by Phytophthora effectors is unknown, many other pathogens secrete a series of effectors to disturb MEK2-SIPK/WIPK activation. Xanthomonas employs at least five effectors (XopE1, XopM, XopQ, AvrBs1, and AvrXv4) to inhibit MEK2DD-mediated cell death (Teper et al. 2015), with XopQ also suppressing SIPK-induced cell death (Teper et al. 2014). CSEP0139 and CSEP0192 are fungi Blumeria graminis f. sp. hordei (Bgh) effectors that inhibit cell death induced by MEK2DD (Li et al. 2021). However, it remains unclear how plants regulate the MEK2-SIPK/WIPK cascade.
The ubiquitin-proteasome degradation system attenuates plant immune responses via an efficient negative feedback loop (Trenner et al. 2022). Ubiquitin is attached to substrates with E3 ubiquitin ligases (Chen and Hellmann. 2013), which are divided into four structural groups: RING (Really Interesting New Gene), HECT (Homologous to E6AP C-Terminus), U-box, and CRL (Cullin-RING Ligases) (Vierstra. 2009; Zhou and Zeng. 2017). KEEP ON GOING (KEG), an Arabidopsis RING-type E3 ubiquitin ligase ubiquitinates MKK4 and MKK5 to attenuate immunity (Gao et al. 2021).
Plant U-box proteins (PUBs) with C-terminal Armadillo (ARM) repeats are the most abundant U-box E3 ligases in plants (Patterson. 2002). ARM-containing PUBs ubiquitinate and degrade immune components, especially protein kinases (Trujillo. 2018). For example, Arabidopsis PUB13 targets include the flg22 receptor FLS2 (FLAGELLIN-SENSING2), the chitin receptor LYK5 (LYSM-CONTAINING RECEPTOR-LIKE KINASE 5), and the phytosulfokine receptor PSKR1 (PHYTOSULFOKINE RECEPTOR 1) (Lu et al. 2011; Liao et al. 2017; Hu et al. 2023). Rice SPL11, a PUB13 ortholog, ubiquitinates a S-domain receptor-like kinase SDS2 (SPL11 cell-death suppressor 2) (Fan et al. 2018). PUB25 and PUB26 negatively regulate immunity by ubiquitinating the unphosphorylated (non-activated) RLCK BIK1 (BOTRYTIS-INDUCED KINASE 1) to facilitate its proteasomal degradation (Wang et al. 2018b).
Given the importance of MAPK signaling in plant immunity and the significance of PUBs in destabilizing MAPKs, we screened Nicotiana benthamiana PUBs using tobacco rattle virus (TRV)-based gene silencing and identified PUB40 as an attenuator of plant resistance to Phytophthora capsici. We further demonstrated that NbPUB40-mediated resistance attenuation is achieved by interacting with and destabilizing the MEK2-SIPK/WIPK (wound-induced protein kinase) cascade, which is an essential MAPK signaling pathway for Solanaceae plant immunity (Mase et al. 2012).
Results
Identification of N. benthamiana ARM PUBs repressing resistance to P. capsici
A total of 47 ARM PUBs were identified in N. benthamiana using hidden Markov model-based profiles (Fig. 1a) (Kourelis et al. 2019). A maximum likelihood tree was constructed using these NbPUBs and 41 Arabidopsis ARM PUBs (Mudgil et al. 2004). Since N. benthamiana is an allotetraploid, many NbPUBs with high similarity occurred in pairs in the phylogenetic tree (Fig. 1b). Accordingly, we constructed 28 TRV gene silencing constructs (TRV:P1–28) targeting 47 ARM NbPUBs (Fig. 1b and Additional file 1: Table S1). TRV:P22-infiltrated N. benthamiana plants exhibited notable growth defect (Additional file 2: Figure S1a) and reduced P22 expression (Additional file 2: Figure S1b), spontaneous cell death spreading from leaf veins (Additional file 2: Figure S1a) as detected by trypan blue staining (Additional file 2: Figure S1c), increased H2O2 accumulation shown by 3,3’-diaminobenzidine tetrahydrochloride (DAB) staining (Additional file 2: Figure S1d), and highly induced expression of pathogenesis-related genes PR1 and PR2 (Additional file 2: Figure S1e). These results demonstrate that the two NbPUBs (NbD002382 and NbD042169) silenced by TRV:P22 may negatively regulate plant immunity, which is consistent with a previous report that Arabidopsis PUB13 suppresses defense responses including cell death (Li et al. 2012b).
Identification of Nicotiana benthamiana ARM PUBs repressing plant immunity. a The screening pipeline for ARM PUBs repressing P. capsici resistance using TRV-based gene silencing. b Phylogenetic analysis of 47 N. benthamiana and 41 Arabidopsis ARM PUBs. A maximum likelihood tree was constructed using IQ-TREE 2 (evolutionary model: JTT + F + I + G4). The blue font represents NbPUB40 and NbPUB40-like. The red font represents the two genes silenced by TRV:P22. Their silencing activated plant autoimmunity. c, d Silencing of NbPUB40 homologs induced by TRV:P12 or TRV:P13 inhibited P. capsici infection. TRV:GFP was used as a negative control for all the assays. TRV:P12/13-treated leaves were inoculated with P. capsici zoospores and photographed after 36–48 h under UV light. Seven leaves were counted in each experiment, and the experiment was repeated three times. Lesion areas were measured by ImageJ. Error bars indicate ± SD (Dunnett’s post hoc test, * P < 0.05, *** P < 0.001). e Relative transcript accumulation levels of NbPUB40 in TRV:GFP- and TRV:P12-treated plants, the expression level of NbPUB40-like in TRV:GFP- and TRV:P13-treated plants. Error bars indicate ± SD (Student’s t-test, *** P < 0.001)
Compared to TRV:GFP-treated control plants, no visible phenotype was induced by the other 27 constructs one week after infiltration. To test their involvement in Phytophthora resistance, we inoculated TRV-treated plants with P. capsici zoospores 18 days after infiltration (Additional file 2: Figure S2). Lesions caused by P. capsici were significantly inhibited by TRV:P12 targeting NbD017068 (NbPUB40) or TRV:P13 targeting NbD030710/NbD036202 (NbPUB40-like) (Fig. 1c, d). RT-qPCR analysis confirmed that P12 was effectively silenced in TRV:P12-treated plants, and P13 was silenced in TRV:P13-treated plants (Fig. 1e). We then generated at least three heterozygous pub40-like mutants and two homozygous pub40 mutant lines (pub40-1 and pub40-2) via CRISPR/Cas9-mediated genome editing. The respective 1-bp insertion and 154-bp deletion in pub40-1 and pub40-2 lead to premature termination of NbPUB40 (Fig. 2a and Additional file 2: Figure S3), which did not cause an obvious difference in growth phenotypes between four-week pub40 mutants and non-transgenic N. benthamiana (Additional file 2: Figure S4). In line with TRV:P12/P13-treated plants, pub40 mutants showed significantly reduced disease symptoms (Fig. 2b, c) and pathogen biomass accumulation after P. capsici inoculation (Fig. 2d). We used RT-qPCR to further detect the expression pattern of NbPUB40 after infection with P. capsici. As expected, the expression of NbPUB40 was significantly inhibited in N. benthamiana after 3 h of infection (Fig. 2e). Collectively, NbPUb40 is a novel repressor of P. capsici resistance in N. benthamian.
