Abstract
Tobacco mosaic virus (TMV; genus Tobamovirus) is one of the most prevailing pathogens that seriously affects the quality and yield of tobacco (Nicotiana tabacum) leaves. Cross-protection using mild strains is a potential strategy for the biological prevention of plant viral diseases. Complementary mutations in attenuated strains may cause attenuated ones to suddenly evolve into virulent strains, which limits the application of cross-protection in practice. To data there has been no study on engineering the complementary mutation sites to generate stable attenuated mutants for cross-protection. In this study, we found that the substitution of the conserved arginine at position 88 (R88) in p126 protein with alanine (A) abolished the cell-to-cell movement and reduced the replication of TMV. However, a spontaneous complementary mutation of serine at position 114 (S114) to lysine (K) in p126 restored TMV virulence. Substitution of S114 with R in p126 restored the systemic infection but not the virulence of TMV, therefore, the mutant TMV-R88A/S114R was an attenuated one. Furthermore, our results showed that TMV-R88A/S114R was a stable attenuated mutant, and could effectively protect tobacco plants against the wild-type TMV infection. This study reports a promising TMV mild mutant for cross-protection in tobacco plants by modifying the complementary mutation site in p126.
Similar content being viewed by others
Background
Tobacco (Nicotiana tabacum) is an economically important crop grown worldwide (Song et al. 2018). However, tobacco production is affected seriously by viral diseases (Scholthof et al. 2011; Jones 2021). Tobacco mosaic virus (TMV, genus Tobamovirus) causes mosaic and deformation symptoms, which seriously threatens the quality of tobacco leaves (Ellis et al. 2020), and has been considered as a limiting factor for tobacco production (Zheng et al. 2013; Ye et al. 2022).
TMV, possesses a single-stranded RNA genome. Its genome is approximately 6.5 kb long and encodes at least four proteins, including 183 kDa (p183), 126 kDa (p126), movement protein (MP), and coat protein (CP) (Saito et al. 1987). TMV p126 includes a helicase (HEL) domain, two non-conserved regions (NONI and NONII), and an N-terminal methyltransferase (MET) domain (Shintaku et al. 1996; Ding et al. 2004). These domains of p126 play essential roles in viral replication and movement (Lewandowski and Dawson 2000; Hirashima and Watanabe 2001), and in counteracting the host RNA silencing defense mechanisms (Ding et al. 2004). Specific amino acids critical for p126 functions and TMV virulence have been identified. Mutation of serine (S) at position 643 to phenylalanine (F) in p126 inhibited the replication but not the cell-to-cell movement of the TMV U1 strain (Lewandowski and Dawson 1993). A single amino acid substitution of lysine (K) at position 669 with arginine (R) affected the RNA silencing suppression activity of p126 (Wang et al. 2012). Mutation of glutamic acid (E) at position 601 to K in p126 attenuated the virulence of the TMV-U1 strain (Bao et al. 1996; Shintaku et al. 1996). Therefore, p126 is an important target for screening attenuated TMV mutants.
The cross-protection phenomenon was first discovered in 1929 (McKinney 1929). RNA silencing and superinfection-exclusion are common explanations of cross-protection (Voinnet 2001; Folimonova et al. 2010; Folimonova 2013; Sanfaçon 2015). Although the exact mechanism of cross-protection remains obscure, it has been applied in controlling several plant viral diseases (Folimonova 2013; Agüero et al. 2018). Natural or artificial symptomless TMV mutants have been isolated and used to control TMV via cross-protection (Holmes 1934; Rast 1972, 1975; Yang et al. 2001). Obtaining genetically stable and attenuated mutants is a prerequisite for the application of cross protection (Ziebell and MacDiarmid 2017; Pechinger et al. 2019; Raja et al. 2022). However, the error-prone nature of RNA viruses will continually introduce mutation to their genomes (Reanney 1982; García-arenal et al. 1984), in which process some attenuated strains may become virulent strains. For examples, a spontaneous reverse mutation of R at position 528 to histidine (H) in p126 increased the virulence of the attenuated strain TMV V-69 (Snegireva et al. 2005); a spontaneous complementary mutation of glycine (G) at position 440 to R in helper component-proteinase (HC-Pro) rescued the virulence of the attenuated mutant of sugarcane mosaic virus (SCMV) (Xu et al. 2020). Therefore, producing stable attenuated mutants is a challenge for the application of cross-protection in controlling plant viral diseases.
In this study, we investigated the variability in progeny viruses of a movement-defective TMV mutant with a mutation in the conserved residue in p126, and found that a spontaneous complementary mutation could restore its virulence. Then, we developed a stable attenuated TMV mutant by engineering the complementary mutation site and evaluated its cross-protective potential in tobacco plants. This study provides an insight on the development of stable attenuated mutants for cross-protection.
Results
Mutation of the highly conserved R88 residue in p126 abolished the systemic movement of TMV in tobacco plants
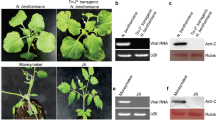
The conserved regions in viral genomes often play important roles in the virulence and symptom development of viruses (Xu et al. 2021; Murai et al. 2022). To screen the highly conserved residues in tobamoviral p126s, the amino acid sequences of 17 tobamoviruses were aligned. Results showed that the amino acid arginine at position 88 (R88) of TMV p126 was highly conserved (Fig. 1a, b).