NbPUB40 negatively regulates plant resistance to P. capsici. a Genotypes of two N. benthamiana pub40 mutants (pub40-1 and pub40-2) generated by CRISPR/Cas9-mediated genome editing. sgRNA sequences are highlighted with underlines. Protospacer adjacent motifs (PAMs) are represented in blue font. Insertion and deletion are in red font and dashed lines. b, c Both pub40 mutants showed increased resistance to P. capsici infection. Leaves infected by P. capsici were photographed two days post-inoculation (dpi) under UV light. Average lesion areas were measured by ImageJ. Data included results from three independent experiments, with at least ten leaves in each experiment. Error bars indicate ± SD (Dunnett’s post hoc test, *** P < 0.001). d Relative biomass was measured by quantitative PCR using NbACT as an internal reference gene. Error bars indicate ± SD (Dunnett’s post hoc test, ** P < 0.01). e The expression pattern of NbPUB40 was analyzed after infection with P. capsici at various time points by quantitative PCR. The NbACT gene was used as the reference gene. Error bars indicate ± SD
NbPUB40 is subject to ubiquitination and proteasomal degradation in planta
An active E3 ubiquitin ligase, such as potato CMPG1 (Bos et al. 2010), usually can ubiquitinate itself for degradation by the 26S proteasome. When N. benthamiana leaves expressing HA-tagged NbPUB40 were treated with the protein synthesis inhibitor cyclohexmide (CHX), NbPUB40 protein abundance was notably reduced in a treatment time-dependent manner (Fig. 3a). NbPUB40 degradation could be suppressed by the proteasome inhibitor PS341 (bortezomib) and MG132, but not by the autophagy inhibitor E64d and DMSO (dimethyl sulfoxide) control (Fig. 3b, c and Additional file 2: Figure S5), indicating that NbPUB40 is subject to proteasomal degradation.
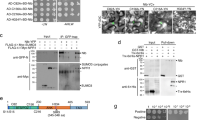
NbPUB40 is subject to ubiquitination and proteasomal degradation in planta. a Inhibition of protein synthesis by CHX revealed in planta degradation of NbPUB40. After transient expression of NbPUB40 for 36 h, N. benthamiana leaves were treated with 50 μM CHX for 0, 0.5, 1, and 2 h before collecting samples for immunoblotting. b NbPUB40 degradation was suppressed by the 26S proteasome inhibitor PS341 and MG132. Leaves expressing NbPUB40-HA were treated with 0.5% DMSO (dimethyl sulfoxide), 50 μM CHX, CHX + DMSO, CHX + 50 μM PS341, CHX + 50 μM E64d, or CHX + 50 μM MG132 for 2 h before sample collection. The anti-HA antibody was used for immunoblotting. Protein band intensities were analyzed using ImageJ. c NbPUB40 degradation was suppressed by PS341 in a time-dependent manner. After transient expression of NbPUB40 for 36 h, leaves were treated with 50 μM CHX for 4 h before sampling and treated with 50 μM PS341 for 4, 3, 2, and 1 h before sampling. The anti-HA antibody was used for immunoblotting. Protein band intensities were analyzed using ImageJ. A simple illustration of the sampling timeline is shown. d Two conserved active sites (represented by red triangles) of the U-box domain in NbPUB40, potato StPUB17, and Arabidopsis AtPUB13 and AtPUB25. e Mutation of C28A or V41I significantly reduced NbPUB40 polyubiquitination
The U-box domain, especially its two highly conserved amino acid residues (Cys and Val), is crucial for the activity of E3 ubiquitin ligases (Gonzalez-Lamothe et al. 2006; Yang et al. 2006). Multiple sequence alignment showed that the U-box domain of NbPUB40 also contains these two conserved residues (Cys28 and Val41, Fig. 3d). In an in-planta ubiquitination assay, FLAG-tagged NbPUB40, NbPUB40-C28A, and NbPUB40-V41I mutants were co-expressed with HA-UBQ (ubiquitin) in N. benthamiana leaves. Ubiquitination signals of extracted total proteins immunoprecipitated with anti-FLAG beads were determined by immunoblots using anti-HA antibody. Compared to wild-type (WT) NbPUB40, C28A or V41I mutation led to a remarkably reduced ubiquitination signal (Fig. 3e). These findings suggested that NbPUB40 might be an active E3 ubiquitin ligase with U-box Cys28 and Val41 being critical for its activity.
NbPUB40 interacts with multiple MAPKs
Next, we identified candidate protein substrates of NbPUB40 by immunoprecipitation-mass spectrometry (IP-MS). Interacting proteins of an unrelated PUB (NbD002382 targeted by TRV:P22) were also identified in parallel to characterize potential false positive targets. Multiple MAPKs were found in the 586 candidate NbPUB40 substrate list, including a Nicotiana protein kinase 1 (NPK1)-like (NbNPL1: NbD027101) MAPKKK, two MAPKKs NbMEK2 (NbD050000) and NbSIPKK (NbD049175), and two MAPKs NbSIPK (NbD046763) and NbWIPK (NbD016498) (Additional file 1: Table S2).
We used split-LUC (luciferase) and co-immunoprecipitation (Co-IP) assays to confirm in vivo interactions between NbPUB40 and the abovementioned MAPK signaling proteins except for NbNPL1, which is lethal to Agrobacterium tumefaciens used for transient expression in N. benthamiana. Co-expression of NbPUB40 with NbSIPKK, NbSIPK, or NbWIPK generated high-intensity luminescence in split-LUC assays (Fig. 4a). For the Co-IP assay, NbPUB40 co-immunoprecipitated with NbSIPKK, NbMEK2, or NbWIPK (Fig. 4b). Since co-expression with NbSIPK dramatically reduced NbPUB40 protein expression in N. benthamiana (Additional file 2: Figure S6a), we successfully purified the other proteins than NPL1 from Escherichia coli and performed GST pull-down assays, where NbPUB40 was able to pull down NbSIPKK NbMEK2, NbSIPK, and NbWIPK in vitro (Fig. 4c). Yeast two-hybrid (Y2H) assays showed NbPUB40 interaction with NbNPL1, NbSIPKK, and NbSIPK (Additional file 2: Figure S6b). Based on these results, NbPUB40 is a key immune component associated with MAPK protein signaling (Fig. 4d).
NbPUB40 interacts with multiple MAPKs. a NbPUB40 was associated with NbSIPKK, NbSIPK, and NbWIPK in the split-LUC assay. The corresponding constructs were transiently expressed in N. benthamiana, and protein expression was confirmed by immunoblot assays. Protein interaction intensities were indicated by relative luminescence units (RLUs) (mean ± SD, n ≥ 6). b Co-IP assays confirmed NbPUB40 interaction with NbSIPKK, NbMEK2, and NbWIPK. GFP-tagged NbPUB40 transiently co-expressed with NbSIPKK-HA, NbMEK2-HA, or NbWIPK-HA. Extracted total plant proteins were immunoprecipitated with GFP-Trap agarose (Chromo Tek). Red asterisks indicate the band of NbPUB40-GFP. c NbPUB40 interact with NbSIPK, NbWIPK, NbMEK2, and NbSIPKK in vitro. Protein interactions were detected by GST pull-down assays with glutathione agarose beads. d A schematic illustration of interactions between PUB40 and MAPKs
The interaction network of NbPUB40-targeted MAPKs
MEK2-SIPK/WIPK is an essential MAPK immunity cascade in N. benthamiana (Sharma et al. 2003; Asai et al. 2008). As a negative regulator of MAPKKKε/MEK2/SIPK-WIPK, SIPKK suppresses SIPK activity (Gomi et al. 2005; Melech-Bonfil and Sessa. 2010). To test whether NbNPL1 interacts with NbSIPKK or NbMEK2, we constructed their interaction network via Co-IP, split-LUC, and Y2H assays. NbSIPKK but not NbMEK2 strongly interacted with NbNPL1 in Y2H (Additional file 2: Figure S7). Both NbMEK2 and NbSIPKK interacted with NbSIPK and NbWIPK in split-LUC and Co-IP assays (Fig. 5a–d). Y2H assays confirmed that NbMEK2 interacts with NbSIPK and NbWIPK, NSIPKK interacts with NbWIPK (Fig. 5e), but did not detect the interaction between NbSIPKK and NbSIPK (Fig. 5f). The interaction relationships are summarized in Fig. 5g.
The interaction network of NbPUB40-targeted MAPKs. a, b Split-LUC assay showed NbMEK2 and NbSIPKK interaction with NbSIPK and NbWIPK. Corresponding constructs were transiently expressed in N. benthamiana, and protein expression was confirmed by immunoblot assays. Protein interaction intensities were indicated by relative luminescence units (RLU) (mean ± SD, n ≥ 6). c, d NbMEK2 and NbSIPKK interacted with NbSIPK and NbWIPK in Co-IP assays. Anti-GFP and anti-HA antibodies were used for the western blots. e, f Interactions between NbMEK2/ NbSIPKK and NbSIPK/ NbWIPK were confirmed by Y2H. T/53, positive control, T/Lam, negative control. AD, pGADT7; BD, pGBKT7. g The interaction network of NbPUB40-targeted MAPK signaling kinases in N. benthamiana
NbPUB40 destabilizes the MEK2-SIPK/WIPK cascade
We then investigated whether NbPUB40 induced the degradation of these MAPKs. Protein abundances of HA-tagged NbMEK2, NbSIPK, and NbWIPK but not NbSIPKK, decreased notably when co-expressed with NbPUB40-GFP (Fig. 6a). Consistently, the pub40 mutant showed increased protein abundances of NbMEK2, NbSIPK, and NbWIPK, but lower NbSIPKK accumulation (Fig. 6b). In in vivo ubiquitination assays, FLAG-tagged NbSIPKK, NbMEK2, NbSIPK, or NbWIPK were co-expressed with HA-UBQ in WT and pub40-2 mutant plants. NbSIPK was later excluded from the assay since it severely affected the stable expression of UBQ in N. benthamiana for an unknown reason (Additional file 2: Figure S8). A strong polyubiquitination signal was detected in the NbSIPKK, NbMEK2, and NbWIPK immunoprecipitated product using anti-HA antibody (Fig. 6c–e). NbMEK2 and NbWIPK were less ubiquitinated in pub40-2 than in WT, while the polyubiquitination levels of NbSIPKK showed no difference. These findings suggested that NbPUB40 targets the MEK2-SIPK/WIPK cascade.
NbPUB40 destabilizes the MEK2-SIPK/WIPK cascade. a NbPUB40 promoted NbMEK2, NbSIPK, and NbWIPK degradation in planta. HA-tagged NbMEK2, NbSIPK, or NbWIPK were co-expressed with NbPUB40-GFP or the GFP control for 36 h. N. benthamiana leaves were treated with 50 μM CHX for 0, 2, and 4 h before collecting samples for immunoblotting assay. Band intensities were analyzed using ImageJ. b Time-course degradation of NbMEK2, NbSIPK, and NbWIPK in WT and pub40. Designated MAPKs were transiently expressed in N. benthamiana and treated with 50 μM CHX for 0, 2, and 4 h before collecting samples for immunoblotting. c, d, e NbMEK2 and NbWIPK exhibited decreased polyubiquitination in pub40. FLAG-tagged NbMEK2, NbWIPK, or NbSIPKK were transiently expressed with HA-UBQ in WT and pub40
The MEK2-SIPK/WIPK cascade contributes to resistance against P. capsici
The MEK2-SIPK/WIPK cascade is essential for resistance to Gram-negative bacteria, tobacco mosaic virus (TMV), and the Colletotrichum orbiculare fungus (Jin et al. 2003; Tanaka et al. 2009; Melech-Bonfil and Sessa. 2010), but seems to have an independent defense mechanism against Phytophthora infection (Wang et al. 2018a). To confirm the role of this cascade in Phytophthora resistance, we constructed TRV vectors to silence relevant MAPKs before P. capsici inoculation. RT-qPCR analysis confirmed the effective silencing of these genes in TRV-treated plants (Additional file 2: Figure S9). Plants treated with TRV:NbMEK2, TRV:NbSIPK, TRV:NbWIPK, or TRV:NbS/WIPK were more susceptible to P. capsici infection than TRV:GFP-treated plants, as reflected by significantly larger lesion areas (Fig. 7a, b) and the higher levels of relative P. capsici biomass (Fig. 7c). These results indicated that MEK2-SIPK/WIPK positively regulates plant resistance to P. capsici.