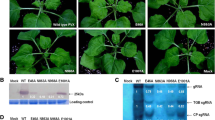
Effect of the conserved arginine at position 88 (R88) mutation inp126 on tobacco mosaic virus virulence. a Schematic representation of TMV genome, showing R88 and serine at position 114 (S114) in TMV p126. p126 contains four domains, including the N-terminal methyltransferase (MET) domain, two non-conserved regions (NONI and NONII) domains, and one helicase (HEL) domain. b Alignment of the partial amino acid sequences of 17 tobamovirus p126s using the BioEdit program version 7.2.5. The highly conserved R88 and the less-conserved S114 in TMV p126 were pointed by the red triangles. The analysis was performed with the p126 sequences of TMV (GenBank accession: MH595921), tomato brown rugose fruit virus (ToBRFV, GenBank accession: MT018320), tomato mosaic virus (ToMV, GenBank accession: NC_002692), cucumber green mottle mosaic virus (CGMMV, GenBank accession: NC_001801), brugmansia mild mottle virus (BrMMV, GenBank accession: NC_010944), kyuri green mottle mosaic virus (KGMMV, GenBank accession: NC_003610), obuda pepper virus (ObPV, GenBank accession: NC_003852), odontoglossum ringspot virus (ORSV, GenBank accession: NC_001728), paprika mild mottle virus (PaMMV, GenBank accession: NC_004106), pepper mild mottle virus (PMMoV, GenBank accession: NC_003630), rehmannia mosaic virus (ReMV, GenBank accession: NC_009041), ribgrass mosaic virus (RMV, GenBank accession: JQ319720), streptocarpus flower break virus (SFBV, GenBank accession: NC_008365), tobacco mild green mosaic virus (TMGMV, GenBank accession: NC_001556), turnip vein-clearing virus (TVCV, GenBank accession: NC_001873), wasabi mottle virus (WMoV, GenBank accession: NC_003355) and zucchini green mottle mosaic virus (ZGMMV, GenBank accession: NC_003878). c Symptoms of Nicotiana tabacum (tobacco) plants infected with the wild-type TMV and its mutant at 15 days post-agroinfiltration (dpai). Mock, tobacco plants inoculated with the empty vector pCB301-Rz. TMV, tobacco plants infected with the wild-type TMV. TMV-R88A, the residue of R88 was substituted with alanine (A) in p126 protein of TMV-R88A mutant. The inoculated (i) and upper (systemic, s) leaves of tobacco plants were pointed by white arrows. d Western blotting analysis of the wild type and mutant TMV CP accumulation levels in the inoculated (i) and upper (systemic, s) leaves at 15 dpai. The sample loadings were shown with the ponceau S staining (PSS). Band intensities were measured using the ImageJ software. Numbers indicated TMV CP accumulation levels normalized to PSS staining. e The sequencing result of p126 RT-PCR products from the inoculated (i) leaves infected with TMV and TMV-R88A mutant. The sites of point mutations in p126 were underlined and the corresponding amino acid residues were indicated. The experiments were repeated three times
To explore the role of R88 residue in regulating TMV virulence, we performed site-directed mutagenesis using primers listed in Additional file 1: Table S1, and obtained a TMV mutant plasmid named pTMV-R88A. This plasmid was transformed into Agrobacterium tumefaciens and inoculated to the lower fully expanded leaves of tobacco plants. The corresponding amino acid of R88 was alanine (A) in p126 derived from the progeny of TMV-R88A mutant. At 15 days post-agroinfiltration (dpai), the upper non-inoculated (systemic) leaves of tobacco plants infected with the wild-type TMV displayed typical mosaic symptoms. However, the upper leaves of tobacco plants inoculated with pTMV-R88A did not show any symptoms (Fig. 1c). Western blotting results showed that the CP accumulation levels of TMV-R88A were about 7% of the wild type TMV in the inoculated (i) tobacco leaves, but CP was not detected in the upper (systemic, s) leaves of pTMV-R88A-inoculated tobacco plants (Fig. 1d). The p126-encoding sequences of TMV progeny from pTMV-inoculated and pTMV-R88A-inoculated tobacco leaves were individually sequenced at 15 dpai. Results showed that only CGA (codon for R88) was replaced by GCA (codon for A) in the p126 coding sequence of TMV-R88A mutant progeny, compared with wild type TMV progeny (Fig. 1e). These results showed that the mutation of the highly conserved residue R88 in p126 to A reduced the accumulation levels of TMV RNA and abolished the systemic movement of TMV in tobacco plants.
A spontaneous complementary mutation of S114 to K in p126 restored the virulence of TMV
At 40 dpai, all the tobacco plants infected with wild type TMV showed severe mosaic symptoms. We noticed that 3 of 14 tobacco plants inoculated with pTMV-R88A displayed obvious mosaic symptoms in the upper leaves (Fig. 2a). Western blotting analysis confirmed the presence of CP in the upper leaves of tobacco plants inoculated with pTMV-R88A (Fig. 2b). To explore the possible reasons for the virulence restoration of TMV-R88A mutant, we sequenced and aligned the full-length coding sequences of TMV-R88A progeny in the upper leaves of these three tobacco plants. The results showed that TCG (the codon for S at position 114 (S114) spontaneously mutated to AAG (codon for K) in the p126 coding sequence of TMV-R88A mutant progeny from these three tobacco plants (Fig. 2c; Additional file 1: Table S2). To explore whether the spontaneous mutation of S114 to K occurred in the inoculated (i) leaves or upper (systemic, s) leaves, we sequenced the p126 coding sequence of TMV-R88A mutant progeny in these leaves. We found that the codon of the amino acid at position 114 of p126 in the upper leaves of pTMV-R88A-inoculated plants was only AAG (codon for K114), while the codons of the amino acid at position 114 of p126 in pTMV-R88A-inoculated leaves were TCG (codon for S114) and AAG (codon for K114) (Fig. 2d), indicating that the mutation of S114 to K occurred in pTMV-R88A-inoculated leaves and only the variant containing K114 could move to the upper leaves.
The spontaneous mutation of S114 to lysine (K) restored the virulence of TMV. a Symptoms caused by wild type and mutant TMV in tobacco plants at 40 dpai. Mock, tobacco plants mock-inoculated with the empty vector pCB301-Rz. TMV, tobacco plants infected the with wild-type TMV. TMV-R88Avariant, tobacco plants infected with the evolved variant of TMV-R88A mutant. The inoculated (i) and upper (systemic, s) leaves of tobacco plants were pointed by white arrows. The numbers of symptomatic/inoculated tobacco plants were listed in brackets. b Western blotting analysis of the wild type and mutant TMV CP accumulation levels in the upper leaves at 40 dpai. The sample loadings were shown with the ponceau S staining (PSS). c Alignment of partial nucleotide sequences of the wild-type TMV and the evolved variant of TMV-R88A mutant using the DNAMAN program version 7.0. The sites of point mutations in p126 were underlined and the corresponding amino acid residues were indicated. d The sequencing result of p126 RT-PCR products from the inoculated (i) and upper (systemic, s) leaves infected with the evolved variant of TMV-R88A mutant. The sites of point mutations in p126 were underlined and the corresponding amino acid residues were indicated. e Symptoms caused by the wild type TMV and its mutants in tobacco plants at 15 dpai. Mock, tobacco plants mock-inoculated with the empty vector pCB301-Rz. TMV, tobacco plants infected with the wild-type TMV. TMV-R88A, the residue of R88 was substituted with A in p126 protein of TMV-R88A mutant. TMV-S114K, the residue of S114 was substituted with K in p126 protein of TMV-S114K mutant. TMV-R88A-S114K, R88 was substituted with A, and S114 was substituted with K in TMV p126. f RT-qPCR analysis of TMV and mutants CP RNA accumulation levels in tobacco upper leaves at 15 dpai. The bar graphs represent the means ± standard deviations of three replicates. Statistically significant differences between means were determined by employing Tukey multiple range test for between-group comparisons. Different letters indicate significant differences (P < 0.05). g Western blotting analysis of the wild type and mutant TMV CP accumulation levels in tobacco upper leaves at 15 dpai. Band intensities were measured using the ImageJ software. Numbers indicated TMV CP accumulation levels normalized to PSS staining
To elucidate whether the mutation of S114 to K was responsible for the virulence restoration of TMV-R88A mutant, we constructed mutated plasmids pTMV-S114K and pTMV-R88A/S114K using primers listed in Additional file 1: Table S1. At 15 dpai, the systemically infected leaves of tobacco plants inoculated with pTMV-S114K and pTMV-R88A/S114K showed severe mosaic symptoms, similar to those inoculated with pTMV, while, as described above, TMV-R88A did not induce any symptoms in the upper leaves (Fig. 2e). Results of reverse transcription quantitative PCR (RT-qPCR) showed that the CP RNA accumulation levels of TMV and TMV-S114K in systemically infected leaves were at similar levels (Fig. 2f), indicating that the mutation of S114 to K alone did not affect TMV replication. TMV CP RNA was not detected in the upper leaves of pTMV-R88A-inoculated plants, but the CP RNA accumulation levels of TMV-R88A/S114K in systemically infected tobacco leaves were at the similar level with those of the wild type TMV (Fig. 2f), indicating that the mutation of S114 to K restored the replication level and rescued the movement defect of TMV-R88A mutant. Western blotting results showed that the CP accumulation levels of TMV, TMV-S114K, and TMV-R88A/S114K were also at a similar level, while CP was not detected in the upper leaves of tobacco plants inoculated with pTMV-R88A at 15 dpai (Fig. 2g).
Taken together, these results indicated that the spontaneous mutation of TCG (codon for S114) to AAG (codon for K114) was a complementary mutation that could restore the replication and rescue the virulence of TMV.
Mutation of S114 to K or R, but not H, rescued the cell-to-cell movement and replication of TMV
The net charge of a viral protein has been reported to be critical for some viruses to maintain their virulence (Kimalov et al. 2004; Chiang et al. 2007; Cong et al. 2019). The spontaneous complementary mutation of TMV-R88A was from the neutral amino acid S114 to positively-charged K114, which restored the net charge of TMV p126. To investigate the effect of the substitution of the neutral amino acid S114 with positively-charged R or H on the virulence of TMV-R88A, we generated plasmids pTMV-R88A/S114R and pTMV-R88A/S114H. These plasmids contain mutations in the codon for S114 residue of p126, resulting in an amino acid change to either R or H in the progeny viruses. At 15 dpai, TMV and TMV-R88A/S114K induced severe mosaic symptoms in the upper leaves, however, the upper leaves of tobacco plants inoculated with pTMV-R88A, pTMV-R88A/S114R, and pTMV-R88A/S114H were asymptomatic (Fig. 3a). Results of RT-qPCR showed that, same as the case of TMV-R88A, the p126 RNA levels of TMV-R88A/S114H were not detected in upper leaves (Additional file 2: Figure S1), indicating that the mutation of S114 to H did not rescue the systemic infection of TMV-R88A mutant. The p126 RNA accumulation levels of TMV-R88A/S114R in upper leaves were significantly lower than those of TMV and TMV-R88A/S114K (Additional file 2: Figure S1), indicating that the mutation of S114 to R rescued the systemic infection but not the RNA accumulation levels of TMV-R88A mutant. Western blotting results showed that the CP accumulation levels of TMV-R88A/S114R were substantially lower than those of TMV and TMV-R88A/S114K, while TMV-R88A and TMV-R88A/S114H were not detected in the upper leaves (Fig. 3b). These results indicated that the net charge of p126 was not responsible for TMV virulence.
The mutation of S114 to K or R rescued the cell-to-cell movement and replication of attenuated TMV. a Symptoms caused by the wild type TMV and its mutants in tobacco plants at 15 dpai. Mock, tobacco plants mock-inoculated with the empty vector pCB301-Rz. TMV, tobacco plants infected with the wild-type TMV. TMV-R88A, the residue of R88 was substituted with A in p126 protein of TMV-R88A mutant. TMV-R88A-S114K, R88 was substituted with A, and S114 was substituted with K in TMV p126. TMV-R88A-S114R, R88 was substituted with A, and S114 was substituted with R in TMV p126. TMV-R88A-S114H, R88 was substituted with A, and S114 was substituted with H in TMV p126. b Western blotting analysis of the wild type and mutant TMV CP accumulation levels in tobacco upper leaves at 15 dpai. Band intensities were measured using the ImageJ software. Numbers indicated TMV CP accumulation levels normalized to PSS staining. c Cell-to-cell movement of TMV-GFP and its four mutants in N. benthamiana leaves at 60 h post agroinfiltration (hpai). Confocal micrographs of N. benthamiana leave cells were taken at 60 hpai. The values are presented as means ± standard deviations from 30 infection foci per treatment. d The number of N. benthamiana leave cells infected with TMV-GFP and its four mutants. The values were presented as means ± standard deviations from 15 infection foci per treatment. e The relative accumulation levels of TMV CP (−) RNA in tobacco leaf protoplasts infected with TMV and its four mutants at 18 h post transfection. Statistically significant differences between means were determined by employing Tukey multiple range test for between-group comparisons. Different letters indicate significant differences (P < 0.05)
To investigate the effects of amino acid residues R88 and S114 in p126 on the cell-to-cell movement of TMV, we constructed mutated plasmids pTMV-GFP-R88A, pTMV-GFP-R88A/S114K, pTMV-GFP-R88A/S114R, and pTMV-GFP-R88A/S114H using primers listed in Additional file 1: Table S1. The Agrobacterium cultures carrying these plasmids were diluted to OD600 = 0.0001 and individually infiltrated into N. benthamiana leaves. GFP fluorescence from TMV-GFP was observed in clusters of multiple cells at 60 h post agroinfiltration (hpai). GFP fluorescence from TMV-GFP-R88A was confined to single leaf cells (Fig. 3c, d), indicating that R88 was crucial for TMV cell-to-cell movement. However, the GFP fluorescence from TMV-GFP-R88A/S114K and pTMV-GFP-R88A/S114R, but not pTMV-GFP-R88A/S114H, spread across multiple adjacent cells (Fig. 3c, d), indicating that the mutation of S114 to K or R, but not H, restored the cell-to-cell movement of TMV. To confirm the possible role of R88 and S114 in TMV replication, we conducted protoplast transfection assays. Protoplasts were isolated from tobacco leaves and then separately transfected with pTMV, pTMV-R88A, pTMV-R88A/S114K, pTMV-R88A/S114R, and pTMV-R88A/S114H. RT-qPCR was performed to monitor viral (−) RNA accumulation in protoplasts at 18 h post transfection. The viral (−) RNA accumulation levels of TMV-R88A/S114R were significantly higher than those of TMV-R88A and TMV-R88A/S114H (P < 0.05), but were significantly lower than those of TMV and TMV-R88A/S114K (P < 0.05, Fig. 3e).
These findings indicated that the mutation of R88 to A in p126 abolished TMV cell-to-cell movement and reduced its replication, but the complementary mutation of S114 to K or R could partially or completely rescue these functions of TMV p126. Furthermore, TMV-R88A/S114R mutant was an attenuated mutant that could systemically infect tobacco plants.
Attenuated mutant TMV-R88A/S114R was stable and could protect tobacco plants against the infection of wild type TMV
At 70 dpai, tobacco plants infected with TMV exhibited severe mosaic and deformation symptoms. However, all the tobacco plants infected with TMV-R88A/S114R did not show any noticeable symptoms in the upper leaves (Fig. 4a). The sequencing results of the p126-coding sequences derived from TMV-R88A/S114R progeny in systemically infected tobacco leaves revealed that no recovery mutation occurred at 70 dpai (Fig. 4b). Western blotting results showed that the CP accumulation levels of TMV-R88A/S114R decreased by about 68% for TMV in tobacco upper leaves (Fig. 4c).