The MEK2-SIPK/WIPK cascade is essential for plant resistance to P. capsici. a Representative leaf lesions caused by TRV:MEK2, TRV:SIPK, TRV:WIPK, or TRV:S/WIPK treatment followed by P. capsici infection. Photos were taken 2 dpi under UV light. b Lesion areas were measured by ImageJ. Independent experiments were repeated three times, and similar results were obtained. Error bars indicate ± SD (Dunnett’s post hoc test, * P < 0.05, ** P < 0.01, *** P < 0.001). c Relative P. capsici biomass was analyzed by qPCR using NbACT as an internal reference. Error bars indicate ± SD (Dunnett’s post hoc test, * P < 0.05, ** P < 0.01, *** P < 0.001). d Transient co-expression of NbPUB40 inhibited NbSIPK-triggered cell death compared to the GFP control. Photos were taken under UV light. Relative conductivity was measured two days after infiltration. This assay was repeated using 10 leaves (Student’s t-test, * P < 0.05). e Relevant protein expression was confirmed by immunoblotting assay. The anti-HA antibody was used to detect HA-tagged NbSIPK. The anti-GFP antibody was used to detect PUB40-GFP and the GFP control. Ponceau staining (RBC) showed equal protein loading. f A schematic diagram of NbPUB40-mediated suppression of plant immunity via polyubiquitinating and destabilizing the MEK2-SIPK/WIPK cascade
NtSIPK has been reported to induce cell death in tobacco (Yang et al. 2001; Zhang and Liu. 2001). We observed a similar phenotype when transiently expressed NbSIPK (Fig. 7d). NbSIPK-induced cell death and electrolyte leakage were significantly suppressed when co-expressed with NbPUB40 (Fig. 7d). Proper expression of all proteins was confirmed by immunoblotting (Fig. 7e). These results led us to explore the effect of PUB40 on MAPK activation, we detected MAPK activation after treating WT and pub40 mutants with P. capsici spores for 6 h, and found that MAPK proteins were strongly activated in pub40 mutants (Additional file 2: Figure S10). These results demonstrated that NbPUB40 attenuates P. capsici resistance by destabilizing the MEK2-SIPK/WIPK cascade in N. benthamiana (Fig. 7f).
Discussion
Arabidopsis PUB40 and its two homologs, PUB41 (AT5G62560) and PUB39 (AT3G47820), are involved in the degradation of BRASSINAZOLE RESISTANT1 (BZR1), which is a transcription factor regulating plant root growth and tolerance to phosphate (Pi) starvation (Kim et al. 2019). In the absence of brassinosteroids (BRs), BRASSINOSTEROID-INSENSITIVE2 (BIN2) phosphorylates and stabilizes PUB40 in roots to enhance its binding to BZR1, which leads to BZR1degradation (Kim et al. 2019). However, whether PUB40 participates in plant immunity is unknown. P. capsici can significantly reduce the yield of many crops due to its broad host range and potent destructiveness (Barchenger et al. 2018). We found that NbPUB40, an ortholog of PUB40 in N. benthamiana (Fig. 1b), acts as a negative regulator of plant P. capsici resistance.
A growing number of studies have shown the importance of other PUB proteins in plant immunity (Trujillo. 2018). Arabidopsis PUB13 and its homologs degrade immune receptors FLS2, PSKR1, and SDS2 (Lu et al. 2011; Fan et al. 2018; Hu et al. 2023). PUB25 and PUB26 ubiquitinate unphosphorylated BIK1, which regulates key signaling events downstream of PRRs (Wang et al. 2018b). PUB22 mediates the degradation of Exo70B2, a subunit of the exocyst complex involved in PTI. PUB22 also interacts with MPK3 but does not promote its degradation (Stegmann et al. 2012; Furlan et al. 2017). Soybean GmSAUL1 represses plant immunity likely by inhibiting the activation of MPK3, but it is unclear whether GmSAUL1 could degrade MPK3 (Li et al. 2023b). There have been no reports of U-box type E3 ubiquitin ligase interacting with MAPK protein to regulate plant immune response. In our study, NbPUB40 directly interacts with MEK2-SIPK/WIPK and SIPKK (Fig. 4c), MEK2-SIPK/WIPK contributes to plant resistance to P. capsici (Fig. 7a). These results indicated a new mechanism by which MAPK proteins are the targets of PUBs as well, demonstrating the significant impact of PUBs on plant immunity.
MAPK proteins are also the main targets of pathogen effectors, particularly in Phytophthora species. For instance, multiple effector proteins from the plant pathogenic oomycete P. infestans have been shown to target potato MAPK cascades. Pi22926, PexRD2, and Pi17316 interact with distinct MAPKKKs (King et al. 2014; Murphy et al. 2018; Ren et al. 2019), while PITG20300 and PITG20303 stabilize potato StMKK1 to negatively regulate plant immunity (Du et al. 2021). Phytophthora sojae effector Avh331 promotes the infection by manipulating the MAPK signaling of A. thaliana and N. benthamiana. (Cheng et al. 2012). Unfortunately, the effector of P. capsici that inhibits MAPK activity has not been reported so far; for this reason, it is important to investigate the negative regulators involved in the plant response to P. capsici infection. We observed that NbPUB40 negatively regulates the infection of P. capsici. As a potentially active E3 ubiquitin ligase (Fig. 3), NbPUB40 is essential for the precise regulation of the target MAPK cascade. NbPUB40 influences the ubiquitination of MEK2-SIPK/WIPK in plants (Fig. 6c, d), the mutation of PUB40 enhances their stability, and the MAPK activation following P. capsici infection in pub40 mutants was significantly stronger than in WT (Additional file 2: Figure S9). These findings clarify the mechanism of NbPUB40 promoting the infection of P. capsici by negatively regulating the protein levels of MEK2, SIPK, and WIPK.
E3 ligase activity is often regulated by phosphorylation. MPK3 phosphorylates Thr62 and Thr88 residues of PUB22 to reduce its autoubiquitination, which leads to increased PUB22 accumulation and inhibition of immune signals (Furlan et al. 2017). The phosphorylation of KEG leads to its degradation (Liu and Stone. 2010). BRI1 phosphorylates the Ser-344 residue of PUB13, which is required for PUB13-mediated degradation and endocytosis of BRI1 (Zhou et al. 2018). In addition, the phosphorylation status of E3 ligase substrates is also linked to degradation. For instance, PUB4, PUB25, and PUB26 specifically ubiquitinate and degrade the non-phosphorylated BIK1 (Wang et al. 2018b; Yu et al. 2022). In our results, the co-expression of NbPUB40 and NbSIPK significantly reduced NbPUB40 protein levels (Additional file 2: Figure S6a), suggesting that NbPUB40 may be phosphorylated by NbSIPK. Whether NbPUB40 is phosphorylated and/or activated by its MAPK substrates and the phosphorylation status of NbPUB40-degradable MAPKs remains to be determined. In addition to NbPUB40, we identified two NbPUB40-like (NbD030710/NbD036202) proteins on the same screen. They likely play similar roles in destabilizing MAPKs and attenuating plant immunity. Their acting mechanisms and possible coordination with NbPUB40 need to be explored in the future.
Conclusion
Plant MAPKs are key modules of immune signaling. Precise regulation is crucial to prevent their hyperactivation. However, only limited studies have investigated the regulation of MAPK protein abundance. In this study, we reported that a U-box E3 ligase NbPUB40 attenuates plant resistance to P. capsici by targeting and destabilizing the MEK2-SIPK/WIPK cascade via the 26S proteasome system.