Attenuated mutant TMV-R88A/S114R was stable and could protect tobacco plants against the wild type TMV infection. a Symptoms of TMV and TMV-R88A/S114R mutant in tobacco plants at 70 dpai. The images represented at least 13 tobacco plants. b The sequencing result of p126 RT-PCR products from the upper leaves infected with TMV-R88A/S114R mutant. The sites of point mutations in p126 were underlined and the corresponding amino acid residues were indicated. c Western blotting analysis of TMV and TMV-R88A/S114R mutant CP accumulation levels in tobacco leaves at 70 dpai. The sample loadings were shown with PSS. Band intensities were measured using the ImageJ software. Numbers indicated TMV CP accumulation levels normalized to PSS staining. d Symptoms of tobacco plants infected with TMV-R88A/S114R mutant during four serial passages at a 20-day interval. The images represented at least 12 tobacco plants. e The sequencing results of p126 RT-PCR products from the upper leaves infected with TMV-R88A/S114R mutant after four serial passages. The sites of point mutations in p126 were underlined and the corresponding amino acid residues were indicated. f Symptoms of tobacco plants challenged with the wild-type TMV at 20 days post-challenge inoculation with intervals of 7 or 14 dpi. Mock, tobacco plants were mock-inoculated with phosphate-buffered saline. Non-protected, tobacco plants inoculated with the empty vector pCB301-Rz. g Western blotting analysis of the CP accumulation levels in tobacco top leaves challenged with the wild-type TMV at 20 days post-challenge inoculation. Band intensities were measured using the ImageJ software. Numbers indicated TMV CP accumulation levels normalized to PSS staining. h With the interval of 14 days, the sequencing results of p126 RT-PCR products from the top leaves of tobacco plants protected by TMV-R88A/S114R mutant at 20 days post-challenge inoculation. The sites of point mutations in p126 were underlined and the corresponding amino acid residues were indicated
To test the genetic stability of TMV-R88A/S114R mutant, serial passages of progeny viruses from infected plants to healthy tobacco plants was performed at a 20-day interval. All the tobacco plants infected with TMV-R88A/S114R mutant did not induce visible symptoms during four serial passages (Fig. 4d). The p126-encoding sequences of TMV-R88A/S114R progeny were sequenced in tobacco plants after four serial passages. The sequencing results indicated that TMV-R88A/S114R were genetically stable in tobacco plants during four serial passages (Fig. 4e). All these results suggested that TMV-R88A/S114R mutant had the potential in cross-protection.
To assess the cross-protection efficacy of TMV-R88A/S114R mutant, we pre-inoculated tobacco plants with TMV-R88A/S114R mutant (Additional file 2: Figure S2). At 7- or 14-day protection intervals, the first fully expanded leaves of the tobacco plants were mechanically inoculated with the wild-type TMV. At 20 days post-challenge inoculation, the non-protected plants and the plants protected 7 days by TMV-R88A/S114R exhibited leaf distortion and mosaic symptoms, while the tobacco plants protected 14 days by TMV-R88A/S114R showed no symptom in the upper leaves (Fig. 4f). Western blotting results showed that the CP accumulation levels in TMV-R88A/S114R-protected plants with a 14-day protection interval were notably lower than those in the non-protected plants and TMV-R88A/S114R-protected plants with a 7-day protection interval (Fig. 4g). At 20 days post-challenge inoculation, the p126-coding sequences of TMV progeny in the top leaves of TMV-R88A/S114R-protected tobacco plants with a 14-day protection interval were determined. The sequencing results showed that the codons of the amino acids at positions 88 and 114 were GCA (codon for A88) and CGA (codon for R144), respectively (Fig. 4h), indicating TMV was completely excluded by TMV-R88A/S114R mutant with an interval of 14 days.
Discussion
In this study we found that the mutation of the conserved R88 in p126 to A abolished TMV movement and reduced its replication. However, a complementary mutation of S114 to K rescued TMV virulence and accumulation levels. We obtained an attenuated mutant TMV-R88A/S114R by substituting the complementary mutation site of S114 with R. Mutant TMV-R88A/S114R did not induce any symptoms in tobacco plants even at 70 dpai and could provide effective protection against the wild-type TMV infection with a 14-day protection interval. Therefore, it is feasible to construct promising attenuated strains by engineering the complementary mutation sites.
TMV p126 is a multifunctional protein and an important target for screening attenuated TMV mutants. The functions of the p126 HEL domain are to hydrolyze ATP and unwind RNA duplexes to promote viral replication (Goregaoker et al. 2001; Goregaoker and Culver 2003). The p126 NONI and NONII domains function in virus replication and symptom development (Bao et al. 1996; Shintaku et al. 1996). Mutation of S at position 643 in the NONII domain of p126 to F affected the replication of the TMV-U1 strain (Lewandowski and Dawson 1993). The MET, NONII, and HEL domains of p126 are involved in the suppression activity of RNA silencing (Ding et al. 2004; Wang et al. 2012). Viral suppressors of RNA silencing are potential candidates for screening attenuated mutants. Substitution of R in the conserved FRNK motif of HC-Pro with I could reduce the virulence of tobacco vein banding mosaic virus (TVBMV), zucchini yellow mosaic virus (ZYMV), and turnip mosaic virus (TuMV) (Wu et al. 2010; Gao et al. 2012; Kung et al. 2014). The p126 MET domain also plays a vital role in viral cell-to-cell movement and interacts with the translation elongation factor 1A (Knapp et al. 2005; Yamaji et al. 2006). A spontaneous mutation of amino acid leucine (L) at position 11 in the p126 MET domain to A attenuated the symptoms of tomato mosaic virus in tobacco and tomato plants (Oshima et al., 1965). In this study, we found that the mutation of R88 to A in p126 MET domain reduced the replication levels of TMV. The mutant TMV-R88A could not be detected from the non-inoculated upper leaves of tobacco plants, but could be detected from the inoculated areas (Fig. 1d and Additional file 2: Figure S3), indicating that this mutant was viable. Microscopy observation results indicated that the mutant TMV-R88A could not move from cell to cell (Fig. 3c). Therefore, we concluded that the mutation of R88 to A in p126 MET domain abolished TMV cell-to-cell movement, thus abolished the systemic movement of TMV.
RNA viral polymerases lack proofreading and repair activity, resulting in high mutation rates (Scheel et al. 2013). Conserved regions of virus genomes are usually responsible for determining virus virulence (Yoon et al. 2006; Liu et al. 2020). The substitution of the conserved cysteine (C) at positions 57 or 60 in the zinc finger-like motif of HC-Pro with A significantly reduced the virulence of SCMV (Xu et al. 2021). Some mutations occurred in the variable region resulting in progeny with enhanced competitiveness (Torres-Barceló et al. 2008; Haikonen et al. 2013; Ambrós et al. 2018). The mutation of R in the conserved FRNK motif of HC-Pro to I reduced SCMV virulence. However, a spontaneous complementary mutation of the non-conserved G at position 440 to R in HC-Pro rescued SCMV virulence (Xu et al. 2020). The substitution of valine (V) at position 192 in HC-Pro with A reduced the virulence of tobacco etch virus (TEV). A compensatory mutation of tyrosine (Y) in position 642 to C restored TEV virulence (Torres-Barceló et al., 2009). In this study, we found that the mutation of the highly conserved R88 to A in p126 affected the virulence and symptom development of TMV (Fig. 1), however, a spontaneous mutation of the less-conserved S114 to K in p126 restored TMV virulence (Fig. 2). The lack of proofreading and repair mechanisms in the polymerases of RNA viruses results in RNA genome populations containing high levels of genetic heterogeneity. Malpica et al. found that TMV MP mutant frequency was 0.02–0.05, and 35% of the sequenced mutants contained two or more mutations (Malpica et al. 2002). In this study, we found that a mutation of R116 to K in the MP coding sequence of the TMV-R88A mutant progeny occurred in one tobacco plant. However, the mutation of S114 to K in the p126 coding sequence of TMV-R88A mutant progeny occurred in all three tobacco plants (Additional file 1: Table S2). To elucidate whether the mutation of S114K in the p126 was responsible for the virulence restoration of TMV-R88A mutant and exclude the effect of other possible mutations, we introduced the mutation of S114K into the original plasmid pTMV-R88A. The double-mutant plasmid pTMV-R88A/S114K induced severe mosaic symptoms in tobacco plants as the wild-type TMV did (Fig. 2e) The CP accumulation levels of TMV-R88A/S114K in the systemically infected leaves of tobacco plant were also restored (Fig. 2g). These results supported the conclusion that the mutation of S114 to K was the key for virulence restoration of the TMV-R88A mutant.