Methods
Plant and microbial materials
Wild-type N. benthamiana and pub40-1 and pub40-2 mutant plants were grown in a glasshouse (25°C, 14-h day/10-h night, and 60% relative humidity). Phytophthora capsici was grown at 25°C on vegetable juice (V8) medium (100 mL filtered V8 juice and 0.2 g calcium carbonate per liter). Escherichia coli DH5α and Agrobacterium tumefaciens GV3101 strains were respectively cultured at 37°C and 28°C on Luria Bertani (LB) medium (5 g yeast extract powder, 10 g tryptone, and 10 g NaCl per liter).
Plasmid vector construction
Designated gene fragments were amplified from N. benthamiana cDNA and ligated into corresponding vectors using ClonExpress® II One Step Cloning Kit (C112). DNA fragments for gene silencing in N. benthamiana were cloned into the pTRV2 vector (Liu et al. 2002). For split-luciferase assays, NbPUB40, NbNPL1, NbSIPKK, NbMEK2, NbWIPK, and NbSIPK coding sequences were inserted into pCAMBIA1300-Cluc-3 × FLAG and pCAMBIA1300-Nluc-HA vectors. For co-immunoprecipitation (Co-IP) assays, constructs were generated using pCAMBIA1300-GFP, pCAMBIA1300-3 × FLAG, and pCAMBIA1300-HA vectors. For yeast two-hybrid assays (Y2H), all designated genes were constructed into pGADT7 and pGBKT7 vectors. PCR Primers used in this study are listed in Additional file 1: Table S1 and Table S3.
CRISPR/Cas9-mediated N. benthamiana genome editing
N. benthamiana pub40 mutants were generated as described previously (Wang et al. 2022). Briefly, two guide RNAs (sgRNA1: GGACAGATGAATTCCTTTGG, sgRNA2: GGCGCGATTTGGGATGATAG) targeting NbPUB40 were designed using the online program CCTop (Stemmer et al. 2015). The pHEE401E construct harboring sgRNAs was transformed into Agrobacterium strain LBA4404. Agrobacterium-mediated leaf disc transformation of N. benthamiana was performed as previously described (Ellis et al. 1987). Gene-edited T0 plants obtained through tissue culture were verified by PCR and sequencing. Seedlings expressing prematurely terminated NbPUB40 were transferred to soil for DNA isolation and genotyping. Homozygous T2 plants were used for subsequent studies.
Virus-induced gene silencing in N. benthamiana
Agrobacterium strains harboring pTRV1 and pTRV2 constructs were mixed with infiltration buffer at a ratio of 1:1 and infiltrated into two-week-old leaves of N. benthamiana. After 18 days, the two largest true leaves were used for P.capsici infection assay. Silencing efficiencies were detected by RT-qPCR, with TRV:GFP used as a control. Primers used for RT-qPCR are listed in Additional file 1: Table S3.
P. capsici infection assay
Fresh mycelial plugs of P. capsici strain LT263 were collected using a cork-borer set (Sigma, Ø 0.5 cm) and inoculated on the back of detached N. benthamiana leaves. Inoculated leaves were placed in dark boxes with high humidity for 36 h. Photos were taken under the ultraviolet lamp. Lesions were measured by ImageJ. Relative P. capsici biomass in infected plant leaves was measured by qPCR (Yu et al. 2012).
RT-qPCR
Total RNA samples were extracted from N. benthamiana leaves pre-treated with TRV constructs. For silencing efficiency evaluation, top leaves were collected 20 days after TRV treatment. TriZol kit (Zoman) and reverse transcription kit (Zoman) were used for RNA extraction and first-strand cDNA synthesis, respectively. qPCR was performed using 2 × HQ SYBR qPCR Mix (Zoman) on QuantStudio 1 (Thermo).
Identification of NbPUB40-interacting proteins
To identify potential NbPUB40 interacting proteins, FLAG-tagged NbPUB40 was transiently expressed in N. benthamiana leaves for 48 h. Total proteins were extracted using lysis buffer: 10 mM Tris–HCl, 150 mM NaCl, 0.5 mM EDTA, 0.5% v/v Triton X-100, 2% (w/v) polyvinylpolypyrrolidone, and 10% (v/v) glycerol. Centrifuged supernatant was incubated with anti-FLAG M2 agarose (Sigma) at 4℃ for 2 h. The beads were then washed with lysis buffer and TBS buffer. Finally, immunoprecipitated proteins were used for LC-MS/MS analysis. Five MAPK proteins with high Qscores were selected as NbPUB40-interacting candidate proteins, as shown in Additional file 1: Table S2.
Co-IP assays
HA and FLAG/GFP-tagged proteins of interest were transiently co-expressed in N. benthamiana. After protein extraction and co-immunoprecipitation, products were incubated with anti-FLAG M2 agarose (Sigma) or GFP-Trap®_A (Chromotek) at 4°C with slight shaking for 2 h. The beads were collected and washed five times with TBS buffer. Eluted proteins were separated in SDS-PAGE and detected with anti-HA, anti-FLAG, or anti-GFP antibodies. The buffer used for protein extraction has been previously described (Zhang et al. 2023).
Split-luciferase complementation assay
Agrobacterium culture harboring Nluc- and Cluc-tagged constructs were co-infiltrated in N. benthamiana. After 48 h, leaf discs were collected in a 96-microplate and incubated in ddH2O, which was then replaced with 1 mM luciferin (Biovision). Relative luciferase activities were measured using Tecan Infinite F200.
Pull-down assay
Recombinant proteins were expressed in E. coli and purified using glutathione agarose beads for GST-tagged proteins or dextrin beads 6FF for MBP-tagged proteins. An amount of 3 μg GST and GST-tagged proteins were incubated with MBP-NbPUB40 with 30 μL glutathione agarose beads in 1 mL GST buffer (25 mM Tris–HCl, 100 mM NaCl, 1 mM DTT, pH 7.5) for 2 h. The glutathione agarose beads were washed 6–7 times with GST wash buffer (25 mM Tris–HCl, 100 mM NaCl, 1 mM DTT, 0.1% Triton, pH 7.5) and eluted with GST buffer containing 15 mM GSH. Eluted proteins were tested by anti-MBP and anti-GST immunoblotting.
Yeast two-hybrid assay
Designated MAPK genes were cloned into pGBKT7 and pGADT7 vectors. Constructs for protein interaction tests were co-transformed into yeast strain AH109. Transformed yeast cells were grown on 2D (Trp-/Leu-) deficient culture medium at 28℃ for 3 days, and then transferred to fresh 2D, 3D (Trp-/Leu-/His-), and 4D (Trp-/Leu-/His-/Ade-) media at 28℃ for 3 days for interaction examinations.
Electrolyte leakage assay
Plant cell death was quantified by measurement of electrolyte leakage (Hatsugai and Katagiri. 2018). N. benthamiana leaf disks were placed in ddH2O at room temperature for 5 h before measured electrical conductivity measurements. Samples were boiled for 20 min and cooled to room temperature for the second round of solution conductivity measurements. Finally, relative electrolyte leakage was calculated by the ratio of sample conductivities before and after boiling. Sample conductivity was measured using a conductivity meter (Mettler Toledo. LE703).