Mild strain-mediated cross-protection is an efficient way to control plant virus diseases (Ziebell and Carr 2010; Cheng et al. 2023; Goh et al. 2023). There are various methods for developing mild strains. The traditional method of identifying attenuated strains is selection from naturally occurring strains. A naturally occurring mild variant of ZYMV-WK could effectively protect against the wild-type ZYMV infection in two cultivars of zucchini squash (Lecoq et al. 1991). Attenuated viruses can be obtained by thermal treatment of infected plants (Desjardins et al. 1959). Random chemical mutagenesis was also used to develop attenuated viruses of TMV and papaya ringspot virus (PRSV) (Rast 1972; Yeh and Cheng 1989). High-throughput sequencing is an emerging tool for discovering potentially cross-protective strains (Cook et al. 2016; Kamitani et al. 2016), but this approach has several limitations (Pechinger et al. 2019). Recently, reverse genetics had been adopted to develop useful mild mutants (Tran et al. 2023). Some attenuated mutants of PRSV and East Asian passiflora virus (EAPV) were constructed by mutating HC-Pro that the pathogenicity determinant of potyviruses (Cheng et al. 2023; Chong et al. 2023). The mild strain should be capable of systemic infection, which is a requirement for its cross-protection (Pechinger et al. 2019). In this study, we found that the mutation of R88 to A in p126 affected the systemic infection of TMV (Fig. 1). Substituting the complementary mutation site of S114 in p126 with R restored the systemic infection, but not virulence, of the movement-defective TMV-R88A mutant (Fig. 3). Furthermore, TMV-R88A/S114R mutant was a stable attenuated strain and have the potential for controlling of wild-type TMV in tobacco plants (Fig. 4).
Conclusions
Our results demonstrate that the conserved R88 residue in the p126 MET domain is involved in the cell-to-cell movement and replication of TMV, and that the attenuated TMV mutant can be a promising tool for cross-protection by engineering the complementary mutation site. These results provide a novel method for the development of attenuated virus mutants for cross-protection.
Methods
Plant growth
N. benthamiana and tobacco N. tabacum cultivar Zhongyan 100 plants were used in this study. The plants were grown in a chamber under controlled conditions at 24°C with a 16-h light and 8-h dark photoperiod, and light intensity of 125 μmol/m2/s.
Plasmid construction
The infectious clone of the wild-type TMV (pTMV) based on TMV Mudanjiang strain (GenBank accession: MH595921) and the GFP-expressing infectious clones of TMV (pTMV-GFP) were kept in the laboratory of Professor Xiangdong Li, Shandong Agricultural University. To introduce mutations into the p126 protein, site-directed mutagenesis was performed as reported previously (Liu and Naismith 2008). Each primer used for constructing the TMV mutants were listed in Additional file 1: Table S1.
Virus inoculation
Each construct was transformed into Agrobacterium tumefaciens strain GV3101 via the freeze-thaw method (Jyothishwaran et al. 2007). The transformed Agrobacterium carrying each construct was cultured overnight at 28°C and collected by centrifugation at 6000 g at 25°C. Then, the Agrobacterium cultures were resuspended in induction buffer (10mM MgCl2, 150μM acetosyringone, and 10mM 2-(N-Morpholino) ethane sulfonic acid (MES)) to a cell concentration with an OD600 of 0.5 and incubated at 28°C for 3 hours. The lower two fully expanded leaves of tobacco plants were infiltrated with the transformed Agrobacterium carrying each construct. The plants inoculated with the empty vector pCB301-Rz were used as negative controls. To determine viral cell-to-cell movement, the Agrobacterium cultures were diluted to an OD600 of 0.0001 and individually infiltrated into N. benthamiana leaves (Yan et al. 2020; Yan et al. 2021). GFP fluorescence from TMV-GFP and its mutants was observed using a Zeiss LSM510 META laser scanning microscope at 60 hpai. GFP was excited at 488 nm and the emitted signal was captured at 505–530 nm. The number of N. benthamiana cells showing fluorescence was manually counted. The average numbers of fluorescent cells from 30 infection foci per treatment were calculated. The experiments were repeated three times.
RNA extraction and RT-qPCR
The total RNA of leaf tissues was isolated using TRIZol reagent from TransGen Biotech Co., Ltd. (Beijing, China), according to the manufacturer’s instructions. 500 ng total RNA was used as a template to synthesis the first-strand cDNA via HiScript II Q RT select SuperMix (+gDNA wiper; Vazyme, Nanjing, China). RT-qPCR was performed using ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) on a LC96 Real-Time System (Roche, Basel, Switzerland). The housekeeping 18S rRNA gene (GenBank accession: HQ384692) in tobacco plants was used as an internal control for the assay (Ellis et al. 2020). The experiments were repeated three times.
Tobacco protoplasts isolation and transfection
Protoplasts were isolated from tobacco leaves and transfected using a polyethylene glycol (PEG)-mediated method (Jiang et al. 2021). Approximately 4 × 105 tobacco protoplasts were gently mixed with 10 μg plasmid DNA and 40% polyethylene glycol (PEG) solution (40% PEG4000 (MW 4000, Fluka), 0.4 M mannitol, and 100 mM CaCl2) at 25°C for 20 min. The protoplasts were gently washed in an equal volume of cold W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM MES, pH 5.7) and collected by centrifugation at 150 g for 1 min. The protoplasts were resuspended in 1 mL W5 solution and incubated at room temperature. The total RNA was extracted from 1 × 105 protoplasts for RT-qPCR analysis at 18 h post transfection. The experiments were repeated three times.
Western blotting
Total proteins of 100 mg leaf tissues were extracted with 200 μL of lysis buffer (100 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.6% SDS (w/v), and 2% β-mercaptoethanol). Protein extracts were separated on 12% SDS-PAGE gels and transferred onto nitrocellulose membrane (Pall Gelman, New York, USA) (Sun and Suzuki 2008). Polyclonal antibodies specific for TMV CP prepared in our laboratory were used as the primary antibodies. The horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma-Aldrich, St. Louis, MO, USA) was used as the secondary antibody. The amounts of TMV CP were quantified by ImageJ software (Wyrsch et al. 2015). The experiments were repeated three times.
Confocal microscopy
To monitor viral intercellular movement in N. benthamiana leaves, the Agrobacterium-infiltrated leaf patches were collected and visualized by a Zeiss LSM510 META laser scanning microscope using the ZEN blue version 2.1. GFP was excited at 488 nm and the emitted signal was captured at 505–530 nm. The images were captured at 60 hpai.
Cross protection assay
The lower two fully expanded leaves of tobacco plants at the five-leaf stage were first infiltrated with the Agrobacterium culture carrying the plasmid of the attenuated TMV mutant for protective inoculation. Tobacco plants inoculated with Agrobacterium carrying pCB301-Rz plasmid were used as non-protected controls. At 5 or 10 days after the protective inoculation (in the following text these periods are referred to as the protective intervals), the top fully expanded tobacco leaves were mechanically challenge-inoculated with the wild-type TMV prepared from crude extracts of infected tobacco leaves. Plants were analyzed by visual observation of leaf, western blotting for TMV CP, and sequencing of p126 coding sequences at 20 days after challenge inoculation.
Stability test
Tobacco plants were infiltrated with the Agrobacterium culture carrying the plasmid of TMV mutant. The p126 coding sequences of TMV mutant progeny in the systemically infected tobacco leaves were sequenced at 70 dpai. In addition, serial passaging of viruses from infected plants to fresh uninfected plants was performed at a 20-day interval. After four serial passages, the p126-coding sequences of TMV mutant progeny in tobacco leaves were sequenced.
Availability of data and materials
Not applicable.
Abbreviations
- dpai:
-
Days post-agroinfiltration
- hpai:
-
h post agroinfiltration
- MES:
-
2-(N-Morpholino) ethane sulfonic acid
- TMV:
-
Tobacco mosaic virus
References
Agüero J, Gómez-Aix C, Sempere RN, García-Villalba J, García-Núñez J, Hernando Y, et al. Stable and broad spectrum cross-protection against Pepino mosaic virus attained by mixed infection. Front Plant Sci. 2018;9:1810. https://doi.org/10.3389/fpls.2018.01810.
Ambrós S, de la Iglesia F, Rosario SM, Butković A, Elena SF, Abergel C. Engineered functional redundancy relaxes selective constraints upon endogenous genes in viral RNA genomes. Genome Biol Evol. 2018;10:1823–36. https://doi.org/10.1093/gbe/evy141.
Bao Y, Carter SA, Nelson RS. The 126-and 183-kilodalton proteins of tobacco mosaic virus, and not their common nucleotide sequence, control mosaic symptom formation in tobacco. J Virol. 1996;70:6378–83. https://doi.org/10.1128/jvi.70.9.6378-6383.1996.
Cheng HW, Lin TT, Huang CH, Raja JA, Yeh SD. Modification of papaya ringspot virus HC-pro to generate effective attenuated mutants for overcoming the problem of strain-specific cross protection. Plant Dis. 2023;107:1757–68. https://doi.org/10.1094/PDIS-05-22-1130-RE.
Chiang CH, Lee CY, Wang CH, Jan FJ, Lin SS, Chen TC, et al. Genetic analysis of an attenuated papaya ringspot virus strain applied for cross-protection. Eur J Plant Pathol. 2007;118:333–48. https://doi.org/10.1007/s10658-007-9130-z.
Chong YH, Do DH, Cheng HW, Raja JA, Ngo XT, Hwang SG, et al. Generation of attenuated mutants of east Asian Passiflora virus for disease management by cross protection. Mol Plant-Microbe Interact. 2023;36:345–58. https://doi.org/10.1094/MPMI-11-22-0238-R.
Cong Q, Wang Y, Liu J, Lan Y, Guo Z, Yang J, et al. Evaluation of potato virus X mild mutants for cross protection against severe infection in China. Virol J. 2019;16:36. https://doi.org/10.1186/s12985-019-1143-7.
Cook G, van Vuuren SP, Breytenbach JH, Burger JT, Maree HJ. Expanded strain-specific RT-PCR assay for differential detection of currently known citrus tristeza virus strains: a useful screening tool. J Phytopathol. 2016;164:847–51. https://doi.org/10.1111/jph.12454.
Desjardins P, Wallace JM, Wollman E, Drake R. A separation of virus strains from a tristeza--seedling-yellows complex by heat treatment of infected lime seedlings. Proceeding of the International Organization of Citrus Virologists Conference Proceedings (1957-2010): Citrus Virus Dis. 1959;1:91–5. https://doi.org/10.5070/C52v6009cn.
Ding X, Liu J, Cheng N, Folimonov A, Hou Y, Bao Y, et al. The tobacco mosaic virus 126-kDa protein associated with virus replication and movement suppresses RNA silencing. Mol Plant-Microbe Interact. 2004;17:583–92. https://doi.org/10.1094/MPMI.2004.17.6.583.
Ellis MD, Hoak JM, Ellis BW, Brown JA, Sit TL, Wilkinson CA, et al. Quantitative real-time PCR analysis of individual flue-cured tobacco seeds and seedlings reveals seed transmission of tobacco mosaic virus. Phytopathology. 2020;110:194–205. https://doi.org/10.1094/PHYTO-06-19-0201-FI.
Folimonova SY. Developing an understanding of cross-protection by Citrus tristeza virus. Front Microbiol. 2013;4:76. https://doi.org/10.3389/fmicb.2013.00076.
Folimonova SY, Robertson CJ, Shilts T, Folimonov AS, Hilf ME, Garnsey SM, et al. Infection with strains of Citrus tristeza virus does not exclude superinfection by other strains of the virus. J Virol. 2010;84:1314–25. https://doi.org/10.1128/jvi.02075-09.
Gao R, Tian Y, Wang J, Yin X, Li X, Valkonen JP. Construction of an infectious cDNA clone and gene expression vector of tobacco vein banding mosaic virus (genus Potyvirus). Virus Res. 2012;169:276–81. https://doi.org/10.1016/j.virusres.2012.07.010.
García-arenal F, Palukaitis P, Zaitlin M. Strains and mutants of tobacco mosaic virus are both found in virus derived from single-lesion-passaged inoculum. Virology. 1984;132:131–7. https://doi.org/10.1016/0042-6822(84)90097-7.
Goh RP, Xie XY, Lin YC, Cheng HW, Raja JA, Yeh SD. Rapid selection of potyviral cross-protection effective mutants from the local lesion host after nitrous acid mutagenesis. Mol Plant Pathol. 2023;24:973–88. https://doi.org/10.1111/mpp.13346.
Goregaoker SP, Culver JN. Oligomerization and activity of the helicase domain of the tobacco mosaic virus 126-and 183-kilodalton replicase proteins. J Virol. 2003;77:3549–56. https://doi.org/10.1128/jvi.77.6.3549-3556.2003.
Goregaoker SP, Lewandowski DJ, Culver JN. Identification and functional analysis of an interaction between domains of the 126/183-kDa replicase-associated proteins of tobacco mosaic virus. Virology. 2001;282:320–8. https://doi.org/10.1006/viro.2001.0831.
Haikonen T, Rajamäki ML, Tian YP, Valkonen JPT. Mutation of a short variable region in HCpro protein of potato virus a affects interactions with a microtubule-associated protein and induces necrotic responses in tobacco. Mol Plant-Microbe Interact. 2013;26:721–33. https://doi.org/10.1094/mpmi-01-13-0024-r.