DAB and trypan blue staining
DAB staining was used to determine the H2O2 accumulation. N. benthamiana leaves were placed in DAB staining solution (1 mg/mL) incubated on a shaker at 100 rpm for 8 h, and then boiled with 95% alcohol for decolorization until the green color faded completely. Trypan blue staining was used to detect cell death. N. benthamiana leaves were boiled in trypan blue staining solution (0.02 g trypan blue, 10 mL lactic acid, and 10 g phenol, dissolved in 10 mL ddH2O) for 5 min, and then transferred into a chloral hydrate solution (2.5 g/mL chloral hydrate solution) for decolorization until the green color faded completely.
In planta ubiquitination assay
Detection of ubiquitination in N. benthamiana was performed as described previously (Wang et al. 2022). The ubiquitin protein (UBQ) of N. benthamiana and FLAG-tagged MAPK proteins were transiently co-expressed in N. benthamiana and immunoprecipitated using anti-FLAG M2 agarose (Sigma). Ubiquitin signals were detected using the anti-HA antibody.
Protein stability in planta
For protein stability determination in N. benthamiana, HA-tagged protein was transiently expressed and treated with 50 μM CHX in a time-dependent manner. To determine NbPUB40 impact on MAPK stability, NbPUB40 or the GFP control was co-expressed with designated MAPKs in N. benthamian leaves treated with 50 μM CHX. Total plant proteins were extracted from leaves with the RIPA buffer added for immunoblot analyses. HA immunoblots were quantified in ImageJ and normalized according to the intensity of Ponceau staining.
Availability of data and materials
Sequences mentioned this study can be found in the Oxford Research Archive using the following accession numbers (Kourelis et al. 2019): NbPUB40, NbD017068; NbNPL1, NbD02710; NbSIPKK, NbD04917; NbMEK2, NbD050000; NbSIPK, NbD04676; NbWIPK, NbD01649.
Abbreviations
- ARM:
-
Armadillo
- BIK1:
-
Botrytis-induced kinase 1
- CHX:
-
Cycloheximide
- CRCK3:
-
Calmodulin-binding receptor-like cytoplasmic kinase 3
- CRL:
-
Cullin-RING Ligases
- ETI:
-
Effector-triggered immunity
- FLS2:
-
Flagellin-sensing2
- GFP:
-
Green fuorescent protein
- LB:
-
Luria Bertani
- LRR:
-
Leucine-rich repeat
- LYK5:
-
LysM-containing receptor-like kinase 5
- MAPKs:
-
Mitogen-activated protein kinases
- MAPKKs:
-
MAPK kinases
- MAPKKKs:
-
MAPKK kinases
- MEK2:
-
Mitogen-activated protein kinase 2
- PAMPs:
-
Pathogen-associated molecular patterns
- PTI:
-
PAMP-triggered immunity
- PRRs:
-
Pattern recognition receptors
- PSKR1:
-
Phytosulfokine receptor 1
- PUB:
-
Plant U-box protein
- RLCKs:
-
Receptor-like cytoplasmic kinases
- SIPK:
-
SA-induced protein kinase
- TRV:
-
Tobacco rattle virus
- WIPK:
-
Wound-induced protein kinase
References
Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–83.
Asai S, Ohta K, Yoshioka H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell. 2008;20:1390–406.
Barchenger DW, Lamour KH, Bosland PW. Challenges and strategies for breeding resistance in Capsicum annuum to the multifarious pathogen, Phytophthora Capsici. Front Plant Sci. 2018;9:628.
Bos JI, Armstrong MR, Gilroy EM, Boevink PC, Hein I, Taylor RM, Zhendong T, Engelhardt S, Vetukuri RR, Harrower B, et al. Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc Natl Acad Sci USA. 2010;107:9909–14.
Chen L, Hellmann H. Plant E3 ligases: flexible enzymes in a sessile world. Mol Plant. 2013;6:1388–404.
Cheng BP, Yu XL, Ma ZC, Dong SM, Dou DL, Wang YC, Zheng XB. Phytophthora sojae effector Avh331 suppresses the plant defence response by disturbing the MAPK signalling pathway. Physiol Mol Plant Pathol. 2012;77:1–9.
Cui H, Tsuda K, Parker JE. Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev Plant Biol. 2015;66:487–511.
Deng XG, Zhu T, Peng XJ, Xi DH, Guo H, Yin Y, Zhang DW, Lin HH. Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana. Sci Rep. 2016;6:20579.
Du Y, Chen X, Guo Y, Zhang X, Zhang H, Li F, Huang G, Meng Y, Shan W. Phytophthora infestans RXLR effector PITG20303 targets a potato MKK1 protein to suppress plant immunity. New Phytol. 2021;229:501–15.
Ellis JG, Llewellyn DJ, Dennis ES, Peacock WJ. Maize Adh-1 promoter sequences control anaerobic regulation: addition of upstream promoter elements from constitutive genes is necessary for expression in tobacco. EMBO J. 1987;6:11–6.
Fan J, Bai P, Ning Y, Wang J, Shi X, Xiong Y, Zhang K, He F, Zhang C, Wang R, et al. The monocot-specific receptor-like kinase SDS2 controls cell death and immunity in rice. Cell Host Microbe. 2018;23:498–510.
Furlan G, Nakagami H, Eschen-Lippold L, Jiang X, Majovsky P, Kowarschik K, Hoehenwarter W, Lee J, Trujillo M. Changes in PUB22 ubiquitination modes triggered by MITOGEN-ACTIVATED PROTEIN KINASE3 dampen the immune response. Plant Cell. 2017;29:726–45.
Gao C, Sun P, Wang W, Tang D. Arabidopsis E3 ligase KEG associates with and ubiquitinates MKK4 and MKK5 to regulate plant immunity. J Integr Plant Biol. 2021;63(2):327–39. https://doi.org/10.1111/jipb.13007.
Gomi K, Ogawa D, Katou S, Kamada H, Nakajima N, Saji H, Soyano T, Sasabe M, Machida Y, Mitsuhara I, et al. A mitogen-activated protein kinase NtMPK4 activated by SIPKK is required for jasmonic acid signaling and involved in ozone tolerance via stomatal movement in tobacco. Plant Cell Physiol. 2005;46:1902–14.
Gonzalez-Lamothe R, Tsitsigiannis DI, Ludwig AA, Panicot M, Shirasu K, Jones JDG. The U-Box protein CMPG1 is required for efficient activation of defense mechanisms triggered by multiple resistance genes in tobacco and tomato. Plant Cell. 2006;18:1067–83.
Group M. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci. 2002;7:301–8.
Hatsugai N, Katagiri F. Quantification of plant cell death by electrolyte leakage assay. Bio-Protoc. 2018;8:e2758.
Hu Z, Fang H, Zhu C, Gu S, Ding S, Yu J, Shi K. Ubiquitylation of PHYTOSULFOKINE RECEPTOR 1 modulates the defense response in tomato. Plant Physiol. 2023;192:2507–22.
Jiang L, Anderson JC, Gonzalez Besteiro MA, Peck SC. Phosphorylation of Arabidopsis MAP Kinase Phosphatase 1 (MKP1) is required for PAMP responses and resistance against bacteria. Plant Physiol. 2017;175:1839–52.
Jin HL, Liu YD, Yang KY, Kim CY, Baker B, Zhang SQ. Function of a mitogen-activated protein kinase pathway in N gene-mediated resistance in tobacco. Plant J. 2003;33:719–31.
Jonak C, Okresz L, Bogre L, Hirt H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol. 2002;5:415–24.
Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–9.
Kanzaki H, Saitoh H, Ito A, Fujisawa S, Kamoun S, Katou S, Yoshioka H, Terauchi R. Cytosolic HSP90 and HSP70 are essential components of INF1-mediated hypersensitive response and non-host resistance to Pseudomonas cichorii in Nicotiana benthamiana. Mol Plant Pathol. 2003;4:383–91.
Kim EJ, Lee SH, Park CH, Kim SH, Hsu CC, Xu S, Wang ZY, Kim SK, Kim TW. Plant U-Box40 mediates degradation of the brassinosteroid-responsive transcription factor BZR1 in Arabidopsis roots. Plant Cell. 2019;31:791–808.
King SR, McLellan H, Boevink PC, Armstrong MR, Bukharova T, Sukarta O, Win J, Kamoun S, Birch PR, Banfield MJ. Phytophthora infestans RXLR effector PexRD2 interacts with host MAPKKK epsilon to suppress plant immune signaling. Plant Cell. 2014;26:1345–59.
Kourelis J, Kaschani F, Grosse-Holz FM, Homma F, Kaiser M, van der Hoorn RAL. A homology-guided, genome-based proteome for improved proteomics in the alloploid Nicotiana benthamiana. BMC Genomics. 2019;20:722.
Li G, Meng X, Wang R, Mao G, Han L, Liu Y, Zhang S. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 2012a;8:e1002767.
Li W, Ahn IP, Ning Y, Park CH, Zeng L, Whitehill JG, Lu H, Zhao Q, Ding B, Xie Q, et al. The U-Box/ARM E3 ligase PUB13 regulates cell death, defense, and flowering time in Arabidopsis. Plant Physiol. 2012b;159:239–50.
Li X, Jin C, Yuan HB, Huang WT, Liu F, Fan RC, Xie JK, Shen QH. The barley powdery mildew effectors CSEP0139 and CSEP0182 suppress cell death and promote B. graminis fungal virulence in plants. Phytopathol Res. 2021;3:7.
Li F, Chen X, Yang R, Zhang K, Shan W, Joosten M, Du Y. Potato protein tyrosine phosphatase StPTP1a is activated by StMKK1 to negatively regulate plant immunity. Plant Biotechnol J. 2023a;21:646–61.
Li JM, Ye MY, Wang C, Ma XH, Wu NN, Zhong CL, Zhang Y, Cheng N, Nakata PA, Zeng L, et al. Soybean GmSAUL1, a bona fide U-Box E3 ligase, negatively regulates immunity likely through repressing the activation of GmMPK3. Int J Mol Sci. 2023b;24:6240.
Liao DH, Cao YR, Sun X, Espinoza C, Nguyen CT, Liang Y, Stacey G. Arabidopsis E3 ubiquitin ligase PLANT U-BOX13 (PUB13) regulates chitin receptor LYSIN MOTIF RECEPTOR KINASE5 (LYK5) protein abundance. New Phytol. 2017;214:1646–56.
Liu HX, Stone SL. Abscisic acid increases Arabidopsis ABI5 transcription factor levels by promoting KEG E3 ligase self-ubiquitination and proteasomal degradation. Plant Cell. 2010;22:2630–41.
Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002;30:415–29.
Lu D, Lin W, Gao X, Wu S, Cheng C, Avila J, Heese A, Devarenne TP, He P, Shan L. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science. 2011;332:1439–42.
Mase K, Mizuno T, Ishihama N, Fujii T, Mori H, Kodama M, Yoshioka H. Ethylene signaling pathway and MAPK cascades are required for AAL toxin-induced programmed cell death. Mol Plant Microbe Interact. 2012;25:1015–25.
Melech-Bonfil S, Sessa G. Tomato MAPKKK epsilon is a positive regulator of cell-death signaling networks associated with plant immunity. Plant J. 2010;64:379–91.
Mudgil Y, Shiu SH, Stone SL, Salt JN, Goring DR. A large complement of the predicted Arabidopsis ARM repeat proteins are members of the U-box E3 ubiquitin ligase family. Plant Physiol. 2004;134:59–66.
Murphy F, He Q, Armstrong M, Giuliani LM, Boevink PC, Zhang W, Tian Z, Birch PRJ, Gilroy EM. The potato MAP3K StVIK is required for the Phytophthora infestans RXLR effector Pi17316 to promote disease. Plant Physiol. 2018;177:398–410.
Patterson C. A new gun in town: the U box is a ubiquitin ligase domain. Science’s STKE. 2002;2002:pe4.
Ren Y, Armstrong M, Qi Y, McLellan H, Zhong C, Du B, Birch PRJ, Tian Z. Phytophthora infestans RXLR effectors target parallel steps in an immune signal transduction pathway. Plant Physiol. 2019;180:2227–39.
Sharma PC, Ito A, Shimizu T, Terauchi R, Kamoun S, Saitoh H. Virus-induced silencing of WIPK and SIPK genes reduces resistance to a bacterial pathogen, but has no effect on the INF1-induced hypersensitive response (HR) in Nicotiana benthamiana. Mol Genet Genom. 2003;269:583–91.
Stegmann M, Anderson RG, Ichimura K, Pecenkova T, Reuter P, Zarsky V, McDowell JM, Shirasu K, Trujillo M. The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell. 2012;24:4703–16.
Stemmer M, Thumberger T, Del Sol KM, Wittbrodt J, Mateo JL. CCTop: An intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS ONE. 2015;10:e0124633.
Su B, Zhang X, Li L, Abbas S, Yu M, Cui Y, Baluska F, Hwang I, Shan X, Lin J. Dynamic spatial reorganization of BSK1 complexes in the plasma membrane underpins signal-specific activation for growth and immunity. Mol Plant. 2021;14:588–603.
Sun T, Nitta Y, Zhang Q, Wu D, Tian H, Lee JS, Zhang Y. Antagonistic interactions between two MAP kinase cascades in plant development and immune signaling. EMBO Rep. 2018;19:e45324.