Hirashima K, Watanabe Y. Tobamovirus replicase coding region is involved in cell-to-cell movement. J Virol. 2001;75:8831–6. https://doi.org/10.1128/JVI.75.18.8831-8836.2001.
Holmes FO. A masked strain of tobacco mosaic virus. Phytopathology. 1934;24:845–73.
Jiang Y, Zheng W, Li J, Liu P, Zhong K, Jin P, et al. NbWRKY40 positively regulates the response of Nicotiana benthamiana to tomato mosaic virus via salicylic acid signaling. Front Plant Sci. 2021;11:603518. https://doi.org/10.3389/fpls.2020.603518.
Jones RA. Global plant virus disease pandemics and epidemics. Plants. 2021;10:233. https://doi.org/10.3390/plants10020233.
Jyothishwaran G, Kotresha D, Selvaraj T, Srideshikan S, Rajvanshi P, Jayabaskaran C. A modified freeze–thaw method for efficient transformation of Agrobacterium tumefaciens. Curr Sci. 2007;93:770–2.
Kamitani M, Nagano AJ, Honjo MN, Kudoh H. RNA-Seq reveals virus–virus and virus–plant interactions in nature. FEMS Microbiol Ecol. 2016;92:176. https://doi.org/10.1093/femsec/fiw176.
Kimalov B, Gal-On A, Stav R, Belausov E, Arazi T. Maintenance of coat protein N-terminal net charge and not primary sequence is essential for zucchini yellow mosaic virus systemic infectivity. J Gen Virol. 2004;85:3421–30. https://doi.org/10.1099/vir.0.80417-0.
Knapp E, Danyluk GM, Achor D, Lewandowski DJ. A bipartite tobacco mosaic virus-defective RNA (dRNA) system to study the role of the N-terminal methyl transferase domain in cell-to-cell movement of dRNAs. Virology. 2005;341:47–58. https://doi.org/10.1016/j.virol.2005.06.032.
Kung Y, Lin P, Yeh S, Hong S, Chua N, Liu L, et al. Genetic analyses of the FRNK motif function of turnip mosaic virus uncover multiple and potentially interactive pathways of cross-protection. Mol Plant-Microbe Interact. 2014;27:944–55. https://doi.org/10.1094/MPMI-04-14-0116-R.
Lecoq H, Lemaire J, Wipf-Scheibel C. Control of zucchini yellow mosaic virus in squash by cross protection. Plant Dis. 1991;75:208–11. https://doi.org/10.1094/pd-75-0208.
Lewandowski DJ, Dawson WO. A single amino acid change in tobacco mosaic virus replicase prevents symptom production. Mol Plant-Microbe Interact. 1993;6:157. https://doi.org/10.1094/MPMI-6-157.
Lewandowski DJ, Dawson WO. Functions of the 126-and 183-kDa proteins of tobacco mosaic virus. Virology. 2000;271:90–8. https://doi.org/10.1006/viro.2000.0313.
Liu H, Naismith JH. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 2008;8:91. https://doi.org/10.1186/1472-6750-8-91.
Liu J, Li X-D, Xu S. Single amino acid substitutions in the coat protein and RNA-dependent RNA polymerase alleviated the virulence of cucumber green mottle mosaic virus and conferred cross protection against severe infection. Virus Genes. 2020;56:228–35. https://doi.org/10.1007/s11262-019-01726-3.
Malpica JM, Fraile A, Moreno I, Obies CI, Drake JW, García-Arenal F. The rate and character of spontaneous mutation in an RNA virus. Genetics. 2002;162:1505–11. https://doi.org/10.1093/genetics/162.4.1505.
McKinney H. Mosaic diseases in the Canary Islands, West Africa and Gibraltar. J Agric Res. 1929;39:577–8.
Murai H, Atsumaru K, Mochizuki T. Effect of mutations in the 2b protein of tomato aspermy virus on RNA silencing suppressor activity, virulence, and virus-induced gene silencing. Arch Virol. 2022;167:471–81. https://doi.org/10.1007/s00705-021-05344-z.
Oshima N, Komochi S, Goto T. Study on control of plant virus diseases by vaccination of attenuated virus.(1) Control of tomato mosaic disease. Hokkaido Natl Agric Exp Stn Res Bull. 1965;85:23-33.
Pechinger K, Chooi KM, MacDiarmid RM, Harper SJ, Ziebell H. A new era for mild strain cross-protection. Viruses. 2019;11:670. https://doi.org/10.3390/v11070670.
Raja JA, Huang CH, Chen CC, Hu WC, Cheng HW, Goh RP, et al. Modification of the N-terminal FWKG-αH1 element of potyviral HC-pro affects its multiple functions and generates effective attenuated mutants for cross-protection. Mol Plant Pathol. 2022;23:947–65. https://doi.org/10.1111/mpp.13201.
Rast ATB. M II-16, an artificial symptomless mutant of tobacco mosaic virus for seedling inoculation of tomato crops. Neth J Plant Pathol. 1972;78:110–2. https://doi.org/10.1007/bf01980475.
Rast ATB. Variability of tobacco mosaic virus in relation to control of tomato mosaic in glasshouse tomato crops by resistance breeding and cross protection. Agric Res Rep. 1975:834.
Reanney D. The evolution of RNA viruses. Ann Rev Microbiol. 1982;36:47–73. https://doi.org/10.1146/annurev.mi.36.100182.000403.
Saito T, Meshi T, Takamatsu N, Okada Y. Coat protein gene sequence of tobacco mosaic virus encodes a host response determinant. Proc Natl Acad Sci USA. 1987;84:6074–7. https://doi.org/10.1073/pnas.84.17.6074.
Sanfaçon H. Plant translation factors and virus resistance. Viruses. 2015;7:3392–419. https://doi.org/10.3390/v7072778.
Scheel TK, Galli A, Li Y-P, Mikkelsen LS, Gottwein JM, Bukh J. Productive homologous and non-homologous recombination of hepatitis C virus in cell culture. PLoS Pathog. 2013;9:e1003228. https://doi.org/10.1371/journal.ppat.1003228.
Scholthof KBG, Adkins S, Czosnek H, Palukaitis P, Jacquot E, Hohn T, et al. Top 10 plant viruses in molecular plant pathology. Mol Plant Pathol. 2011;12:938–54. https://doi.org/10.1111/j.1364-3703.2011.00752.x.
Shintaku MH, Carter SA, Bao Y, Nelson RS. Mapping nucleotides in the 126-kDa protein gene that control the differential symptoms induced by two strains of tobacco mosaic virus. Virology. 1996;221:218–25. https://doi.org/10.1006/viro.1996.0368.
Snegireva P, Istomina E, Shiyan A. A single reverse mutation in the 126/183-kDa replicase gene of the attenuated tomato strain V-69 of tobacco mosaic virus increases the virus pathogenicity. Russ J Genet. 2005;41:32–9. https://doi.org/10.1007/PL00022107.
Song Y, Liu L, Wang Y, Valkenburg DJ, Zhang X, Zhu L, et al. Transfer of tomato immune receptor Ve1 confers Ave1-dependent Verticillium resistance in tobacco and cotton. Plant Biotechnol J. 2018;16:638–48. https://doi.org/10.1111/pbi.12804.
Sun L, Suzuki N. Intragenic rearrangements of a mycoreovirus induced by the multifunctional protein p29 encoded by the prototypic hypovirus CHV1-EP713. RNA. 2008;14:2557–71. https://doi.org/10.1261/rna.1125408.
Torres-Barceló C, Daròs JA, Elena SF. Compensatory molecular evolution of HC-Pro, an RNA-silencing suppressor from a plant RNA virus. Mol Biol Evol. 2009;27:543-51. https://doi.org/10.1093/molbev/msp272.
Torres-Barceló C, Martín S, Daròs J-A, Elena SF. From hypo-to hypersuppression: effect of amino acid substitutions on the RNA-silencing suppressor activity of the tobacco etch potyvirus HC-pro. Genetics. 2008;180:1039–49. https://doi.org/10.1534/genetics.108.091363.
Tran TTY, Cheng HW, Nguyen VH, Yeh SD. Modification of the helper component proteinase of papaya ringspot virus Vietnam isolate to generate attenuated mutants for disease management by cross protection. Phytopathology. 2023;113:334–44. https://doi.org/10.1094/PHYTO-05-22-0168-R.
Voinnet O. RNA silencing as a plant immune system against viruses. Trends Genet. 2001;17:449–59. https://doi.org/10.1016/S0168-9525(01)02367-8.
Wang LY, Lin SS, Hung TH, Li TK, Lin NC, Shen TL. Multiple domains of the tobacco mosaic virus p126 protein can independently suppress local and systemic RNA silencing. Mol Plant-Microbe Interact. 2012;25:648–57. https://doi.org/10.1094/MPMI-06-11-0155.
Wu HW, Lin SS, Chen KC, Yeh SD, Chua NH. Discriminating mutations of HC-pro of zucchini yellow mosaic virus with differential effects on small RNA pathways involved in viral pathogenicity and symptom development. Mol Plant-Microbe Interact. 2010;23:17–28. https://doi.org/10.1094/MPMI-23-1-0017.
Wyrsch I, Domínguez-Ferreras A, Geldner N, Boller T. Tissue-specific FLAGELLIN-SENSING 2 (FLS2) expression in roots restores immune responses in Arabidopsis fls2 mutants. New Phytol. 2015;206:774–84. https://doi.org/10.1111/nph.13280.
Xu XJ, Li HG, Cheng DJ, Liu LZ, Geng C, Tian YP, et al. A spontaneous complementary mutation restores the RNA silencing suppression activity of HC-pro and the virulence of sugarcane mosaic virus. Front Plant Sci. 2020;11:1279. https://doi.org/10.3389/fpls.2020.01279.
Xu XJ, Zhu Q, Jiang SY, Yan ZY, Geng C, Tian YP, et al. Development and evaluation of stable sugarcane mosaic virus mild mutants for cross-protection against infection by severe strain. Front Plant Sci. 2021;12:788963. https://doi.org/10.3389/fpls.2021.788963.
Yamaji Y, Kobayashi T, Hamada K, Sakurai K, Yoshii A, Suzuki M, et al. In vivo interaction between tobacco mosaic virus RNA-dependent RNA polymerase and host translation elongation factor 1A. Virology. 2006;347:100–8. https://doi.org/10.1016/j.virol.2005.11.031.
Yan ZY, Cheng DJ, Liu LZ, Geng C, Tian YP, Li XD, et al. The conserved aromatic residue W122 is a determinant of potyviral coat protein stability, replication, and cell-to-cell movement in plants. Mol Plant Pathol. 2020;22:189–203. https://doi.org/10.1111/mpp.13017.
Yan ZY, Xu XJ, Fang L, Geng C, Tian YP, Li XD. Multiple aromatic amino acids are involved in potyvirus movement by forming π-stackings to maintain coat protein accumulation. Phytopathol Res. 2021;3:10. https://doi.org/10.1186/s42483-021-00088-9.
Yang G, Qiu B, Wei J, Liu G. Effects of mutated replicase and movement protein genes on attenuation of tobacco mosaic virus. Sci China Ser C Life Sci. 2001;44:628–36. https://doi.org/10.1007/BF02879357.
Ye J, Song J, Gao Y, Lu X, Pei W, Li F, et al. An automatic fluorescence phenotyping platform to evaluate dynamic infection process of tobacco mosaic virus-green fluorescent protein in tobacco leaves. Front Plant Sci. 2022;13:968855. https://doi.org/10.3389/fpls.2022.968855.
Yeh SD, Cheng YH. Use of resistant Cucumis metuliferus for selection of nitrous-acid induced attenuated strains of papaya ringspot virus. Phytopathology. 1989;79:1033–9. https://doi.org/10.1094/Phyto-79-1257.
Yoon JY, Ahn HI, Kim M, Tsuda S, Ryu KH. Pepper mild mottle virus pathogenicity determinants and cross protection effect of attenuated mutants in pepper. Virus Res. 2006;118:23–30. https://doi.org/10.1016/j.virusres.2005.11.004.
Zheng L, Changsheng X, Yongguang X, Anquan C, Dengrong Z, Mingfu C, et al. Occurrence regularity and control of tobacco mosaic virus (TMV) in Wuxi tobacco area of China in 2011. Plant Dis Pests. 2013;4:23. https://doi.org/CNKI:SUN:PDSP.0.2013-01-008
Ziebell H, Carr JP. Cross-protection: a century of mystery. Adv Virus Res. 2010;76:211–64. https://doi.org/10.1016/S0065-3527(10)76006-1.
Ziebell H, MacDiarmid R. Prospects for engineering and improvement of cross-protective virus strains. Curr Opin Virol. 2017;26:8–14. https://doi.org/10.1099/vir.0.83138-0.
Acknowledgements
We are grateful to Dr. Hongxia Zhang, Engineering Research Institute of Agriculture and Forestry, Ludong University, for his help.
Funding
This work was supported by funds from ‘Taishan Scholar’Construction Project (TS2022-028; 202101-KN275).
Author information
Authors and Affiliations
Contributions
XX and XL designed the experiments; XX, XH, XM, and SJ performed the experiments; XX, XS, QZ, and YT analyzed the data; XX, CG, and XL wrote the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1: Table S1.
The name and sequence of primers used in this study. Table S2. Variability in the coding sequences of TMV-R88A mutant progeny at 40 dpai.
Additional file 2: Figure S1.
RT-qPCR analysis of TMV and mutants p126 RNA accumulation levels in tobacco upper leaves at 15 dpai. Figure S2. Western blotting analysis of the CP accumulation levels in tobacco top leaves infected with TMV and TMV-R88A/S114R mutant at 7 and 14 dpai. Figure S3. Western blotting analysis of the CP accumulation levels in the inoculated leaves infected with TMV, TMV-R88A, and TMV-R88A/S114H mutants at 5 dpai.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, X., Huan, X., Mu, X. et al. Engineering of the complementary mutation site in tobacco mosaic virus p126 to develop a stable attenuated mutant for cross-protection. Phytopathol Res 6, 27 (2024). https://doi.org/10.1186/s42483-024-00246-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42483-024-00246-9