Tanaka S, Ishihama N, Yoshioka H, Huser A, O’Connell R, Tsuji G, Tsuge S, Kubo Y. The Colletotrichum orbiculare SSD1 mutant enhances Nicotiana benthamiana basal resistance by activating a mitogen-activated protein kinase pathway. Plant Cell. 2009;21:2517–26.
Teper D, Salomon D, Sunitha S, Kim JG, Mudgett MB, Sessa G. Xanthomonas euvesicatoria type III effector XopQ interacts with tomato and pepper 14-3-3 isoforms to suppress effector-triggered immunity. Plant J. 2014;77:297–309.
Teper D, Sunitha S, Martin GB, Sessa G. Five Xanthomonas type III effectors suppress cell death induced by components of immunity-associated MAP kinase cascades. Plant Signal Behav. 2015;10:e1064573.
Trenner J, Monaghan J, Saeed B, Quint M, Shabek N, Trujillo M. Evolution and functions of plant U-Box proteins: from protein quality control to signaling. Annu Rev Plant Biol. 2022;73:93–121.
Trujillo M. News from the PUB: plant U-box type E3 ubiquitin ligases. J Exp Bot. 2018;69:371–84.
Tsuda K, Somssich IE. Transcriptional networks in plant immunity. New Phytol. 2015;206:932–47.
Tsuda K, Mine A, Bethke G, Igarashi D, Botanga CJ, Tsuda Y, Glazebrook J, Sato M, Katagiri F. Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genet. 2013;9:e1004015.
Vierstra RD. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol. 2009;10:385–97.
Wang C, He X, Wang X, Zhang S, Guo X. ghr-miR5272a-mediated regulation of GhMKK6 gene transcription contributes to the immune response in cotton. J Exp Bot. 2017;68:5895–906.
Wang H, Chen Y, Wu X, Long Z, Sun C, Wang H, Wang S, Birch PRJ, Tian Z. A potato STRUBBELIG-RECEPTOR FAMILY member, StLRPK1, associates with StSERK3A/BAK1 and activates immunity. J Exp Bot. 2018a;69:5573–86.
Wang J, Grubb LE, Wang J, Liang X, Li L, Gao C, Ma M, Feng F, Li M, Li L, et al. A regulatory module controlling homeostasis of a plant immune kinase. Mol Cell. 2018b;69:493–504.
Wang N, Yin Z, Zhao Y, Wang J, Pei Y, Ji P, Daly P, Li Z, Dou D, Wei L. An F-box protein attenuates fungal xylanase-triggered immunity by destabilizing LRR-RLP NbEIX2 in a SOBIR1-dependent manner. New Phytol. 2022;236:2202–15.
Yang KY, Liu YD, Zhang SQ. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA. 2001;98:741–6.
Yang CW, Gonzalez-Lamothe R, Ewan RA, Rowland O, Yoshioka H, Shenton M, Ye H, O’Donnell E, Jones JD, Sadanandom A. The E3 ubiquitin ligase activity of arabidopsis PLANT U-BOX17 and its functional tobacco homolog ACRE276 are required for cell death and defense. Plant Cell. 2006;18:1084–98.
Yu X, Tang J, Wang Q, Ye W, Tao K, Duan S, Lu C, Yang X, Dong S, Zheng X, Wang Y. The RxLR effector Avh241 from Phytophthora sojae requires plasma membrane localization to induce plant cell death. New Phytol. 2012;196:247–60.
Yu G, Derkacheva M, Rufian JS, Brillada C, Kowarschik K, Jiang S, Derbyshire P, Ma M, DeFalco TA, Morcillo RJL, et al. The Arabidopsis E3 ubiquitin ligase PUB4 regulates BIK1 and is targeted by a bacterial type-III effector. EMBO J. 2022;41:e107257.
Zhang S, Liu Y. Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. Plant Cell. 2001;13:1877–89.
Zhang M, Zhang S. Mitogen-activated protein kinase cascades in plant signaling. J Integr Plant Biol. 2022;64:301–41.
Zhang H, Li F, Li Z, Cheng J, Chen X, Wang Q, Joosten M, Shan W, Du Y. Potato StMPK7 is a downstream component of StMKK1 and promotes resistance to the oomycete pathogen Phytophthora infestans. Mol Plant Pathol. 2021;22:644–57.
Zhang Y, Yin Z, Pi L, Wang N, Wang J, Peng H, Dou D. A Nicotiana benthamiana receptor-like kinase regulates Phytophthora resistance by coupling with BAK1 to enhance elicitin-triggered immunity. J Integr Plant Biol. 2023;65:1553–65.
Zhou B, Zeng L. Conventional and unconventional ubiquitination in plant immunity. Mol Plant Pathol. 2017;18:1313–30.
Zhou J, Liu D, Wang P, Ma X, Lin W, Chen S, Mishev K, Lu D, Kumar R, Vanhoutte I, et al. Regulation of Arabidopsis brassinosteroid receptor BRI1 endocytosis and degradation by plant U-box PUB12/PUB13-mediated ubiquitination. Proc Natl Acad Sci USA. 2018;115:e1906–15.
Zipfel C. Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol. 2009;12:414–20.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural Science Foundation of China (32100155 and 32202251), the Fundamental Research Funds for the Central Universities (KYQN2023039), the Natural Science Foundation of Jiangsu Province (BK20221000), and the China Agriculture Research System (CARS-21). Mention of trade names or commercial products in this publication is solely for providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Contributions
ZY conceived and designed the experiments. YZ, JW, LP, and NW performed experiments and analyzed data. YZ, ZY, and DD wrote the manuscript with editing by HP and GX.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
42483_2024_249_MOESM1_ESM.xlsx
Additional file 1: Table S1. pTRV2 construct primer information of PUB genes. Table S2. LC–MS/MS identified five potential NbPUB40 interacting proteins. Table S3. Primers used in this study.
42483_2024_249_MOESM2_ESM.docx
Additional file 2: Figure S1. TRV based silencing of P22 in N. benthamiana. Figure S2. Screening of NbPUBs involved in P. capsici resistance via TRV-induced gene silencing. Figure S3. The sequence of pub40 mutants showing premature termination. Figure S4. The growth phenotypes of pub40 mutants. Figure S5. NbPUB40 degradation was suppressed by PS341 or MG132 in a time-dependent manner. Figure S6. NbPUB40 interacts with multiple MAPKs. Figure S7. NbNPL1 interacts with NbSIPKK in yeast two-hybrid assay. Figure S8. NbSIPK disturbs stable expression of UBQ. Figure S9. MAPK gene silencing efficiencies in corresponding TRV-treated plants. Figure S10. MAPK activity was detected in WT, pub40-1, and pub40-2 lines at the indicated time points after infection with P. capsici.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Wang, J., Pi, L. et al. PUB40 attenuates Phytophthora capsici resistance by destabilizing the MEK2-SIPK/WIPK cascade in Nicotiana benthamiana. Phytopathol Res 6, 31 (2024). https://doi.org/10.1186/s42483-024-00249-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42483-024-00249-6